Introduction
It is well accepted that the pre-operative
administration of anticholinergic drugs can prevent adverse
consequences of autonomic nervous system imbalance during surgery.
Penehyclidine hydrochloride (penehyclidine) is a long-acting
cholinergic receptor blocker manufactured in China (1). The main anesthetic mechanism of
penehyclidine is selective blocking of central and peripheral M1
and M3 muscarinic acetylcholine receptors and N1 and N2 nicotinic
acetylcholine receptors, without obvious effect on the M2 receptors
distributed in the heart or presynaptic nerve membranes (1–3). The
inhibition of M1 and M3 receptors leads to the direct inhibition of
parasympathetic nerve effects and reflexive regulation of
sympathetic nerves, thus stabilizing autonomic nerves and resulting
in a central sedative effect (4). N1
receptors are responsible for modulating the release of
neurotransmitters from neurons (5).
The activation of N2 receptors results in muscle depolarization,
the induction of action potentials and muscle contraction (5); therefore, blockade of N1 and N2
receptors leads to the inhibition of the overexcitation of
autonomic nerves, and muscle relaxation. The activation of M2
receptors could modulate heart rate (HR) by affecting the
conduction of electrical impulses through the atrioventricular node
(6). Penehyclidine has been
demonstrated to exert little or no effect on M2 receptors (2,7,8) in rats and humans, and thus has little
influence on HR.
Spectral analysis of the electrocardiogram is a
non-invasive approach to monitor the sympathetic and
parasympathetic outflow in various clinical situations (9). It has been demonstrated that HR
variability (HRV), the slight fluctuation of the R-R interval
between consecutive heartbeats, can be used to assess the autonomic
control of the heart (10). HRV has
great potential to detect cardiac sympathetic and vagus nervous
system fluctuations (11), as well
as to evaluate the influence of anticholinergic drugs (12). Among the HRV indices, total power
(TP) represents the total power of the autonomic nervous
activities; low-frequency power (LF), the frequency range from 0.04
to 0.15 Hz, demonstrates the sympathetic alteration of HR and, to a
lesser extent, indicates parasympathetic activity; high-frequency
power (HF), the frequency range from 0.15 to 0.45 Hz, is a measure
of vagal nerve activity (13,14). The
LF/HF ratio indicates the balance of sympathetic and
parasympathetic activities (10,13,14). An
elevated LF/HF ratio reflects enhanced sympathetic activity, while
a low ratio indicates parasympathetic nerve activity.
The objective of the present study was to explore
the effect of different doses of penehyclidine on sympathovagal
balance, as determined by HRV, in patients undergoing gynecological
hysteroscopic surgery.
Materials and methods
General information
Following approval from the Ethics Committee of
Jilin University (Changchun, China; clinical trial registration no.
H20020606), 180 patients undergoing gynecological hysteroscopic
surgery [American Society of Anesthesiologists (ASA) grade I–II]
were enrolled in this prospective study conducted between January
and September 2012 at the First Hospital of Jilin University
(Changchun, China). Informed consent was obtained from each
patient. Patients with elevated body temperature, contraindication
to saddle anesthesia, allergy to cholinergic drugs, history of
glaucoma, liver or kidney dysfunction, an estimated blood loss of
>600 ml during surgery and patients who were undergoing
treatment with other anticholinergic drugs were excluded from this
study.
Anesthesia methods
Subsequent to obtaining venous access, the patients
were administrated sodium chloride solution prior to anesthesia.
The patients were randomly divided into three groups (n=60/group):
Penehyclidine 0.5 mg (group I), penehyclidine 1.0 mg (group II) or
saddle anesthesia (control). Randomization was performed using
sealed envelopes containing computer-generated random numbers.
Penehyclidine (Force Fest Pharmaceutical Co., Chengdu, China) was
administered intravenously 10 min before the induction of
anesthesia. The control patients were given 0.8 ml 0.5% bupivacaine
(Wellhope Pharmaceutical Co., Ltd., Shanghai, China) intrathecally.
All patients were administered midazolam (2 mg; Jiangsu Enhua
Pharmaceutical Co., Ltd., Xuzhou, China) and fentanyl (1 µg/kg;
Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China) prior
to the start of surgery. Propofol (4–6 mg/kg/h; Libang
Pharmaceutical Co., Ltd., Xian, China) was injected continuously
during surgery. A single dose of fentanyl (0.05 mg) was used when
necessary. The ambient temperature was maintained at 20–25°C.
HRV analysis
HRV was measured and recorded prior to the
initiation of anesthesia (T0), following the induction
of anesthesia (T1), at the start of surgery
(T2) and following the completion of the surgery
(T3). HR was monitored continuously using a
multifunctional compact patient monitor (M8004A; Philips Medizin
Systeme Böblingen GmbH, Böblingen, Germany). An HXD-I
multi-function monitor (Huaxiang Co., Harbin, China) was used to
monitor other parameters of HRV. Spectral analysis of HRV was
performed following the manufacturer's instructions. HRV was
assessed in classic frequency bands, including TP, LF (0.04–0.15
Hz), HF (0.15–0.45 Hz) and the ratio of LF to HF (LF/HF).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean for parametric variables with confirmed normality. Data
were analyzed using SPSS statistical software version 13.0 (SPSS
Inc., Chicago, IL, USA) and Prism 4.0 (GraphPad Software, Inc., San
Diego, CA, USA). One-way analysis of variance (ANOVA) and the
Mann-Whitney U and Fisher's exact tests, as appropriate, were used
to compare general patient information. Two-way ANOVA with repeated
measures (inter-group comparisons) and one-way ANOVA (intra-group
comparisons) were used to determine the significance of differences
in HR and HRV. Post hoc analysis was performed using the Bonferroni
and Turkey tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
Patient baseline characteristics are shown in
Table I. Sixty patients were
enrolled in each group. No significant inter-group differences were
observed in age, weight, height, ASA classification or baseline HR,
oxygen saturation or systolic and diastolic blood pressure
(P>0.05). Table II summarizes
the HR and HRV data in the three study groups at different times.
No statistically significant differences were observed among the
three groups in the baseline (T0) HR and HRV variables,
including TP, LF, HF and LF/HF.
 | Table I.Demographic and baseline
characteristics of the three study groups. |
Table I.
Demographic and baseline
characteristics of the three study groups.
|
| Group I | Group II | Group III | P-value |
|---|
| Age (years) | 48.45±2.96 | 41.95±1.65 | 39.25±2.76 | 0.063 |
| Weight (kg) | 57.85±1.74 | 58.50±1.38 | 60.50±1.90 | 0.766 |
| Height (cm) | 158.79±0.84 | 158.65±0.89 | 161.15±0.92 | 0.251 |
| Baseline HR
(bpm) | 74.56±3.07 | 75.95±2.49 | 80.75±2.38 | 0.179 |
| Baseline
SpO2 (%) | 98.55±0.32 | 99.25±0.85 | 99.10±0.19 | 0.221 |
| Baseline SBP
(mmHg) | 137.50±3.68 | 139.25±3.88 | 126.30±7.53 | 0.431 |
| Baseline DBP
(mmHg) | 96.25±16.42 | 77.40±2.09 | 80.0±2.11 | 0.379 |
| ASA status
(n/%) |
|
|
| 0.187 |
| I | 22/36.6 | 27/45.0 | 32/53.3 |
|
| II | 38/63.3 | 33/55.0 | 28/46.7 |
|
 | Table II.HR and HRV analysis of the three
groups at different time-points. |
Table II.
HR and HRV analysis of the three
groups at different time-points.
| Parameter | Group | T0 | T1 | T2 | T3 |
|---|
| HR (bpm) | Group I |
74.65±3.07 |
69.00±2.51 |
67.85±2.32 |
71.30±2.99 |
|
| Group II |
75.95±2.49 |
72.30±2.08 |
77.15±2.43 |
77.80±2.13 |
|
| Control |
80.75±2.38 |
77.80±2.35 |
70.30±1.74 |
77.00±2.01 |
| TP
(msec2/Hz) | Group I |
2449.37±357.68 |
2050.14±309.28 |
3001.14±586.05 |
1912.65±328.27 |
|
| Group II |
4950.66±2069.66 |
4040.75±1681.27 |
9895.51±5385.66a |
2223.78±713.72 |
|
| Control |
3217.81±606.84 |
4478.49±438.04 |
4961.22±662.26 |
3116.45±671.18 |
| LF
(msec2/Hz) | Group I |
649.47±121.62 |
624.84±153.66b |
711.47±135.92a |
502.95±80.95 |
|
| Group II |
1361.24±670.31 |
1210.635±594.44 |
2118.955±492.60 |
484.62±123.20 |
|
| Control |
1102.01±293.21 |
1996.14±329.25 |
1813.89±296.82a |
1100.96±314.10 |
| HF
(msec2/Hz) | Group I |
648.89±130.67 |
587.59±153.26b |
994.81±262.35a,b |
540.68±89.01 |
|
| Group II |
1460.595±606.39 |
813.35±127.63 |
2738.22±585.43b |
705.84±208.88 |
|
| Control |
885.87±198.48 |
1984.14±297.27 |
2977.37±523.46a |
1124.15±426.01 |
| LF/HF | Group I |
1.23±0.15 |
1.11±0.13 |
1.00±0.11 |
1.17±0.21 |
|
| Group II |
0.94±0.10 |
0.94±0.11 |
0.74±0.07 |
0.76±0.08 |
|
| Control |
1.34±0.21 |
1.05±0.13 |
0.71±0.08 |
1.17±0.19 |
Study data
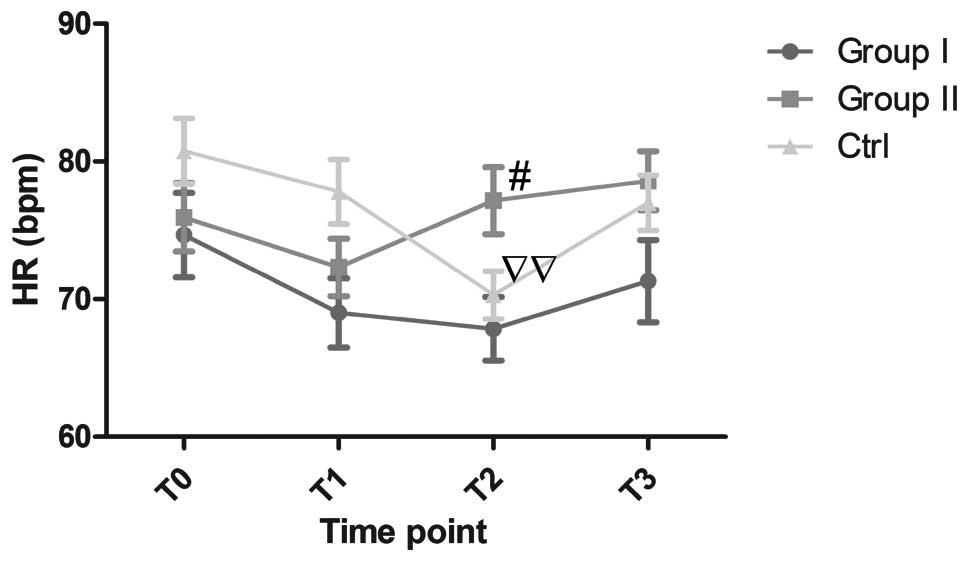
Changes in HR observed in the study groups are shown
in Fig. 1. No notable changes in HR
were observed during surgery in either groups I or II; however, the
HR was significantly lower at T2 than that at
T0 in the control group (70.30±1.74 vs. 80.75±2.38,
P<0.01), and the HR at T2 was significantly higher in
group II than that in group I (77.15±2.43 vs. 67.85±2.32,
P<0.05). No significant differences in HR were observed in
groups I or II compared with the control group. No statistically
significant differences in TP were observed between either group I
or II and the control group (Fig.
2); however, the TP was elevated in group II compared with that
in group I at T2 (9,895.51±5,385.66 vs. 3,001.14±586.05,
P<0.01). The LF and HF values in group I were significantly
lower compared with those in the control group at T1
(LF, 624.84±153.66 vs. 1,996.14±329.25, P<0.01; HF,
587.59±153.26 vs. 1,984.14±297.27, P<0.05) and T2
(LF, 711.47±135.92 vs. 1,813.89±296.82, P<0.05; HF,
994.81±262.35 vs. 2,977.37±523.46, P<0.001). Furthermore, the
patients in group II had higher LF and HF values at T2
than the patients in group I (LF, 2,118.955±492.60 vs.
711.47±135.92, P<0.01; HF, 2,738.22±585.43 vs. 994.81±262.35,
P<0.05). The HF value was higher at T2 than that at
T0 in the control group (2,977.37±523.46 vs.
885.87±198.48, P<0.01). The HF and LF values did not change
significantly within groups I and II during the study.
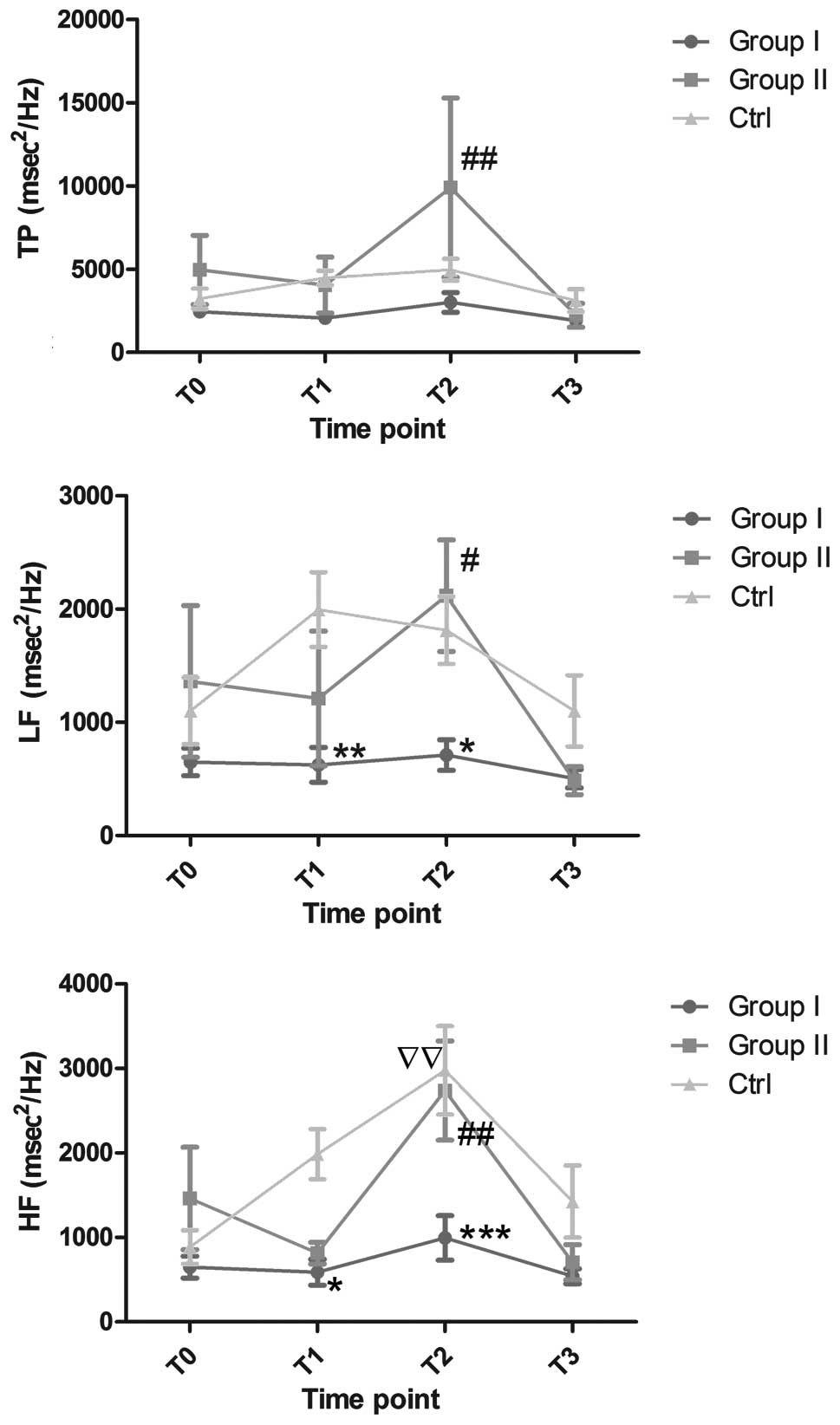 | Figure 2.Changes in TP, LF and HF
(msec2/Hz) in the three study groups. *P<0.05,
**P<0.01 and ***P<0.001, versus the control group;
#P<0.05 and ##P<0.01, versus group I
(all inter-group comparisons were performed at corresponding
time-points). ssP<0.01, T2 versus
T0 within the control group. Ctrl, control group; TP,
total power; LF, low-frequency power; HF, high-frequency power;
T0, prior to the initiation of anesthesia;
T1, following the induction of anesthesia;
T2, at the commencement of surgery; T3,
following the completion of surgery. |
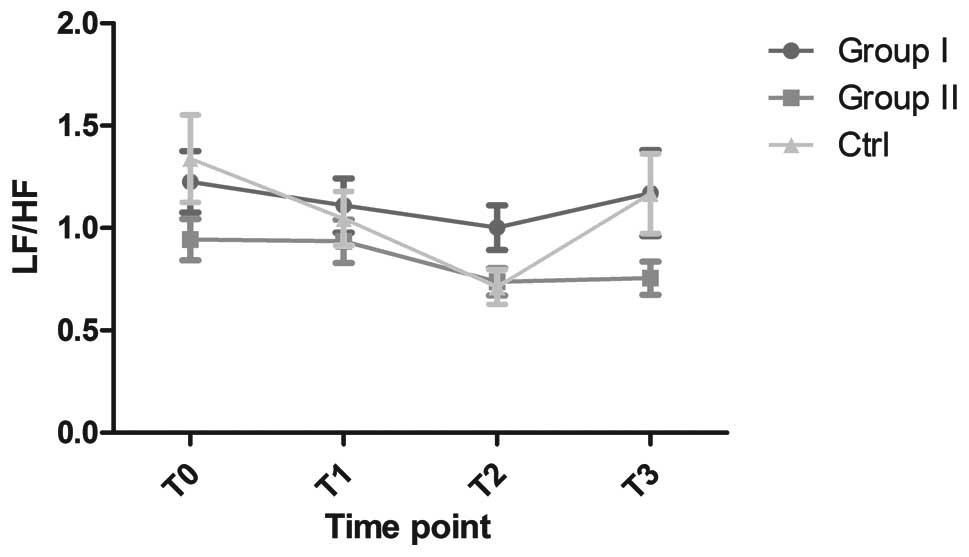
No significant differences in the LF/HF ratio were
observed among the three groups (Fig.
3); the ratio remained constant throughout the study. Despite
the fact that no significant differences were found in the control
group at different times, the LF/HF gradually decreased between
T0 and T2, and then increased to the starting
level. LF/HF was more stable, with little fluctuation, in groups I
and II.
Discussion
The present study demonstrated that the
pre-operative administration of penehyclidine resulted in a more
stable HR following the induction of anesthesia and during surgery
than the low-dose spinal anesthesia combined with intravenous
anesthesia administered to the control group. Within group I, the
administration of 0.5 mg penehyclidine was followed by the stable
evolution of HR and HRV parameters, including TP, LF, HF and LF/HF,
over the whole study period. The administration of 1.0 mg
penehyclidine in group II resulted in higher TP, LF and HF values
at the start of surgery compared with the values in group I. In the
control group, the HF showed a significant increase at the start of
surgery compared with the baseline HF level. At the completion of
surgery, the HF had decreased to a level similar to the baseline.
Compared with group I, the control group had markedly higher LF and
HF values following the induction of anesthesia and during the
surgery. All three study groups demonstrated stable LF/HF ratios,
with only slight fluctuations observed in groups I and II.
Overall, prior to anesthesia, the activity of
parasympathetic and sympathetic nerves, as indicated by the HF, LF
and LF/HF, showed no inter-group differences. All types of
anesthetics can modulate hemodynamic status by blocking sympathetic
output (15), and different
anesthetic approaches can have a variety of effects on HRV and HR
(16).
It is well known that pain and psychological stress
may be associated with enhanced HR through the activation of the
autonomic system (17–19), and feasible pain control and
anesthesia may modulate those adaptive responses (17,20,21).
Saddle anesthesia, which is performed at the lowest block level of
subarachnoid or spinal anesthesia, is widely used in gynecological
surgery, including hysteroscopy (22,23). The
combination of low-dose saddle anesthesia and intravenous
anesthesia, which improves intra-operative management and the
post-operative quality of analgesia (13), is widely applied in gynecological
surgeries in China (24); however, a
common complication associated with spinal anesthesia is sinus
bradycardia (25,26). Geffin and Shapiro (25) reported 12 cases of sinus bradycardia
and asystole during spinal anesthesia, and the acute event appeared
only 15 min after administering the anesthetic injection. Lesser
et al (26) also reported
>600 cases of bradycardia during neuraxial anesthesia, among
which 46 cases were severe (HR <40 bpm). A reduction in HR was
also detected in the present study during the surgery. Despite the
fact that no incident of bradycardia occurred, the potential risk
with this approach should be noted. The occurrence of these
complications may be due to an imbalance between the
parasympathetic and sympathetic modulation of the HR (13). In the present study, penehyclidine,
which is known to have a weak effect on M2 receptors (2), was administered pre-operatively. Upon
the administration of 0.5 or 1.0 mg penehyclidine, no clinically
significant changes in HR were observed, but 1.0 mg penehyclidine
was superior to 0.5 mg penehyclidine in maintaining HR during
surgery.
The fluctuation of LF is associated with sympathetic
activity, and represents the modulation of HR evoked by the
parasympathetic arterial baroreceptor reflex (10). LF is therefore affected by both
parasympathetic and sympathetic activities, particularly the
modulation of sympathetic activity (27). A significantly lower LF was observed
in patients receiving 0.5 mg penehyclidine compared with those
under spinal anesthesia both following the induction of anesthesia
and during surgery. This decline suggests an attenuated response to
sympathetic activation. It has been established that high-intensity
pain occurs with sympathetic activation (28); therefore, decreased LF may indicate
effective pain control or a notable anti-muscarinic effect of 0.5
mg penehyclidine (29).
Concomitant with the reduced LF in patients
receiving 0.5 mg penehyclidine, a parallel reduction in HF
occurred. This result may reflect decreased vagal control of HRV in
group I patients compared with patients given low-dose spinal
anesthesia combined with intravenous anesthesia. This may be a
consequence of the significant decline in respiratory sinus
arrhythmia, which is the major component of the HF spectral band
(14). In contrast to the low HF of
patients receiving penehyclidine, an elevated HF was observed in
patients with spinal anesthesia. This elevation of HF was also
observed by Hanss et al (30). The results in the study by Hanss
et al demonstrated that the main cause of parasympathetic
activity excitation was a reflexive shift to parasympathetic
activity in response to the inhibition of sympathetic activity
(30); however, the results of the
present study revealed a parallel elevation of LF and HF. The exact
mechanism of this phenomenon was not investigated in this study,
but uncontrolled alterations in other factors, such as ambient
temperature or noise, physical and psychological stress, or
differences in ethnic background, may explain the discrepancies.
Generally, anesthetics are assumed to suppress the autonomic system
not only by depressing the excitatory sympathetic activity induced
by surgery, but also by inhibiting parasympathetic activity
(16,31). The results of the present study
indicate that 0.5 mg penehyclidine, when combined with spinal and
intravenous anesthesia, was more effective at accomplishing this
than 1.0 mg penehyclidine.
The combined alterations of LF and HF resulted in an
overall stable LF/HF ratio during the whole anesthetic period. In
the control group, despite the fact that no significant difference
was observed during the experimental period, it is possible that
the LF/HF ratio may have been affected by saddle anesthesia, as the
ratio declined gradually between T0 and T2.
It has been reported in patients undergoing transurethral surgery
that the LF/HF ratio decreased significantly following spinal
anesthesia with bupivacaine regardless of the addition of fentanyl
(13,32). The authors of these studies suggested
that this spinal-anesthesia-associated reduction was the result of
an imbalance in the sympathovagal system. Similar findings have
also been reported in females scheduled for elective Cesarean
section under spinal anesthesia with bupivacaine (30). In the present study, an elevated
baseline LF/HF ratio dropped during spinal anesthesia, and an
attenuation of sympathetic activity was observed. These changes
were not evident in the groups injected with either 0.5 or 1.0 mg
penehyclidine. These results suggest excellent maintenance of
autonomic system balance by penehyclidine.
To the best of our knowledge, this is one of the few
investigations of the effect of penehyclidine on HR and HRV
indices; however, several limitations should be noted. Firstly, the
exact role of HRV analysis remains controversial since it is
unclear precisely what HRV measures. HRV may reflect the modulation
of the HR by the sympathetic and parasympathetic systems, but it is
not an accurate indicator of the absolute levels of autonomic
activity (33). Despite this, HRV is
widely accepted as a useful tool to measure the effect of autonomic
alterations on HR during anesthesia (34). A second limitation was that the
mechanisms of autonomic modulation were not fully accounted for in
this study. Other factors, including non-neural mechanisms and
psychological effects, could not be assessed by HRV signals
(35). Thirdly, this study only
measured the perioperative HRV alterations; thus, the long-term
effects of penehyclidine cannot be evaluated. Finally, it is not
known whether similar observations are likely to apply to patients
undergoing other types of surgeries.
In conclusion, the present study demonstrates that
0.5 mg penehyclidine stabilizes potential fluctuations in HRV,
without significantly altering the autonomic nerve modulation of
HR. Penehyclidine at a dose of 1.0 mg may be superior to 0.5 mg
penehyclidine in maintaining a stable HR, and reduces the incidence
of bradycardia; however, the higher dose is less effective in
maintaining sympathetic and parasympathetic balance.
References
|
1
|
Xiao HT, Liao Z and Tong RS: Penehyclidine
hydrochloride: a potential drug for treating COPD by attenuating
Toll-like receptors. Drug Des Devel Ther. 6:317–322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Ren Y, Zhu Y, et al: Effect of
penehyclidine hydrochloride on the incidence of intra-operative
awareness in Chinese patients undergoing breast cancer surgery
during general anaesthesia. Anaesthesia. 68:136–141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun YJ, Song DD, Diao YG, Zhou J and Zhang
TZ: Penehyclidine hydrochloride preserves the intestinal barrier
function in patients undergoing cardiopulmonary bypass. J Thorac
Cardiovasc Surg. 146:179–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patterson TA, Lipton JR, Bennett EL and
Rosenzweig MR: Cholinergic receptor antagonists impair formation of
intermediate-term memory in the chick. Behav Neural Biol. 54:63–74.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossman AC: The physiology of the
nicotinic acetylcholine receptor and its importance in the
administration of anesthesia. AANA J. 79:433–440. 2011.PubMed/NCBI
|
|
6
|
Harvey RD: Muscarinic receptor agonists
and antagonists: effects on cardiovascular function. Handb Exp
Pharmacol. 299–316. 2012.PubMed/NCBI
|
|
7
|
Xiao HT, Liao Z, Meng XM, Yan XY, Chen SJ
and Mo ZJ: Underlying mechanism of penehyclidine hydrochloride on
isolated rat uterus. Fundam Clin Pharmacol. 23:419–421. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Zhuang Y, Ouyang F, Zhang A, Zeng
B and Gu M: Penehyclidine enhances the efficacy of tropisetron in
prevention of PONV following gynecological laparoscopic surgery. J
Anesth. 26:864–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed. Heart rate variability.
Standards of measurement, physiological interpretation and clinical
use. Task Force of the European Society of Cardiology and the North
American Society of Pacing and Electrophysiology. Eur Heart J.
17:354–381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akselrod S, Gordon D, Ubel FA, Shannon DC,
Berger AC and Cohen RJ: Power spectrum analysis of heart rate
fluctuation: a quantitative probe of beat-to-beat cardiovascular
control. Science. 213:220–222. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Iorio C, Cafiero T and Di Minno RM: The
effects of pneumoperitoneum and head-up position on heart rate
variability and QT interval dispersion during laparoscopic
cholecystectomy. Minerva Anestesiol. 76:882–889. 2010.PubMed/NCBI
|
|
12
|
Sugiura H, Chinushi M, Komura S, Hirono T
and Aizawa Y: Heart rate variability is a useful parameter for
evaluation of anticholinergic effect associated with inducibility
of atrial fibrillation. Pacing Clin Electrophysiol. 28:1208–1214.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujiwara Y, Kurokawa S, Shibata Y, Asakura
Y, Harado M and Komatsu T: Sympathovagal effects of spinal
anaesthesia with intrathecal or intravenous fentanyl assessed by
heart rate variability. Acta Anaesthesiol Scand. 53:476–482. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
No authors listed. Heart rate variability:
standards of measurement, physiological interpretation and clinical
use. Task Force of the European Society of Cardiology and the North
American Society of Pacing and Electrophysiology. Circulation.
93:1043–1065. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banz VM, Jakob SM and Inderbitzin D:
Review article: improving outcome after major surgery:
pathophysiological considerations. Anesth Analg. 112:1147–1155.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riznyk L, Fijałkowska M and Przesmycki K:
Effects of thiopental and propofol on heart rate variability during
fentanyl-based induction of general anesthesia. Pharmacol Rep.
57:128–134. 2005.PubMed/NCBI
|
|
17
|
Lowe NK: The nature of labor pain. Am J
Obstet Gynecol. 186:(5 Suppl Nature). S16–S24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alici G, Ozkan B, Acar G, et al:
Evaluation of autonomic functions by heart rate variability after
stenting in patients with carotid artery stenosis. Ann Noninvasive
Electrocardiol. 18:126–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato R, Mizuno M, Miura T, et al:
Angiotensin receptor blockers regulate the synchronization of
circadian rhythms in heart rate and blood pressure. J Hypertens.
31:1233–1238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaneshiro B, Grimes DA and Lopez LM: Pain
management for tubal sterilization by hysteroscopy. Cochrane
Database Syst Rev. 8:CD0092512012.PubMed/NCBI
|
|
21
|
Griffis CA, Crabb Breen E, Compton P, et
al: Acute painful stress and inflammatory mediator production.
Neuroimmunomodulation. 20:127–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Florio P, Puzzutiello R, Filippeschi M, et
al: Low-dose spinal anesthesia with hyperbaric bupivacaine with
intrathecal fentanyl for operative hysteroscopy: a case series
study. J Minim Invasive Gynecol. 19:107–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cesur M, Alici HA, Erdem AF, Borekci B and
Silbir F: Spinal anesthesia with sequential administration of plain
and hyperbaric bupivacaine provides satisfactory analgesia with
hemodynamic stability in cesarean section. Int J Obstet Anesth.
17:217–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo SB, Lin M and Hao ZB: The application
of combination of spinal anesthesia and intraveous anesthesia in
gynecological laproscopy. Zhong Guo Xian Dai Yao Wu Ying Yong.
3:902009.(In Chinese).
|
|
25
|
Geffin B and Shapiro L: Sinus bradycardia
and asystole during spinal and epidural anesthesia: a report of 13
cases. J Clin Anesth. 10:278–285. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lesser JB, Sanborn KV, Valskys R and
Kuroda M: Severe bradycardia during spinal and epidural anesthesia
recorded by an anesthesia information management system.
Anesthesiology. 99:859–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malliani A, Pagani M, Lombardi F and
Cerutti S: Cardiovascular neural regulation explored in the
frequency domain. Circulation. 84:482–492. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carr DB and Goudas LC: Acute pain. Lancet.
353:2051–2058. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weissman A, Torkhov O, Weissman AI and
Drugan A: The effects of meperidine and epidural analgesia in labor
on maternal heart rate variability. Int J Obstet Anesth.
18:118–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanss R, Ohnesorge H, Kaufmann M, et al:
Changes in heart rate variability may reflect sympatholysis during
spinal anaesthesia. Acta Anaesthesiol Scand. 51:1297–1304. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakatsuka I, Ochiai R and Takeda J:
Changes in heart rate variability in sevoflurane and nitrous oxide
anesthesia: effects of respiration and depth of anesthesia. J Clin
Anesth. 14:196–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujiwara Y, Sato Y, Shibata Y, Asakura Y,
Nishiwaki K and Komatsu T: A greater decrease in blood pressure
after spinal anaesthesia in patients with low entropy of the RR
interval. Acta Anaesthesiol Scand. 51:1161–1165. 2007.PubMed/NCBI
|
|
33
|
Haney MF and Wiklund U: Can heart rate
variability become a screening tool for anesthesia-related
hypotension? Acta Anaesthesiol Scand. 51:1289–1291. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vettorello M, Colombo R, De Grandis CE,
Costantini E and Raimondi F: Effect of fentanyl on heart rate
variability during spontaneous and paced breathing in healthy
volunteers. Acta Anaesthesiol Scand. 52:1064–1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parati G, Mancia G, Di Rienzo M and
Castiglioni P: Point: cardiovascular variability is/is not an index
of autonomic control of circulation. J Appl Physiol (1985).
101:676–678; discussion 681–682. 2006. View Article : Google Scholar : PubMed/NCBI
|