Introduction
Triple-negative breast cancer (TNBC), defined by a
lack of estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor receptor 2 (HER-2) expression, is a
clinically distinct subgroup accounting for 12–24% of breast cancer
cases (1,2). The majority of these tumors have an
inherent aggressiveness, an increased likelihood of distant
recurrence, mortality within 5 years of the diagnosis and a poor
prognosis (3). Since TNBC is not
sensitive to hormone therapy and there is a lack of HER2-targeted
therapies, chemotherapy is the only systemic treatment of choice;
however, no formal treatment guidelines exist regarding the
specific most appropriate systemic regimen for TNBC. Chemotherapy
drugs can be administered as a conventional monotherapy or in
combination and constitute an important therapeutic tool. The
efficacy of anthracycline- and taxane-based neoadjuvant therapies
has been confirmed for the treatment of TNBC (4); however, previous research has shown
that treatment with anthracycline carries a poor prognosis
(5). The improvement of overall
survival (OS) rates among patients with advanced TNBC has therefore
become a hotspot in breast cancer research.
In a study by Liu et al (6), it was reported that TNBC exhibited
increased sensitivity to platinum-based drugs. Oxaliplatin (OXA),
as a third-generation platinum-based drug, has no complete
cross-resistance with cisplatin or carboplatin and shows synergy
with 5-fluorouracil (5-FU) (7),
although OXA is effective even in those cases showing resistance to
5-FU. 5-FU is one of most commonly used drugs for breast cancer.
S-1 is an oral 5-FU anti-cancer drug with superior efficacy to
tegafur (FT) and 5-FU (8). As a
monotherapy, S-1 has been associated with a high rate of efficacy,
mild adverse effects and good tolerance (9). The aim of this retrospective study,
therefore, was to evaluate the effect of an OXA- and S-1-based
chemotherapy regimen in the treatment of advanced TNBC.
Materials and methods
Patients
A retrospective review was conducted on female
patients with advanced TNBC (confirmed by pathological and
immunohistochemical staining), who were treated with OXA plus S-1
at the Taixing People's Hospital (Taixing, China) between January
2011 and January 2013. The patients were eligible if they were
confirmed to have stage IIIC or IV disease that was unsuitable for
surgery or if they had developed a metastasis following surgery.
Metastatic lesions were measured objectively by ultrasound,
computed tomography scans or magnetic resonance imaging. Other
inclusion criteria were as follows: Karnofsky Performance Status
(KPS) score, ≥60; expected survival, ≥3 months; adequate heart,
lung and renal function; white blood cell (WBC) count,
>3.5×109/l; granulocyte (GRAN) count,
>1.5×109/l; platelet (PLT) count,
>75×109/l; liver function, <1.5-fold the upper
limit of normal; essentially normal electrocardiograms; no
peripheral neuropathy; no previous history of treatment with OXA
plus S-1; no use of any chemotherapy drugs in the past month and
ability to provide informed consent. The study was approved by the
Institutional Review Board of the Taixing Peoples Hospital Ethics
Committee, and all patients provided written informed consent.
Treatment, response assessments and
follow-up
OXA (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu,
China) was administered intravenously and 135 mg/m2 was
infused over 120 min on day 1. S-1 (Shandong New Time
Pharmaceutical Co., Ltd., Shandong, China) was given orally twice
daily for the first 2 weeks of a 3-week cycle; the dose of S-1
administered each time was calculated according to the patient's
body surface area as follows: <1.25 m2, 40 mg;
1.25–1.5 m2, 50 mg; and >1.5 m2, 60 mg.
During the chemotherapy, the efficacy assessment was repeated
biweekly. Cycles were repeated every 21 days for a total of 6
cycles unless disease progression was noted, in which case the
regimen was modified. For patients with bone metastasis,
chemotherapy and bisphosphonate treatment could be used
simultaneously, while brain radiotherapy was added upon the
occurrence of brain metastasis. Prior to chemotherapy,
premedication consisted of a 5-hydroxytryptamine 3 receptor
antagonist, and liver- and stomach-protecting treatment was
administered. The adverse events of leukopenia and thrombocytopenia
were treated with granulocyte colony-stimulating factor and
interleukin-11, respectively. The next course of treatment was
delayed when blood analysis revealed the following: WBC
<3.5×109/l, GRAN <1.5×109/l or PLT
<75×109/l. The doses of the chemotherapy drugs were
reduced by 25% in the patients who experienced grade 4
myelosuppression or grade 3 or worse peripheral neuropathy.
All patients were required to undergo complete
examinations (routine blood, urine and stool; liver and renal
functions; blood glucose; electrocardiography; tumor markers) prior
to and following the chemotherapy. Routine blood examinations were
repeated weekly and detailed records were made regarding the KPS
score and weight changes during the treatment. Each patient
received ≥2 cycles of chemotherapy therapy, and clinical responses
were confirmed based on 2 assessments performed ≥2 cycles apart.
The clinical responses were rated according to the Response
Evaluation Criteria in Solid Tumors (10) and assigned to one of five groups:
Complete response (CR), partial response (PR), marginal response,
stable disease (SD) and progressive disease (PD). The overall
response rate (ORR) was calculated as a ratio of the CR and PR for
the entire patient population, while the disease control rate (DCR)
was calculated as a ratio of the CR, PR and SD for the entire
patient population. Toxicities were characterized according to the
National Cancer Institute Common Toxicity Criteria (11), which were divided into five grades.
Progression-free survival (PFS) was calculated from the day of
commencement of OXA plus S-1 administration until the day of
documented progression or mortality. The OS was calculated from the
start date of OXA plus S-1 to the date of mortality or last
follow-up. Patients were followed up by means of letters and
telephones until March 2014.
Statistical analysis
All statistical calculations were carried out using
SPSS Windows version 19.9 (IBM SPSS, Armonk, NY, USA). PFS and OS
were analyzed according to the Kaplan-Meier estimates and were
compared in log-rank tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Table I shows the
characteristics of the patients. A total of 52 patients who
underwent OXA plus S-1 therapy were evaluated. The median age of
the patients was 53 years (range, 31–72 years). The clinical stage
of the patients was advanced to the extent that 16 (30.8%) were in
stage IIIC and 36 (69.2%) were in stage IV. Liver, lymph node,
lung, adrenal, mediastinum, brain, abdominal, bone and other
metastases were found in 32, 28, 20, 10, 8, 8, 8, 6 and 6 patients,
respectively. A total of 16 patients had a single metastatic site,
and 36 patients had ≥2 metastatic sites. Fourteen patients (26.9%)
had previously received taxane chemotherapy, 10 (19.2%) had
previously received anthracycline chemotherapy and 12 (23.1%) had
previously been treated with both anthracycline and taxane.
 | Table I.Patient characteristics (n=52). |
Table I.
Patient characteristics (n=52).
| Characteristic | n (%) |
|---|
| Age,
yearsa | 53 (31–72) |
| Clinical stage |
|
|
IIC | 16 (30.8) |
|
III | 36 (69.2) |
| Metastatic
site |
|
|
Liver | 32 (61.5) |
| Lymph
node | 28 (53.8) |
|
Lung | 20 (38.5) |
|
Adrenal | 10 (19.2) |
|
Mediastinum | 8 (15.4) |
|
Brain | 8 (15.4) |
|
Abdominal | 8 (15.4) |
|
Bone | 6 (11.5) |
|
Others | 6 (11.5) |
| Prior
chemotherapy |
|
|
Taxane | 14 (26.9) |
|
Anthracycline | 10 (19.2) |
|
Anthracycline and taxane | 12 (23.1) |
Clinical response
In total, 224 cycles of chemotherapy were performed.
The median for each patient was 4 cycles, and the range was 2–6
cycles. Treatment delay occurred in 12 patients for <7 days due
to chemotherapy toxicity. Two patients required dose reductions due
to chemotherapy-induced toxicity; however, the toxicities did not
lead to the complete termination of chemotherapy in any case, and
there were no treatment-related mortalities. All 52 patients were
evaluated for treatment efficacy. The follow-up assessments were
completed by December 31, 2013, and the median follow-up time was
13.7 months (range 3.6–36.0 months). Four patients was lost to
follow-up. Out of the 52 patients, 2 exhibited a CR, while PRs, SDs
and PDs were noted in 16, 18 and 16 patients, respectively. The ORR
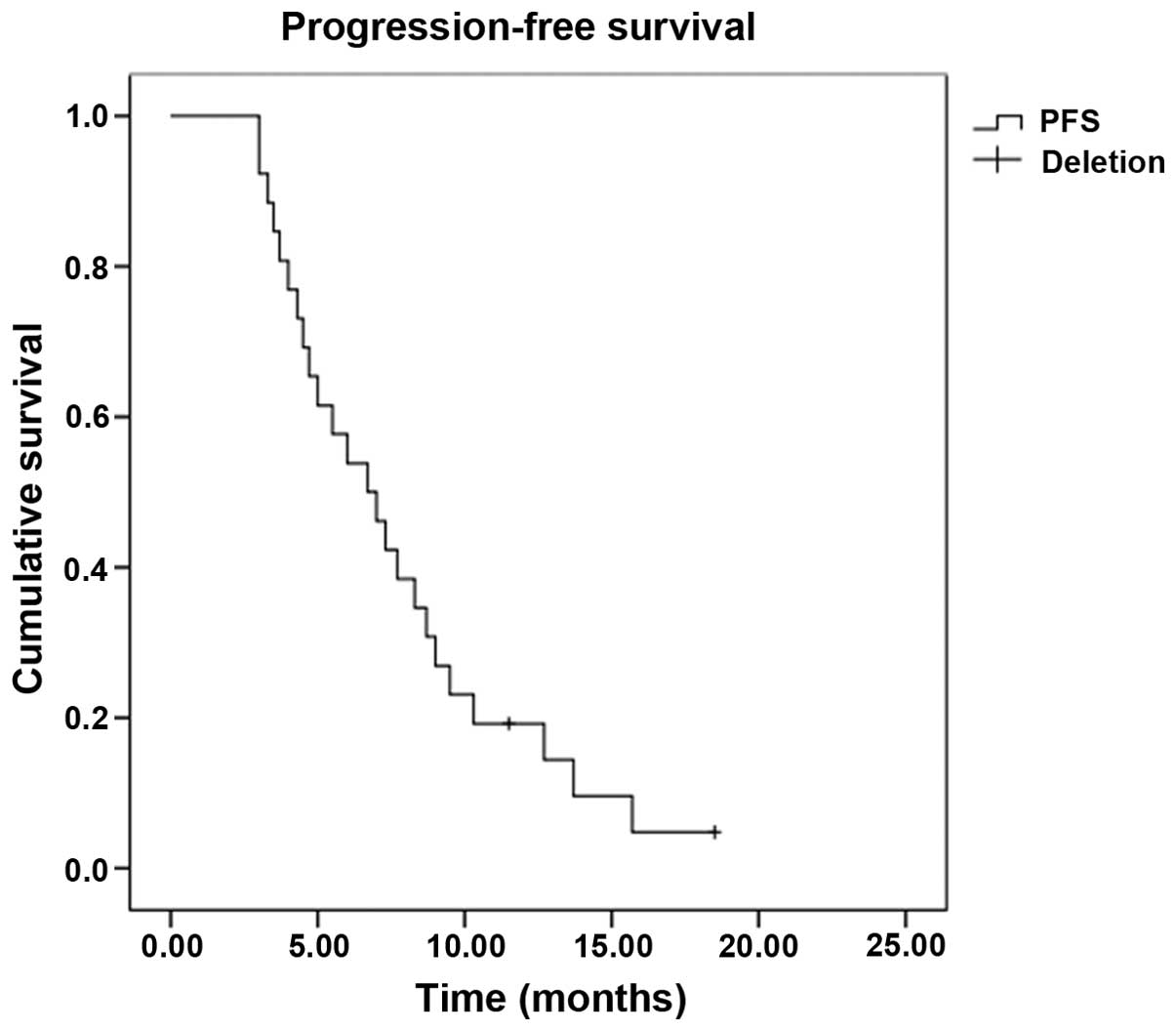
was 34.6% and the DCR was 69.2%. The median PFS time was 6.7 months
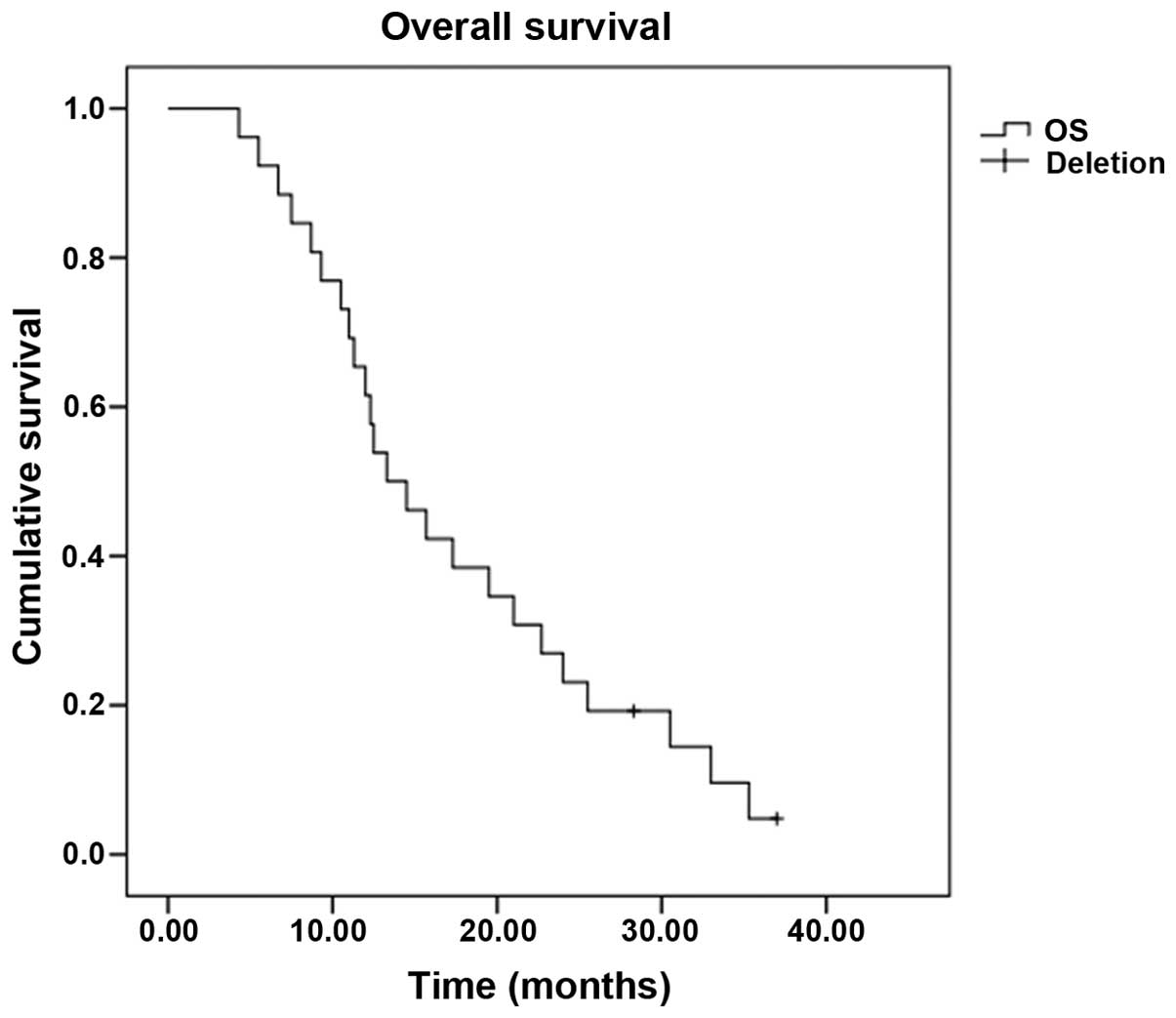
[95% confidence interval (CI), 4.5–9.0] (Fig. 1), and the OS time was 13.3 months
(95% CI, 9.1–17.5) (Fig. 2). Subset
analysis based on the clinical characteristics of the patients
showed that patients who were premenopausal, had infiltrating
ductal carcinoma and had previously received anthracycline or
taxane chemotherapy exhibited a prolongation of survival, although
the comparisons were statistically insignificant (P>0.05).
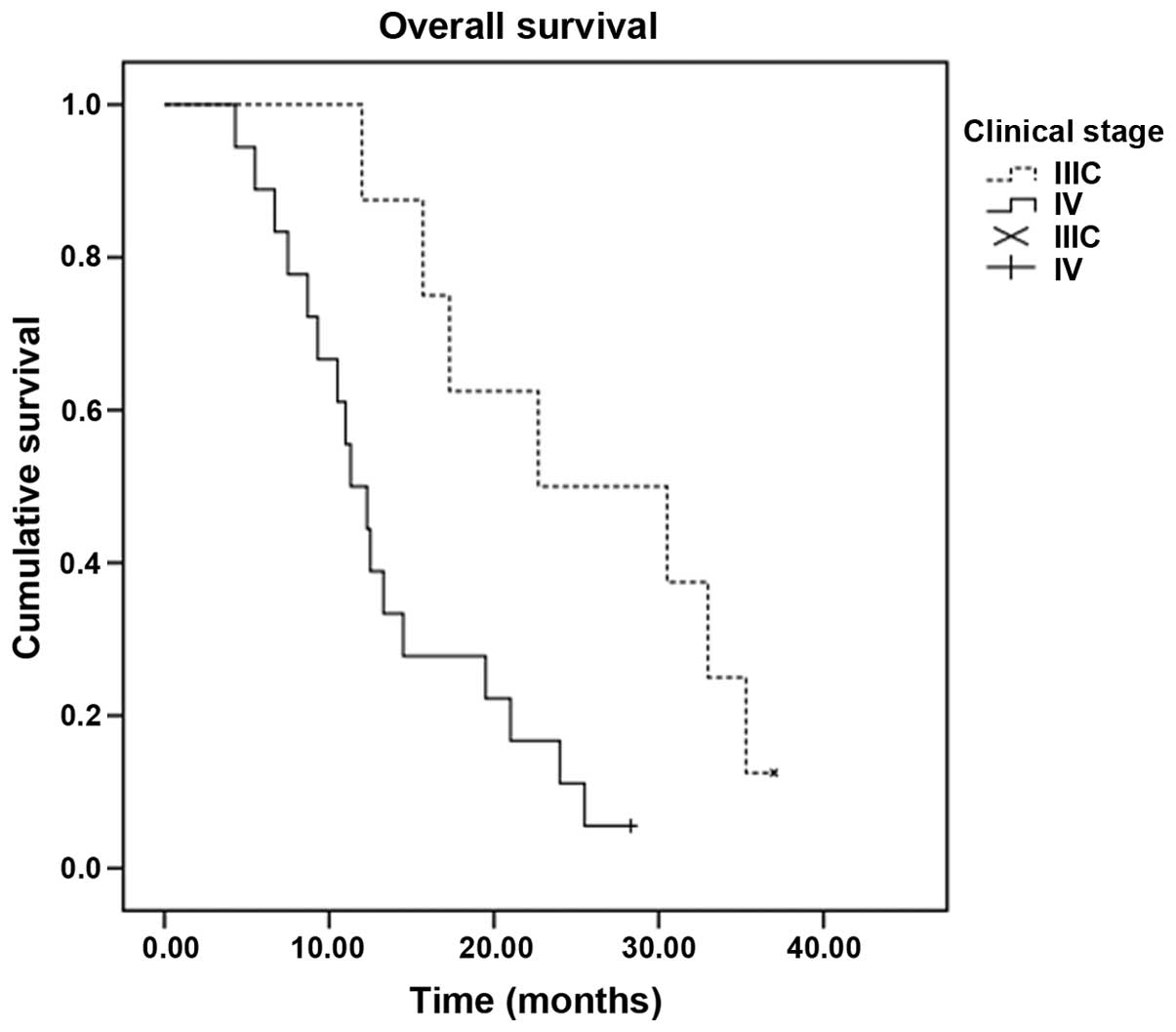
Patients with stage IIIC disease had a statistically better median
OS time than patients with stage IV disease (22.7 vs. 11.3 months,
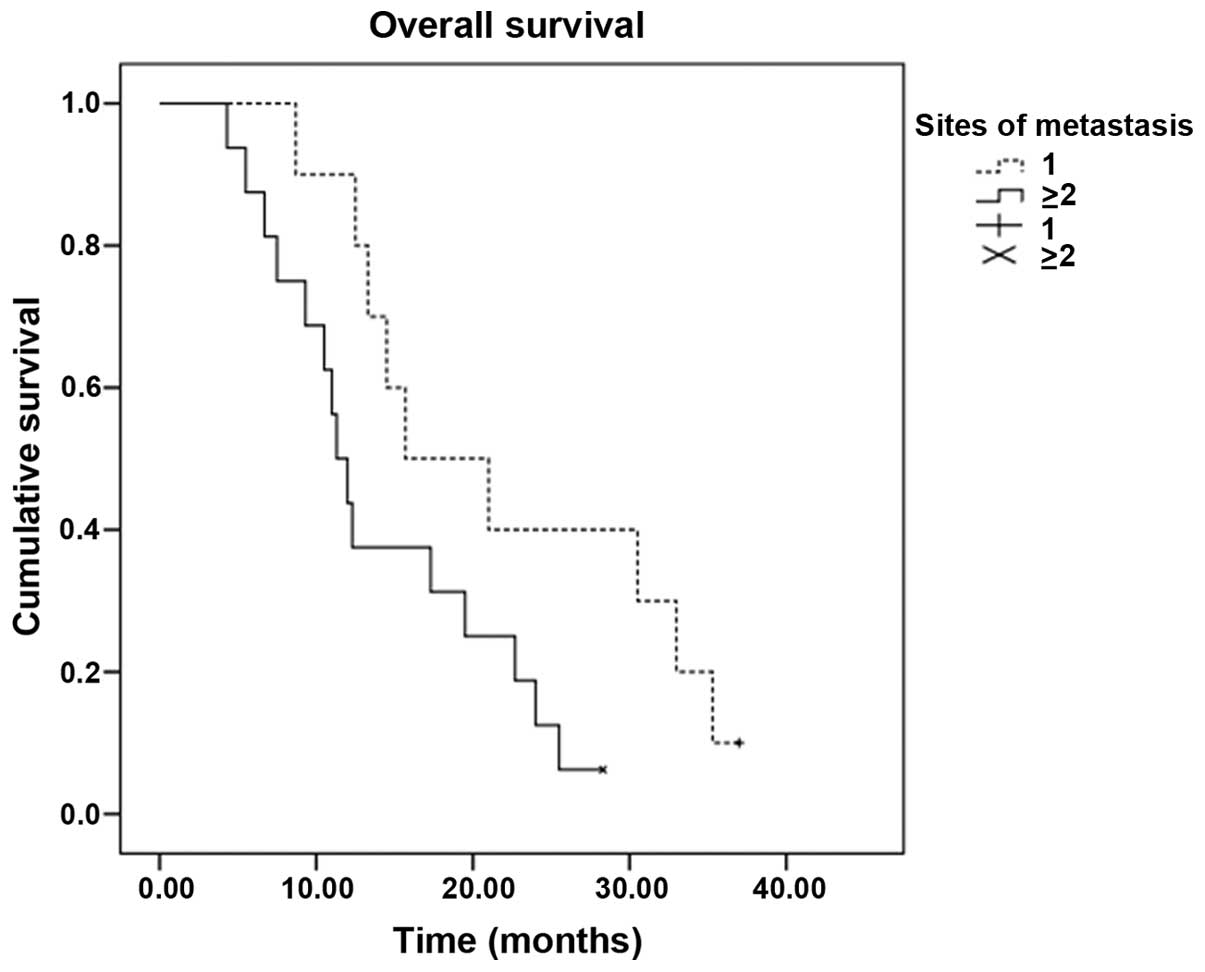
P=0.010). In addition, the median OS time of patients with a single
metastatic site (15.7 months; 95% CI, 5.6–25.8) was superior to
that of patients with ≥2 metastatic sites (11.3 months; 95% CI,
9.3–13.3) (P=0.048) (Table II,
Figs. 3 and 4).
 | Table II.Subset analysis of OS time
(n=52). |
Table II.
Subset analysis of OS time
(n=52).
| Variables | No. of
patients | Median OS
(months) | 95% CI | χ2 | P-value |
|---|
| Menstruation |
|
|
| 0.000 | 0.987 |
|
Pre | 28 | 13.3 | 7.1–19.5 |
|
|
|
Post | 24 | 12.5 | 8.3–16.7 |
|
|
| Clinical stage |
|
|
| 6.668 | 0.010 |
|
IIC | 16 | 22.7 | 4.4–41.0 |
|
|
|
III | 36 | 11.3 | 8.6–14.0 |
|
|
| Histological
type |
|
|
| 0.000 | 0.993 |
|
Infiltrating ductal | 40 | 13.3 | 8.9–17.7 |
|
|
|
Others | 12 | 12.3 | 2.7–21.9 |
|
|
| Number of organs
involved |
|
|
| 3.923 | 0.048 |
| 1 | 20 | 15.7 | 5.6–25.8 |
|
|
| ≥2 | 32 | 11.3 | 9.3–13.3 |
|
|
| Previous
chemotherapya |
|
|
| 0.193 | 0.661 |
|
Yes | 30 | 14.5 | 5.0–24.0 |
|
|
| No | 22 | 12.5 | 7.4–17.6 |
|
|
Toxicity
The three most frequently observed adverse effects
in the group were hematological toxicities, peripheral neuropathy
and gastrointestinal reactions. Alopecia, liver dysfunction and
hand-foot syndrome were mainly grade 1 or 2. Grade 3 or 4
neutropenia occurred in 6 patients (11.5%), while 4 patients (7.7%)
experienced grade 3 nausea and vomiting and 2 patients (3.8%)
experienced grade 3 peripheral neuropathy. There were no
treatment-related mortalities (Table
III).
 | Table III.Adverse events. |
Table III.
Adverse events.
| Event | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) | Grades 3+4 (%) |
|---|
| Anemia | 8 | 4 | 0 | 0 | 0.0 |
| Neutropenia | 20 | 8 | 4 | 2 | 11.5 |
|
Thrombocytopenia | 4 | 2 | 0 | 0 | 0.0 |
|
Nausea/vomiting | 20 | 8 | 4 | 0 | 7.7 |
| Diarrhea | 16 | 6 | 0 | 0 | 0.0 |
| Alopecia | 10 | 4 | 0 | 0 | 0.0 |
| Stomatitis | 6 | 4 | 0 | 0 | 0.0 |
| Phlebitis | 6 | 0 | 0 | 0 | 0.0 |
| Liver
dysfunction | 10 | 6 | 0 | 0 | 0.0 |
| Peripheral
neuropathy | 20 | 6 | 2 | 0 | 3.8 |
| Hand-foot
syndrome | 12 | 6 | 0 | 0 | 0.0 |
| Pigmentation | 10 | 6 | 0 | 0 | 0.0 |
| Electrocardiogram
changes | 4 | 0 | 0 | 0 | 0.0 |
Discussion
Human breast cancer can be subdivided into five
molecular subtypes, distinguished by pervasive differences in their
gene expression patterns: Luminal subtype A, luminal subtype B,
normal breast-like, HER-2 overexpression and basal-like subtype
(12). Basal-like breast cancer, by
definition, is characterized by the absence of immunostaining for
ER, PR and HER2, as well as the overexpression of epidermal growth
factor receptor (EGFR) and cytokeratins (CK5/6, CK17 and CK14)
(2). TNBC is a clinically and
molecularly heterogeneous disease that encompasses more than one
entity. Although TNBC and basal-like breast cancer types share
numerous clinical and pathological characteristics, they are not
identical, with basal-like tumors accounting for ~75% of TNBCs
(13). According to a previous
study, ~75% of TNBCs can be classified as BRCA1-related breast
cancers, while TNBCs account for 80–90% of BRCA1-related breast
cancers (14). TNBC exhibits
distinct biological and clinical behavior. The majority of the
tumors are high-grade, ductal carcinomas, which are associated with
a larger size. Furthermore, the tumor cells exhibit an absence of
androgen receptor, E-cadherin and cyclin D expression, as well as
positive expression of basal cytokeratins (CK5/6, CK17), EGFR and
p53 (2).
TNBC has an increased likelihood of recurrence,
distant metastasis and mortality within 5 years of the diagnosis,
the peak being within the first 3 years (15). Several studies have shown an
increased rate of visceral metastasis, particularly spinal cord,
brain, liver and lung metastasis, versus bone metastasis (13,16,17).
Following a diagnosis of metastatic disease, poor prognosis has
also been reported (16). There are
numerous predictive factors of poor prognosis, although the triple
negative status itself (ER−, PR− and
HER2−) is also associated with a poor prognosis
(17,18). p53 mutation has been reported to be
associated with anthracycline- (19)
and platinum- (20) based
chemotherapy resistance and a poor prognosis of breast cancer
(21,22). The lymph node metastasis status has a
significant impact on the prognosis of patients with TNBC.
Hernandez-Aya et al (23)
proposed that the OS rate of patients with TNBC exhibiting evidence
of axillary lymph node metastasis is lower than that of patients
with TNBC with no tumor metastasis. Previous studies (24,25) have
reported that whether the expression of ER, PR and HER-2 is
concordant between metastatic axillary lymph nodes and primary
lesion is an independent risk factor for TNBC.
Since patients with TNBC can benefit neither from
hormone therapies nor from HER2-targeted therapies, they are more
sensitive to chemotherapy than patients with other types of breast
cancer. As early as 1978, a retrospective study reported that
triple-negative patients had an enhanced response rate to
chemotherapy as compared with non-triple-negative patients
(26). Keam et al (27) analyzed 145 patients with stage II and
III breast cancer who received neoadjuvant chemotherapy, and their
results revealed that patients with a triple-negative phenotype
showed a higher response rate (RR) than patients with a
non-triple-negative phenotype (83.0 vs. 62.2%), as well as a higher
pathologically complete RR (17.0 vs. 3.1%); both these differences
were statistically significant (P<0.05). Deleted or mutated
BRCA1 in most cases of TNBC may render them particularly
susceptible to alkylating agents and platinum drugs that act by
destroying the DNA (28). Currently,
platinum drugs have been used in clinical trials of TNBC and have
proven to be significantly sensitive among patients with TNBC.
Patients who received platinum-based chemotherapy had a longer
overall survival compared with patients that were administered
conventional non-PBC regimens (14.5 vs. 10 months, P=0.041)
(29). Sirohi et al (30) retrospectively reviewed 328 breast
cancer patients (62 with TNBC) treated with platinum-based
chemotherapy and found that the CR rates were significantly higher
for the patients with TNBC (88%) than for patients with non-TNBC
(51%). In addition, the 5-year OS rates were 64 and 85%, the 5-year
disease-free survival rates were 57 and 72% and the ORRs were 41
and 31% for patients with TNBC and those with non-TNBC,
respectively. For patients with advanced breast cancer, TNBC
patients had a significantly prolonged PFS time of 6 months,
compared with 4 months for patients with non-TNBC (30). From the analysis above, it can be
concluded that, compared with non-TNBC, TNBC has an increased
sensitivity to platinum-based chemotherapy.
OXA is a third-generation platinum chemotherapy drug
that is safer and more potent than cisplatin. There are certain
similarities in mechanism between OXA and cisplatin, but their
chemical structures are different. OXA can bind tightly to DNA
>10 times faster than cisplatin and exhibits considerably more
potent cytotoxicity and broad-spectrum antitumor activity. In
addition, OXA has no complete cross-resistance with cisplatin or
carboplatin and is still effective in certain cases showing
resistance to cisplatin or anthracycline (7,31,32,33).
5-FU and OXA have a synergistic effect, and S-1 and
capecitabine are 5-FU derivatives. Chemotherapy oral administration
of 5-FU has become more convenient compared with intravenous
administration, and does not cause phlebitis, although S-1 and
capecitabine differ in terms of pharmacological and side effects
(34,35). A randomized, multicenter phase II
trial of capecitabine versus S-1 in patients with metastatic
gastric cancer demonstrated that there were no significant
differences in clinical outcomes between capecitabine and S-1, with
the exception of hand-foot syndrome and stomatitis, which were more
frequently found in the capecitabine study arm (36). A number of clinical trials have shown
that capecitabine is effective in patients with metastatic breast
cancer (MBC) (37,38). S-1, a third-generation 5-FU
derivative, is a combination of FT, 5-chloro-2,4-dihydropyrimidine
(CDHP) and potassium oxonate (OXO). 5-FU is a derivative of FT, and
is produced in liver mitochondria. CDHP acts to reversibly
antagonize the activity of dihydropyrimidine dehydrogenase (DPD),
which is the rate-limiting enzyme for 5-FU degradation. By
inhibiting DPD, CDHP can therefore prolong the bioavailability of
the 5-FU in the serum and tumors, achieving better curative effects
than continuous intravenous infusion of 5-FU and preventing
complications, such as phlebitis, caused by intravenous
administration. The addition of OXO significantly decreases the
non-hematological toxicological reactions of S-1 (39). Since its listing in Japan in 1999,
numerous studies have indicated the efficacy and favorable
side-effect profile of S-1 (40,41). In
a study investigating S-1 as a third-line or greater chemotherapy
regimen in patients with MBC, one PR (3%) and two SDs (5%) were
observed, and the median time to progression (TTP) was 84 days
(42). A Japanese study showed that
the ORR was 27.8%, the median survival time was 19.2 months and the
TTP was 6.2 months in patients with MBC who did not respond to
capecitabine-based chemotherapy and then received S-1 (43). A new regimen for 16 elderly patients
with advanced MBC revealed that chemotherapy of 2 weeks' S-1
administration followed by 1 weeks' rest was effective; the RR was
31.2%, and the TTP and OS time were 5.1 and 17.9 months,
respectively (44).
Previous studies have indicated that S-1 and OXA are
suitable single agents for the multi-line treatment of advanced
breast cancer and TNBC (3,9,45). On
the basis of the demonstrated efficacy, the present study
retrospectively reviewed 52 patients with advanced TNBC who
received S-1 combined with OXA. The results were encouraging: The
ORR was 34.6%, the median PFS time was 6.7 months (95% CI, 4.5–9.0)
and the median OS time was 13.3 months (95% CI, 9.1–17.5). Subset
analysis showed that patients who were premenopausal, had
infiltrating ductal carcinoma and had previously been treated with
anthracycline or taxane had a prolonged survival time, although
statistical analysis revealed a lack of significance (P>0.05).
The results therefore showed that prolonged survival in the present
OXA- and S-1-based regimen was not significantly associated with
menstrual states, pathologic types or prior chemotherapy; however,
significant associations were found with clinical stage and the
number of metastatic sites. Patients with stage IIIC disease had a
significantly enhanced median OS time compared with patients with
stage IV disease (22.7 vs. 11.3 months, P=0.010). The median OS
time of patients with a single metastatic site (15.7 months; 95%
CI, 5.6–25.8) was significantly longer than that of patients with
≥2 metastatic sites (11.3 months; 95% CI, 9.3–13.3) (P=0.048). A
heavy tumor load, ≥2 metastatic sites and a late disease stage may
therefore lead to poor prognosis. Further studies are required to
analyze the OS times associated with different metastatic sites in
a larger cohort of TNBC patients.
No unexpected adverse events occurred in this study.
Both hematological and non-hematological grade 3/4 toxicities were
experienced by the patients: The main hematological toxicity was
neutropenia, which occurred in 11.5% of patients, while the main
non-hematological toxicities were nausea and vomiting (7.7%) and
peripheral neuropathy (3.8%). Grade 1/2 toxicities, included
diarrhea, liver dysfunction, stomatitis, anemia and hand-foot
syndrome. The study showed that oral administration of S-1 markedly
shortened the hospital stay of the patients with advanced TNBC,
making it suitable for use as a palliative chemotherapeutic agent
to improve the patients' quality of life.
In conclusion, S-1 plus OXA is a safe, active and
well-tolerated combination and should be further investigated as a
standard treatment alternative for patients with advanced TNBC.
Since there are no published data on the efficacy of a combination
of S-1 and OXA as a treatment for advanced TNBC, and as the present
results may have some bias and limitations due to the small size of
the sample, a large, randomized, prospective clinical study is
warranted to confirm the results.
References
|
1
|
Vrdoljak E, Miše BP, Lukić B, et al:
Long-lasting control of triple-negative metastatic breast cancer
with the novel drug combination ixabepilone and capecitabine - case
report. Onkologie. 33:53–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rakha EA and Ellis IO:
Triple-negative/basal-like breast cancer: Review. Pathology.
41:40–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fei F, Chen C, Xue J, et al: Efficacy and
safety of docetaxel combined with oxaliplatin as a neoadjuvant
chemotherapy regimen for Chinese triple-negative local advanced
breast cancer patients. A prospective, open, and unicentric Phase
II clinical trial. Am J Clin Oncol. 36:545–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan DS, Marchió C, Jones RL, et al: Triple
negative breast cancer: Molecular profiling and prognostic impact
in adjuvant anthracycline-treated patients. Breast Cancer Res
Treat. 111:27–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu M, Mo QG, Wei CY, et al:
Platinum-based chemotherapy in triple-negative breast cancer: A
meta-analysis. Oncol Lett. 5:983–991. 2013.PubMed/NCBI
|
|
7
|
Sumpter K, Harper-Wynne C, Cunningham D,
et al: Report of two protocol planned interim analyses in a
randomised multicentre phase III study comparing capecitabine with
fluorouracil and oxaliplatin with cisplatin in patients with
advanced oesophagogastric cancer receiving ECF. Br J Cancer.
92:1976–1983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Cao Y, Wu L, et al: S-1-based
therapy versus 5-FU-based therapy in advanced gastric cancer: A
meta-analysis. Med Oncol. 28:1004–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hara F, Kiyoto S, Takahashi M, et al:
Efficacy and safety of S-1 in patients with metastatic breast
cancer: Retrospective review in a single institution. Oncology.
79:273–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: Development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rastelli F, Biancanelli S, Falzetta A, et
al: Triple-negative breast cancer: Current state of the art.
Tumori. 96:875–888. 2010.PubMed/NCBI
|
|
14
|
Turner N, Tutt A and Ashworth A: Hallmarks
of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 4:814–819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fornier M and Fumoleau P: The paradox of
triple negative breast cancer: Novel approaches to treatment.
Breast J. 18:41–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Laurentiis M, Cianniello D, Caputo R,
et al: Treatment of triple negative breast cancer (TNBC): Current
options and future perspectives. Cancer Treat Rev. 36:(Suppl 3).
S80–S86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rakha EA, El-Sayed ME, Green AR, et al:
Prognostic markers in triple-negative breast cancer. Cancer.
109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rhee J, Han SW, Oh DY, et al: The
clinicopathologic characteristics and prognostic significance of
triple-negativity in node-negative breast cancer. BMC Cancer.
8:3072008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geisler S, Lønning PE, Aas T, et al:
Influence of TP53 gene alterations and c-erbB-2 expression on the
response to treatment with doxorubicin in locally advanced breast
cancer. Cancer Res. 61:2505–2512. 2001.PubMed/NCBI
|
|
20
|
Gadhikar MA, Sciuto MR, Alves MV,
Pickering CR, Osman AA, Neskey DM, Zhao M, Fitzgerald AL, Myers JN
and Frederick MJ: Chk1/2 inhibition overcomes the cisplatin
resistance of head and neck cancer cells secondary to the loss of
functional p53. Mol Cancer Ther. 12:1860–1873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dookeran KA, Dignam JJ, Ferrer K, et al:
p53 as a marker of prognosis in African-American women with breast
cancer. Ann Surg Oncol. 17:1398–1405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chae BJ, Bae JS, Lee A, et al: p53 as a
specific prognostic factor in triple-negative breast cancer. Jpn J
Clin Oncol. 39:217–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hernandez-Aya LF, Chavez-Macgregor M, Lei
X, et al: Nodal status and clinical outcomes in a large cohort of
patients with triple-negative breast cancer. J Clin Oncol.
29:2628–2634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lower EE, Glass E, Blau R and Harman S:
HER-2/neu expression in primary and metastatic breast cancer.
Breast Cancer Res Treat. 113:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lower EE, Glass EL, Bradley DA, et al:
Impact of metastatic estrogen receptor and progesterone receptor
status on survival. Breast Cancer Res Treat. 90:65–70. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lippman ME, Allegra JC, Thompson EB, et
al: The relation between estrogen receptors and response rate to
cytotoxic chemotherapy in metastatic breast cancer. N Engl J Med.
298:1223–1228. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keam B, Im SA, Kim HJ, et al: Prognostic
impact of clinicopathologic parameters in stage II/III breast
cancer treated with neoadjuvant docetaxel and doxorubicin
chemotherapy: Paradoxical features of the triple negative breast
cancer. BMC Cancer. 7:2032007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roy V, Pockaj BA, Allred JB, et al: A
Phase II trial of docetaxel and carboplatin administered every 2
weeks as preoperative therapy for stage II or III breast cancer:
NCCTG study N0338. Am J Clin Oncol. 36:540–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Villarreal-Garza C, Khalaf D, Bouganim N,
et al: Platinum-based chemotherapy in triple-negative advanced
breast cancer. Breast Cancer Res Treat. 146:567–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sirohi B, Arnedos M, Popat S, et al:
Platinum-based chemotherapy in triple-negative breast cancer. Ann
Oncol. 19:1847–1852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Virag P, Perde-Schrepler M, Fischer-Fodor
E, et al: Superior cytotoxicity and DNA cross-link induction by
oxaliplatin versus cisplatin at lower cellular uptake in colorectal
cancer cell lines. Anticancer Drugs. 23:1032–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dunn TA, Schmoll HJ, Grünwald V, Bokemeyer
C and Casper J: Comparative cytotoxicity of oxaliplatin and
cisplatin in non-seminomatous germ cell lines. Invest New Drugs.
15:109–114. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang M, Jiang H, Wang S, et al: Efficacy
of docetaxel combined with oxaliplatin by different administration
routes in treatment of anthracycline-resistant metastatic breast
cancer. Zhong Guo Zhong Liu Lin Chuang Yu Kang Fu. 12:527–529.
2005.(In Chinese).
|
|
34
|
Thuss-Patience PC, von Minckwitz G,
Kretzschmar A, et al: Oxaliplatin and 5-fluorouracil for heavily
pretreated metastatic breast cancer: A preliminary phase II study.
Anticancer Drugs. 14:549–553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miura K, Kinouchi M, Ishida K, et al: 5-fu
metabolism in cancer and orally-administrable 5-fu drugs. Cancers
(Basel). 2:1717–1730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JL, Kang YK, Kang HJ, et al: A
randomised multicentre phase II trial of capecitabine vs S-1 as
first-line treatment in elderly patients with metastatic or
recurrent unresectable gastric cancer. Br J Cancer. 99:584–590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv H, Yan M, Zhang M, et al: Efficacy of
capecitabine-based combination therapy and single-agent
capecitabine maintenance therapy in patients with metastatic breast
cancer. Chin J Cancer Res. 26:692–697. 2014.PubMed/NCBI
|
|
38
|
Gelmon K, Chan A and Harbeck N: The role
of capecitabine in first-line treatment for patients with
metastatic breast cancer. Oncologist. 11:(Suppl 1). 42–51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shirasaka T, Tsukuda M, Inuyama Y and
Taguchi T: New oral anticancer drug, TS-1 (S-1) - from bench to
clinic. Gan To Kagaku Ryoho. 28:855–864. 2001.(In Japanese).
PubMed/NCBI
|
|
40
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boku N: Gastrointestinal Oncology Study
Group of Japan Clinical Oncology Group: Chemotherapy for metastatic
disease: Review from JCOG trials. Int J Clin Oncol. 13:196–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shien T, Shimizu C, Akashi-Tanaka S, et
al: Clinical efficacy of S-1 in pretreated metastatic breast cancer
patients. Jpn J Clin Oncol. 38:172–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamamoto D, Iwase S, Yoshida H, et al:
Efficacy of S-1 in patients with capecitabine-resistant breast
cancer-Japan Breast Cancer Research Network (JBCRN) 04-1 trial.
Anticancer Res. 30:3827–3831. 2010.PubMed/NCBI
|
|
44
|
Fujii K, Kosaka J, Mouri Y, et al:
Chemotherapy of a 2-week S-1 administration followed by 1-week rest
for advanced and metastatic breast cancer. Gan To Kagaku Ryoho.
38:1467–1470. 2011.(In Japanese). PubMed/NCBI
|
|
45
|
Saeki T: Clinical benefit of S-1 in
metastatic breast cancer. Gan To Kagaku Ryoho. 33:(Suppl 1).
202–206. 2006.(In Japanese). PubMed/NCBI
|