Introduction
Due to the progressive aging of populations
worldwide, osteoporosis is a growing public health concern, with
increasing prevalence among aging individuals, particularly
postmenopausal women. Although osteoporosis has been recognized as
a disease entity for almost a century, therapeutic approaches are
limited, since the pathogenesis of postmenopausal osteoporosis
(PMOP) is complex and not yet fully elucidated. Thus, recent
progress towards understanding the role of iron accumulation in
PMOP is crucial, since it may expose the underlying mechanisms and
aid the treatment of this bone disease.
Iron is one of the most abundant transition metals
in the human body, and serves a key function in numerous biological
processes, including oxygen transport, DNA synthesis and energy
production (1). However, excessive
iron is deleterious to organ function (2). If the iron concentration in the
circulation exceeds the binding capacity of transferrin, an
iron-binding blood plasma glycoprotein, then free iron or
non-transferrin-bound iron becomes abnormally enriched in various
organs, including the liver, heart, brain and pancreas (3). As a consequence, organs are subject to
potentially irreversible damage. Previously, Weinberg (4,5)
hypothesized that iron overload is a risk factor for osteoporosis.
In women, the levels of iron in the form of ferritin (an iron
storage protein) have been observed to increase markedly following
menopause (6). Furthermore, previous
studies have indicated that increasing iron concentrations
contribute towards the development of PMOP by enhancing bone
resorption and suppressing bone formation, a mode of action which
is independent from that of estrogen (7,8). A
reduction in iron levels, using either hepcidin (a negative
regulator of iron absorption) or an iron chelator, targets the
underlying cause and may provide a viable therapeutic option for
mitigating the iron accumulation associated with PMOP. The aim of
the present review was to investigate the role of iron accumulation
in the development of PMOP and to evaluate the use of iron
mitigation as a potential therapy for this clinical condition.
Iron accumulation in postmenopausal
women
Iron overload is defined as the presence of high
serum ferritin concentrations of ≥300 µg/l in men and ≥200 µg/l in
women (9). In recent years, an
increasing number of studies have investigated the associations
among ferritin, estrogen and PMOP, in order to determine the reason
for the enhanced risk of developing osteoporosis in women compared
with men. By compiling studies on the levels of ferritin and sex
hormones in various populations, it was concluded that as women
age, their serum levels of estrogen decrease, while serum ferritin
levels increase (10). These results
demonstrated a negative correlation between ferritin and estrogen
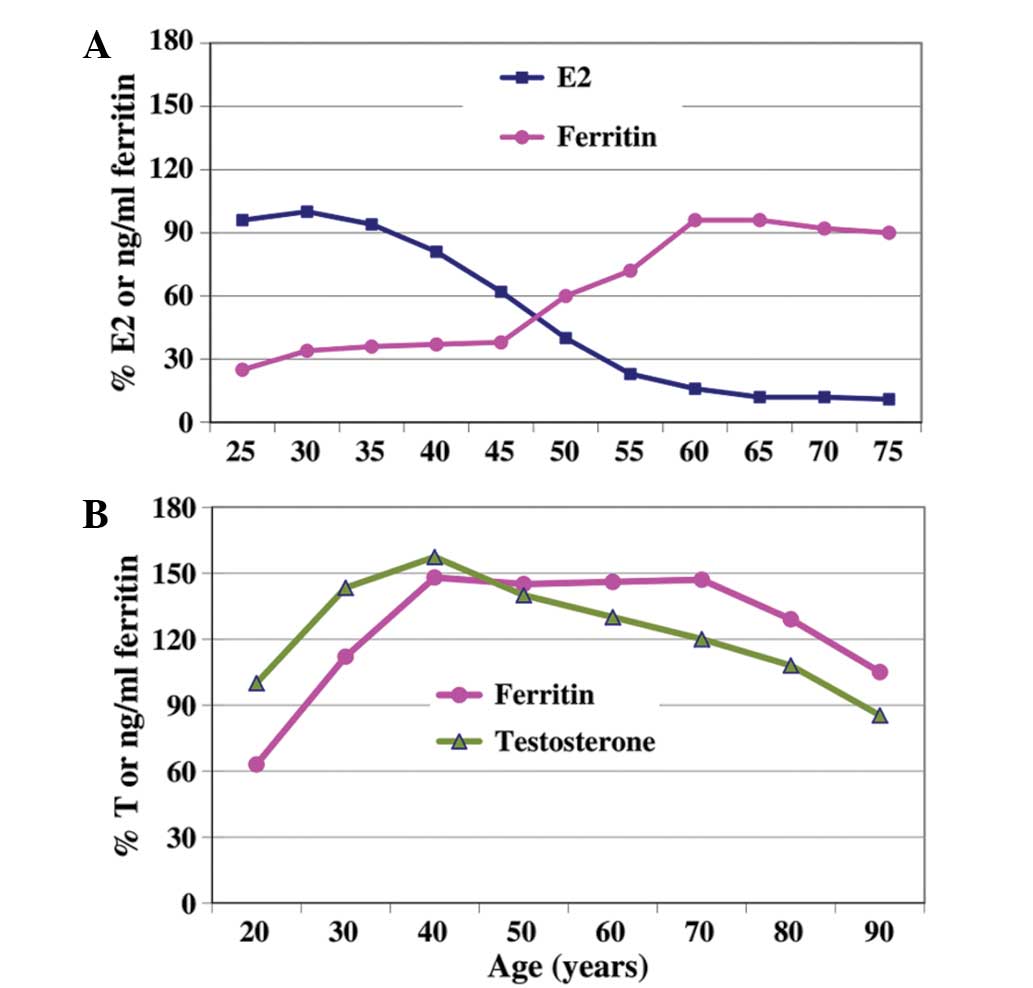
levels during the menopausal transition period (Fig. 1A) (6).
With regard to the changes in ferritin and testosterone levels in
men, a synchronized pattern was observed as the men age, in which
ferritin levels decreased gradually following ʻandropauseʼ
(Fig. 1B) (11). However, serum ferritin levels in
women and men did not reach levels defined as iron overload.
Collectively, these results indicated that iron accumulation was a
common process in aging women, but not iron overload, which may
account for the observed differences between genders in the
incidence of osteoporosis. Our retrospective study indicated that
women aged >70 years with a hip fracture possessed higher serum
ferritin levels and significantly reduced bone mineral density
(BMD) in the lumbar spine and hip, as compared with a control group
(12). In order to eliminate the
possibility that osteoporosis itself, but not iron accumulation,
exerted an effect on bone metabolism, a team of scientists in Seoul
conducted a three-year longitudinal health promotion center-based
study on 1,729 subjects, which included 789 middle-aged men and 940
postmenopausal women (13). Subjects
with illnesses known to affect ferritin levels or bone metabolism,
such as inflammatory diseases, chronic liver diseases or a history
of transfusion, were excluded from the study. The results revealed
a linear association between vertebral fracture prevalence and
serum ferritin levels in women; however, this correlation was not
observed in the male subjects (14),
which partially supported the previous observations (12). In addition, previous studies have
demonstrated that in healthy individuals, increased serum ferritin
levels were associated with an accelerated rate of bone loss, which
was most marked in women aged >45 years (13,14).
Notably, levels of serum ferritin were markedly increased in the
women aged >45 years, as is shown in Fig. 1A. Assuming these results were not a
coincidence, 45 years of age, typically during the perimenopausal
period, appears to be a critical time point at which the routine
examination of biological markers of iron levels may be advisable,
in order to monitor the development of iron accumulation.
Involvement of estrogen in iron
homeostasis
On the basis of the aforementioned clinical results,
an investigation into the interaction between estrogen and iron
levels was conducted. Menstruation is a key process in women of a
reproductive age, which is characterized by periodic fluctuations
in estrogen and the discharge of blood. For menstruating women, the
excretion of endogenous iron occurs primarily through blood loss,
resulting in reduced levels of ferritin and an increased prevalence
of iron deficiency (15,16). Following menopause, iron is no longer
lost through menstruation, and the metal ion increasingly
accumulates in the body. However, the interaction between estrogen
and iron is not exclusively a result of the effects of estrogen on
menstrual blood flow. Through investigating the effect of estrogen
on hepcidin, a negative regulator of iron absorption, estrogen was
observed to transcriptionally suppress the expression of hepcidin
by binding to the estrogen response element in the hepcidin
promoter (17,18). Notably, this process provides a
compensatory mechanism through which estrogen prevents the rapid
reduction in body iron in menstruating women, in addition to
mitigating the accumulation of iron in postmenopausal women.
Iron overload and abnormal bone
metabolism
A number of experimental models of iron overload
have been established in vivo in order to confirm the
adverse effect of iron on bone metabolism. Tsay et al
(7) generated a group of
iron-overloaded mice via injection with iron dextran for two
months. The results indicated that the iron-overloaded mice
exhibited alterations in bone microarchitecture, including the
trabecular number, thickness and bone volume fraction, in addition
to an increase in bone resorption, as compared with the control
group. Similarly, postmenopausal rats fed an iron lactate diet for
4 weeks exhibited a significant increase in urinary
deoxypyridinoline, indicating an increase in bone resorption
activity (19). In an additional
study, pigs were administered 300 mg iron dextran per day
intramuscularly for 36 days, after which the pigs appeared to have
accumulated large iron deposits in the osteoblasts and bone matrix.
Furthermore, the bone mineralization and formation in the pigs were
shown to have significantly decreased (8).
The mechanisms underlying the impact of iron on bone
metabolism are yet to be fully elucidated. However, in vitro
data indicated that iron-induced bone damage was predominantly
attributable to the function of iron in catalyzing the formation of
reactive oxygen species (ROS) via the Fenton reaction (20). Wnt signaling is essential for bone
formation through the stimulation of osteoblastogenesis (21). However, ROS are able to antagonize
Wnt signaling in osteoblast precursors by diverting β-catenin from
T cell factor to Forkhead Box O-mediated transcription, thereby
attenuating bone formation (22).
Furthermore, a previous study indicated that ferric ion promotes
the differentiation of osteoclasts and increases bone resorption
via the generation of ROS (23). In
summary, the risk of osteoporosis is increased through the
suppression of bone formation and enhancing bone resorption.
Reducing iron overload for the prevention of
bone loss
A previous study reported that iron overload was
associated with osteoporosis in ovariectomized (OVX) rats (24). When the OVX rats were fed orally with
a bone-targeted chelator (1-N-Docosyl-triethylenetetramine
pentaacetic acid), bone loss was alleviated significantly in the
chelator-treated OVX rats when compared with the untreated-OVX
controls (24,25). Desferrioxamine (DFO), an iron
chelator isolated from Streptomyces pilosus, is currently
used in clinical practice for the treatment of iron overload in
patients with thalassemia, hemochromatosis and sickle cell anemia
(26–28). Experimental results have indicated
that DFO is able to inhibit osteoclastic differentiation, which has
been associated with reduced mitochondrial biogenesis and the
production of ROS (29).
Furthermore, OVX rats treated with DFO have been shown to exhibit
reduced bone resorption and an improved three-dimensional bone
structure (29). In addition, our
unpublished preliminary data indicated that OVX rats
intraperitoneally treated with DFO for three months presented with
significantly increased BMD values, accompanied with reduced serum
ferritin levels. On the basis of the knowledge that menopause
results in iron accumulation, which may independently increase the
risk of osteoporosis, the manipulation of iron levels using an iron
chelator is hypothesized to be a viable therapeutic approach for
the treatment of PMOP.
Hepcidin treatment: A potential approach for
the reduction of iron overload
Iron homeostasis is closely regulated at the point
of iron absorption and storage. Hepcidin, a peptide hormone
produced by the liver, is the master regulator of iron homeostasis
(30). Hepcidin functions by
inhibiting the efflux of cellular iron into the circulation through
the transmembrane protein receptor, ferroportin. To date,
ferroportin is the only known cellular iron exporter in
vertebrates, and is known to be highly expressed in cells involved
with iron handling, such as duodenal enterocytes, which absorb iron
from the diet, and splenic macrophages, which recycle iron from
senescent erythrocytes (31).
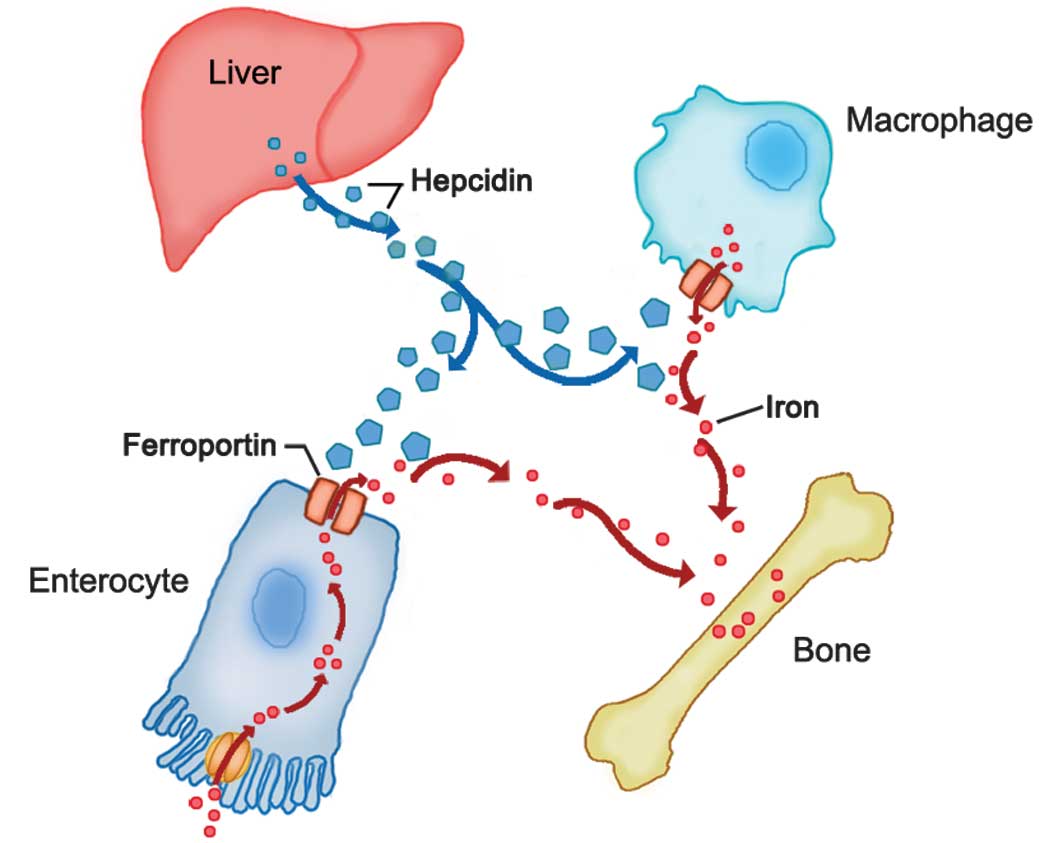
Hepcidin binds to ferroportin on the surface of duodenal
enterocytes and splenic macrophages, and induces the
internalization and lysosomal degradation of ferroportin, thereby
reducing the body's iron stores and iron deposition in the bone
(Fig. 2) (32). Therefore, if the hepcidin-induced
downregulation of ferroportin is inadequate or ineffective,
ferroportin activity is upregulated and iron overload may occur
(33–35).
The Hfe gene encodes a membrane protein that is
implicated in the stimulation of hepcidin expression (37). In a previous study using
Hfe−/− mice, the trabeculae surface was found to be
markedly labeled with Prussian blue (used for detecting ferric
iron), indicating a considerable quantity of iron deposition in the
skeletal tissues. In addition, the Hfe−/− mice
manifested an osteoporotic phenotype characterized by low bone mass
and impaired bone microarchitecture, in addition to an increased
number of osteoclasts along the trabeculae surfaces (38). The results suggested that hepcidin
deficiency increases the bone iron content and reduces the quantity
of bone tissue. Furthermore, constitutive activation of hepcidin
expression or treatment with synthetic hepcidin has been
demonstrated to prevent iron overload and the corresponding
complications in Hfe−/− mice (39,40). In
mice with β-thalassemia, increasing hepcidin expression was shown
to induce a reduction in iron content and an improvement in anemia
(41). Collectively, these results
indicate that hepcidin may possess therapeutic potential for
iron-overload diseases (42–44).
In a rat model of osteoporosis, liver hepcidin gene
expression was observed to reduce over time, which further
suggested that the development of osteoporosis was associated with
reduced levels of hepcidin (45).
Furthermore, increased mineralization and reduced rates of
apoptosis were observed in human osteoblasts treated with hepcidin
(46). In addition, a previous study
observed that hepcidin was able to increase the intracellular
calcium concentration in cultured osteoblasts, an effect that was
more evident in cells growing in a high iron concentration
environment (47). By reducing the
calcium influx from extracellular spaces using nimodipine (a
specific L-type Ca2+ channel blocker) or EDTA (an
extracellular calcium chelator), hepcidin-mediated calcium inflow
was found to occur predominantly via L-type Ca2+
channels (48). Furthermore, the
intracellular calcium induced by hepcidin was sourced primarily
from the endoplasmic reticulum, which is triggered by calcium
influx (47). Thus, increased levels
of intracellular calcium may be associated with the
anti-osteoporosis effect of hepcidin. Furthermore, considering that
postmenopausal women exhibit enhanced iron accumulation, we
hypothesize that hepcidin may provide a viable therapeutic option
for the prevention and treatment of PMOP by reducing the iron
content in the body and enhancing osteoblast mineralization. In
recent years, a patent was filed in the USA detailing the treatment
of osteoporosis with hepcidin in perimenopausal and postmenopausal
women (49). However, further
studies are required to validate this hypothesis.
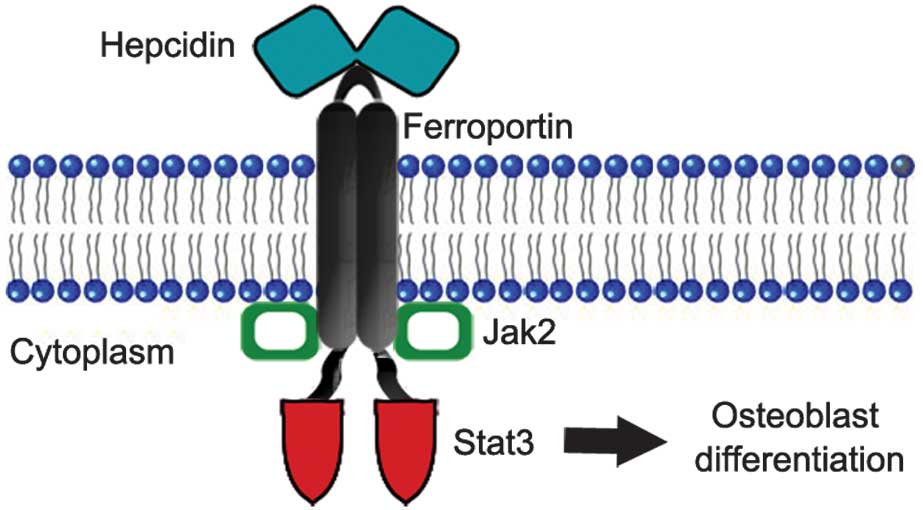
Notably, there is a potential receptor-based
mechanism through which hepcidin may interact with osteoblasts.
Previous studies have indicated that the mechanism underlying
hepcidin-mediated internalization of ferroportin may result from
the activation of the Janus kinase 2/signal transducer and
activator of transcription 3 (JAK2/STAT3) signaling pathway
(50,51). Furthermore, activation of the
JAK2/STAT3 pathway has been reported to promote osteoblast
differentiation (52,53), while inhibition of the JAK2/STAT3
pathway using the JAK2 inhibitor, AG490, has been shown to reduce
human osteoblast differentiation and mineralization (54). Recently, our research group
recognized that ferroportin can be detected in human hFOB 1.19
cultured cells, which indicates that osteoblasts are a potential
target of hepcidin activity (55).
Based on these collective results, a possible mechanism through
which hepcidin stimulates osteoblast differentiation was proposed
(Fig. 3).
Future prospects
In the previous decade, research into iron
metabolism and bone metabolism has progressed rapidly; the results
of which have improved the understanding of the pathogenesis
underlying PMOP (44). The
maintenance of iron homeostasis in postmenopausal women has been
recognized as crucial, and indicates the therapeutic potential of
the manipulation of iron levels for treating PMOP. An artificial,
biologically active form of hepcidin, known as ʻminihepcidinʼ, has
been developed by Preza et al (56).
The following are the key recommendations for
clinical research and practice, based on the present review.
Firstly, well-designed prospective studies are required to
investigate whether DFO or other iron chelators are able to
mitigate bone loss in patients with iron overload conditions, such
as thalassemia, hemachromatosis and sickle cell anemia. Secondly,
the symptoms of iron overload are insensitive and nonspecific,
which differs from the activity of other biologically important
metal ions, such as potassium. Therefore, routine examination of
the biochemical markers of iron stores may be advisable in order to
predict the future patient risk of PMOP. As aforementioned, the
optimum age for initiating iron store examination in an aging
population is ~45 years. Thirdly, the epidemiological profile of
iron deficiency remains among the most prevalent micronutrient
deficiencies worldwide, increasing the risk of diminished bone
metabolism in animals and humans (57,58).
Therefore, the maintenance of normal iron levels is essential in
clinical practice for healthy bone homeostasis.
Acknowledgements
The authors thank Dr Yi-Lin Yan and Dr Han Wang for
critically reading the manuscript. The study was supported by
grants from the National Natural Science Foundation of China (nos.
81273090 and 81302438), the Special Program for Clinic of Jiangsu
Province (no. BL2014044) and the Natural Science Foundation of
Soochow University (no. SDY2013A33).
References
|
1
|
MacKenzie EL, Iwasaki K and Tsuji Y:
Intracellular iron transport and storage: From molecular mechanisms
to health implications. Antioxid Redox Signal. 10:997–1030. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olivieri NF, Liu PP, Sher GD, et al: Brief
report: Combined liver and heart transplantation for end-stage
iron-induced organ failure in an adult with homozygous
beta-thalassemia. N Engl J Med. 330:1125–1127. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y and
Kato J: Body iron metabolism and pathophysiology of iron overload.
Int J Hematol. 88:7–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinberg ED: Iron loading: A risk factor
for osteoporosis. Biometals. 19:633–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinberg ED: Role of iron in osteoporosis.
Pediatr Endocrinol Rev. 6:(Suppl 1). 81–85. 2008.PubMed/NCBI
|
|
6
|
Jian J, Pelle E and Huang X: Iron and
menopause: Does increased iron affect the health of postmenopausal
women? Antioxid Redox Signal. 11:2939–2943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsay J, Yang Z, Ross FP, et al: Bone loss
caused by iron overload in a murine model: Importance of oxidative
stress. Blood. 116:2582–2589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Vernejoul MC, Pointillart A, Golenzer
CC, et al: Effects of iron overload on bone remodeling in pigs. Am
J Pathol. 116:377–384. 1984.PubMed/NCBI
|
|
9
|
Adams PC and Chakrabarti S:
Genotypic/phenotypic correlations in genetic hemochromatosis:
Evolution of diagnostic criteria. Gastroenterology. 114:319–323.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zacharski LR, Ornstein DL, Woloshin S and
Schwartz LM: Association of age, sex, and race with body iron
stores in adults: Analysis of NHANES III data. Am Heart J.
140:98–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang X, Xu Y and Partridge NC: Dancing
with sex hormones, could iron contribute to the gender difference
in osteoporosis? Bone. 55:458–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu YJ, Sirois P and Li K: Iron overload
plays a unique role in osteoporosis. Blood (E-letter). http://www.bloodjournal.org/content/116/14/2582.e-letters#iron-overload-plays-a-unique-role-in-osteoporosisAccessed.
May 6–2015
|
|
13
|
Kim BJ, Ahn SH, Bae SJ, et al: Iron
overload accelerates bone loss in healthy postmenopausal women and
middle-aged men: A 3-year retrospective longitudinal study. J Bone
Miner Res. 27:2279–2290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim BJ, Lee SH, Koh JM and Kim GS: The
association between higher serum ferritin level and lower bone
mineral density is prominent in women ≥45 years of age (KNHANES
2008–2010). Osteoporos Int. 24:2627–2637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clark SF: Iron deficiency anemia. Nutr
Clin Pract. 23:128–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zimmermann MB and Hurrell RF: Nutritional
iron deficiency. Lancet. 370:511–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Q, Jian J, Katz S, Abramson SB and
Huang X: 17beta-Estradiol inhibits iron hormone hepcidin through an
estrogen responsive element half-site. Endocrinology.
153:3170–3178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou Y, Zhang S, Wang L, et al: Estrogen
regulates iron homeostasis through governing hepatic hepcidin
expression via an estrogen response element. Gene. 511:398–403.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Isomura H, Fujie K, Shibata K, et al: Bone
metabolism and oxidative stress in postmenopausal rats with iron
overload. Toxicology. 197:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalinowski DS and Richardson DR: The
evolution of iron chelators for the treatment of iron overload
disease and cancer. Pharmacol Rev. 57:547–583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bennett CN, Longo KA, Wright WS, et al:
Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl
Acad Sci USA. 102:3324–3329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almeida M, Han L, Martin-Millan M, O'Brien
CA and Manolagas SC: Oxidative stress antagonizes Wnt signaling in
osteoblast precursors by diverting beta-catenin from T cell factor-
to forkhead box O-mediated transcription. J Biol Chem.
282:27298–27305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia P, Xu YJ, Zhang ZL, et al: Ferric ion
could facilitate osteoclast differentiation and bone resorption
through the production of reactive oxygen species. J Orthop Res.
30:1843–1852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu G, Men P, Kenner GH and Miller SC:
Age-associated iron accumulation in bone: implications for
postmenopausal osteoporosis and a new target for prevention and
treatment by chelation. Biometals. 19:245–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu G, Men P, Kenner GH and Miller SC:
Therapeutic effects of an oral chelator targeting skeletal tissue
damage in experimental postmenopausal osteoporosis in rats.
Hemoglobin. 32:181–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maggio A, Filosa A, Vitrano A, et al: Iron
chelation therapy in thalassemia major: A systematic review with
meta-analyses of 1520 patients included on randomized clinical
trials. Blood Cells Mol Dis. 47:166–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fabio G, Minonzio F, Delbini P, Bianchi A
and Cappellini MD: Reversal of cardiac complications by deferiprone
and deferoxamine combination therapy in a patient affected by a
severe type of juvenile hemochromatosis (JH). Blood. 109:362–364.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalpatthi R, Peters B, Kane I, et al:
Safety and efficacy of high dose intravenous desferrioxamine for
reduction of iron overload in sickle cell disease. Pediatr Blood
Cancer. 55:1338–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishii KA, Fumoto T, Iwai K, et al:
Coordination of PGC-1beta and iron uptake in mitochondrial
biogenesis and osteoclast activation. Nat Med. 15:259–266. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ganz T: Hepcidin, a key regulator of iron
metabolism and mediator of anemia of inflammation. Blood.
102:783–788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Donovan A, Brownlie A, Zhou Y, et al:
Positional cloning of zebrafish ferroportin1 identifies a conserved
vertebrate iron exporter. Nature. 403:776–781. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemeth E, Tuttle MS, Powelson J, et al:
Hepcidin regulates cellular iron efflux by binding to ferroportin
and inducing its internalization. Science. 306:2090–2093. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lesbordes-Brion JC, Viatte L, Bennoun M,
et al: Targeted disruption of the hepcidin 1 gene results in severe
hemochromatosis. Blood. 108:1402–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nicolas G, Bennoun M, Devaux I, et al:
Lack of hepcidin gene expression and severe tissue iron overload in
upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad
Sci USA. 98:8780–8785. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hadziahmetovic M, Song Y, Ponnuru P, et
al: Age-dependent retinal iron accumulation and degeneration in
hepcidin knockout mice. Invest Ophthalmol Vis Sci. 52:109–118.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roetto A, Papanikolaou G, Politou M, et
al: Mutant antimicrobial peptide hepcidin is associated with severe
juvenile hemochromatosis. Nat Genet. 33:21–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahmad KA, Ahmann JR, Migas MC, et al:
Decreased liver hepcidin expression in the Hfe knockout mouse.
Blood Cells Mol Dis. 29:361–366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guggenbuhl P, Fergelot P, Doyard M, et al:
Bone status in a mouse model of genetic hemochromatosis. Osteoporos
Int. 22:2313–2319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nicolas G, Viatte L, Lou DQ, et al:
Constitutive hepcidin expression prevents iron overload in a mouse
model of hemochromatosis. Nat Genet. 34:97–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moran-Jimenez MJ, Mendez M, Santiago B, et
al: Hepcidin treatment in Hfe-/- mice diminishes plasma iron
without affecting erythropoiesis. Eur J Clin Invest. 40:511–517.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gardenghi S, Ramos P, Marongiu MF, et al:
Hepcidin as a therapeutic tool to limit iron overload and improve
anemia in beta-thalassemic mice. J Clin Invest. 120:4466–4477.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Andrews NC: Closing the iron gate. N Engl
J Med. 366:376–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ganz T and Nemeth E: The
hepcidin-ferroportin system as a therapeutic target in anemias and
iron overload disorders. Hematology Am Soc Hematol Educ Program.
2011:538–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li GF, Pan YZ, Sirois P, Li K and Xu YJ:
Iron homeostasis in osteoporosis and its clinical implications.
Osteoporos Int. 23:2403–2408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma Y, Xu YJ, Wang AD, Yu C, Wang B, Zhang
P and Zhang ZD: A preliminary report of expression of hepcidin gene
in SD rats osteoporosis model. Su Zhou Da Xue Zue Bao. 26:367–369.
2006.(In Chinese).
|
|
46
|
Zhang P, Xu YJ, Zhao DY, et al: Increased
intracellular iron and mineralization of cultured hFOB 1.19 cells
following hepcidin activation through ferroportin-1. Saudi Med J.
31:1303–1308. 2010.PubMed/NCBI
|
|
47
|
Li GF, Xu YJ, He YF, et al: Effect of
hepcidin on intracellular calcium in human osteoblasts. Mol Cell
Biochem. 366:169–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu Y, Li G, Du B, et al: Hepcidin
increases intracellular Ca2+ of osteoblast hFOB1.19
through L-type Ca2+ channels. Regul Pept. 172:58–61.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xi Huang: Treatment of osteoporosis in
peri- and post-menopausal women with hepcidin. US Patent 0,204,122.
Filed. February 11–2010 issued. August 12–2010
|
|
50
|
De Domenico I, Lo E, Ward DM and Kaplan J:
Hepcidin-induced internalization of ferroportin requires binding
and cooperative interaction with Jak2. Proc Natl Acad Sci USA.
106:3800–3805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Domenico I, Zhang TY, Koening CL, et
al: Hepcidin mediates transcriptional changes that modulate acute
cytokine-induced inflammatory responses in mice. J Clin Invest.
120:2395–2405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bellido T, Borba VZ, Roberson P and
Manolagas SC: Activation of the Janus kinase/STAT (signal
transducer and activator of transcription) signal transduction
pathway by interleukin-6-type cytokines promotes osteoblast
differentiation. Endocrinology. 138:3666–3676. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nishimura R, Moriyama K, Yasukawa K, Mundy
GR and Yoneda T: Combination of interleukin-6 and soluble
interleukin-6 receptors induces differentiation and activation of
JAK-STAT and MAP kinase pathways in MG-63 human osteoblastic cells.
J Bone Miner Res. 13:777–785. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barhanpurkar AP, Gupta N, Srivastava RK,
et al: IL-3 promotes osteoblast differentiation and bone formation
in human mesenchymal stem cells. Biochem Biophys Res Commun.
418:669–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu Y, Zhang W, Zhang P, et al:
Downregulation of ferroportin 1 expression in hFOB1.19 osteoblasts
by hepcidin. Inflammation. 35:1058–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Preza GC, Ruchala P, Pinon R, et al:
Minihepcidins are rationally designed small peptides that mimic
hepcidin activity in mice and may be useful for the treatment of
iron overload. J Clin Invest. 121:4880–4888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Katsumata S, Tsuboi R, Uehara M and Suzuki
K: Dietary iron deficiency decreases serum osteocalcin
concentration and bone mineral density in Rats. Biosci Biotechnol
Biochem. 70:2547–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maurer J, Harris MM, Stanford VA, et al:
Dietary iron positively influences bone mineral density in
postmenopausal women on hormone replacement therapy. J Nutr.
135:863–869. 2005.PubMed/NCBI
|