Introduction
Atherosclerosis, a systemic disease that usually
affects large- and medium-sized elastic and muscular arteries, is
the underlying pathology of most cardiovascular diseases. The onset
and pathogenesis of atherosclerosis have not yet been fully
established. Studies have concluded that the activation of the
inflammatory pathways may be important in the pathogenesis of the
disease (1,2). It has been shown that atherosclerotic
risk factors can cause vascular endothelial injuries, inducing the
endothelial expression of adhesion molecules, such as vascular cell
adhesion molecule-1 (VCAM-1), and subsequently initiate
inflammatory responses (3,4).
VCAM-1 belongs to the immunoglobulin superfamily,
which is expressed in vascular endothelial cells. VCAM-1 promotes
the adhesion of leukocytes to endothelial cells (5), and accelerates the migration of the
leukocytes along the endothelial surface. In addition, VCAM-1 has
been linked with the pathogenesis of atherosclerosis. De Caterina
et al (6) showed that soluble
VCAM-1 (sVCAM-1) levels were directly associated with carotid
intima-media thickness and could be used to evaluate prognosis.
Zeitler et al (7) also found
that sVCAM-1 levels were significantly elevated in patients
suffering from coronary heart disease and acute myocardial
infarction. Although these findings concerning the role of sVCAM-1
in coronary heart disease are encouraging, the sVCAM-1 level only
represents the proteins expressed on cell surfaces that are shed
into the blood. It is therefore of great importance to investigate
the expression levels of VCAM-1 in arterial tissues, and to
elucidate the association between arterial VCAM-1 expression and
the disease pathogenesis. Based on this, the aim of the present
study was to investigate the expression levels of VCAM-1 in the
aortic tissues from patients undergoing coronary artery bypass
graft (CABG) surgery for coronary heart disease, and to explore the
association between VCAM-1 expression and the pathogenesis of
atherosclerosis.
Materials and methods
Patients
Thirty-four patients undergoing CABG [26 males and 8
females, aged 48–76 years (mean, 62±7 years)] were included in the
study; all patients had been admitted to the Shandong Provincial
Qianfoshan Hospital (Ji'nan, China) between December 2008 and
February 2012. In the present study, indications for CABG surgery
included left main lesions or bifurcation lesions insensitive to
medical treatment, severe proximal left anterior descending artery
stenosis, three-vessel disease, particularly when accompanied by
cardiac dysfunction or diabetes mellitus, and intervention failure.
The exclusion criteria were as follows: Any type of cancer, liver
and/or kidney dysfunction, and chronic infectious, autoimmune,
acute cerebrovascular and peripheral vascular diseases. Following
admission, the patients received conventional anti-atherosclerotic
treatment. A detailed medical history, including details of the
present illness, past illnesses and family history, was completed,
and physical examination and routine laboratory tests were carried
out in order to establish a clinical diagnosis. Special attention
was paid to cardiovascular risk factors, including smoking status,
hypertension and diabetes mellitus. Out of the 34 patients, 18
patients were smokers, 20 had hypertension and 11 had diabetes
mellitus.
The control group consisted of renal artery
specimens, which were collected from 12 kidney transplant donors.
As indicated by comprehensive physical examinations, these kidney
transplant donors were free from organic diseases and did not have
a history of coronary heart disease, hypertension or diabetes
mellitus. Furthermore, all control subjects were non-smokers and
without long-term medication. Prior written and informed consent
was obtained from every participant and the study was approved by
the Ethics Review Board of the Shandong Provincial Qianfoshan
Hospital.
Biochemical determination
The morning after admission, 6 ml venous blood was
collected from each subject in a fasting state. The colorimetric
endpoint method was used to determine the levels of serum
triglycerides (TG) and total cholesterol (TC), and the chemical
modification-enzymatic method was used to detect the serum levels
of low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C). The levels of lipoprotein (a) [Lp
(a)], apolipoprotein (Apo) AI and Apo-B were measured by
immunoturbidimetry. A MODULAR biochemical analysis system (Roche
Diagnostics AG, Basel, Switzerland) was used for the aforementioned
analyses.
Coronary angiography and SYNTAX
scoring
Coronary angiography was performed via the right
femoral artery using the Judkins technique (8). The lesions were directly exposed,
usually in the 45° left anterior oblique and 30° right anterior
oblique projections, in order to perform left and right coronary
angiography. During the coronary angiography, the complexity of the
coronary artery disease was determined by the synergy between
percutaneous coronary intervention with Taxus and the cardiac
surgery (SYNTAX) score. From the baseline diagnostic angiogram,
each coronary lesion producing ≥50% diameter stenosis in vessels of
≥1.5 mm was scored separately and added together to provide the
overall SYNTAX score. The SYNTAX score was calculated using
dedicated software (version 2.11; www.syntaxscore.com) (9).
Sample preparation and
immunohistochemistry
Full-thickness aortic wall tissue samples were
collected from the patients during the CABG surgery, and control
renal artery tissues were obtained from kidney transplantation
cases. The sample preparation and immunohistochemistry protocols
were in accordance with those described in a previous study
(10). Briefly, the samples were
fixed in 10% formalin. Following alcohol dehydration and xylene
clearing, the samples were embedded in paraffin and cut into
slices. The sections were subsequently dewaxed with xylene and
treated with citrate antigen retrieval buffer and 3% hydrogen
peroxide. Rabbit anti-human VCAM-1 polyclonal antibody (1:300,
bs-0920R; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China) was used to incubate the sections at 4°C overnight.
Horseradish peroxidase-labeled goat anti-mouse/rabbit
immunoglobulin G conjugates were used for incubation for 30 min at
room temperature. The peroxidase-diaminobenzidine reaction and
hematoxylin staining were then performed. The sections were sealed
with neutral resin and visualized with a fluorescence microscope.
Three high-power fields were randomly selected from each slice and
the average optical density (OD) was measured in each field.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used to
perform the statistical analysis. Following testing for the normal
distribution of variables, the normally distributed continuous
variables were analyzed with the Student's t-test. The correlation
between two variables was determined by a simple regression
analysis. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Measurement of blood lipid levels in
atherosclerotic patients
The serum lipid levels of 34 atherosclerotic
patients are presented in Table I.
As expected, the preoperative serum TG, TC, LDL-C, Lp (a) and (Apo)
B levels in the atherosclerotic patients were significantly
elevated, whereas the serum HDL-C and Apo-AI levels were
significantly decreased, compared with the normal reference values
(P<0.05) (Table I).
 | Table I.Blood lipid levels in patients with
coronary heart disease. |
Table I.
Blood lipid levels in patients with
coronary heart disease.
|
| Atherosclerosis | Reference values | t-test | P-value |
|---|
| TG (mmol/l) |
2.25±1.21 |
1.13±0.29 | 5.431 | <0.001 |
| TC (mmol/l) |
5.13±1.50 |
4.42±0.79 | 2.755 | 0.010 |
| LDL-C (mmol/l) |
3.35±1.23 |
2.59±0.26 | 3.597 | 0.002 |
| HDL-C (mmol/l) |
1.14±0.33 |
1.46±0.24 | −5.385 | <0.001 |
| Lp (a) (mg/dl) |
32.31±32.09 |
14.40±6.64 | 3.255 | 0.003 |
| Apo-AI (g/l) |
1.08±0.26 |
1.30±0.15 | −4.659 | <0.001 |
| Apo-B (g/l) |
1.07±0.46 |
0.85±0.13 | 2.755 | 0.024 |
Expression levels of VCAM-1 in
arterial tissues in atherosclerotic patients
To investigate the expression levels of VCAM-1 in
the arterial tissues of atherosclerotic patients,
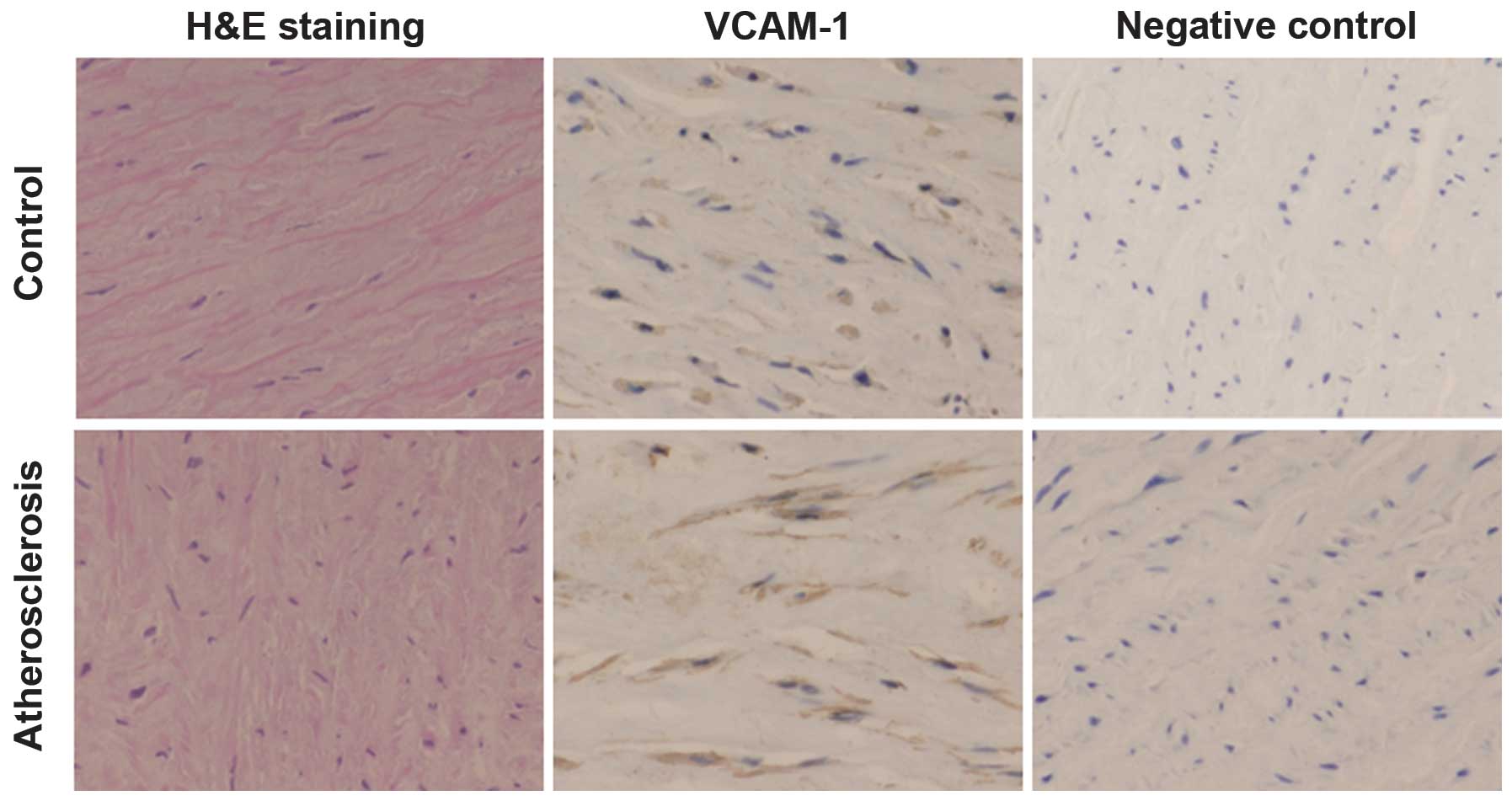
immunohistochemistry was carried out. As shown in Fig. 1, brown staining of VCAM-1 was
observed in the endothelial and smooth muscle cells. The expression
level of VCAM-1 in the arterial tissues of the atherosclerotic
patients was 0.23±0.06 OD units (range, 0.12–0.40 OD units), which
was significantly higher than that in the control group tissues
(0.08±0.03 OD units; range, 0.06–0.10 OD units). These results
suggested that the expression levels of VCAM-1 in the arterial
tissues were significantly elevated in atherosclerotic patients
compared with those in the control subjects.
To further determine the association between the
aortic VCAM-1 expression and the blood lipid indicators in
atherosclerotic patients, correlation analysis was performed. The
results showed that, in those patients, the expression levels of
VCAM-1 in the aortic tissues were positively correlated with the
serum levels of TG (r=0.347, P=0.046), TC (r=0.469, P=0.005), LDL-C
(r=0.463, P=0.006), Lp (a) (r=0.507, P=0.002) and Apo-B (r=0.384,
P=0.025), while a statistically insignificant negative correlation
was observed between the aortic VCAM-1 expression and the serum
HDL-C levels (r=-0.319, P=0.066). These results suggested that the
elevated expression of VCAM-1 in the aortic tissues was associated
with the pathophysiological changes in atherosclerosis.
Correlation between aortic VCAM-1
expression and coronary lesion severity in atherosclerotic
patients
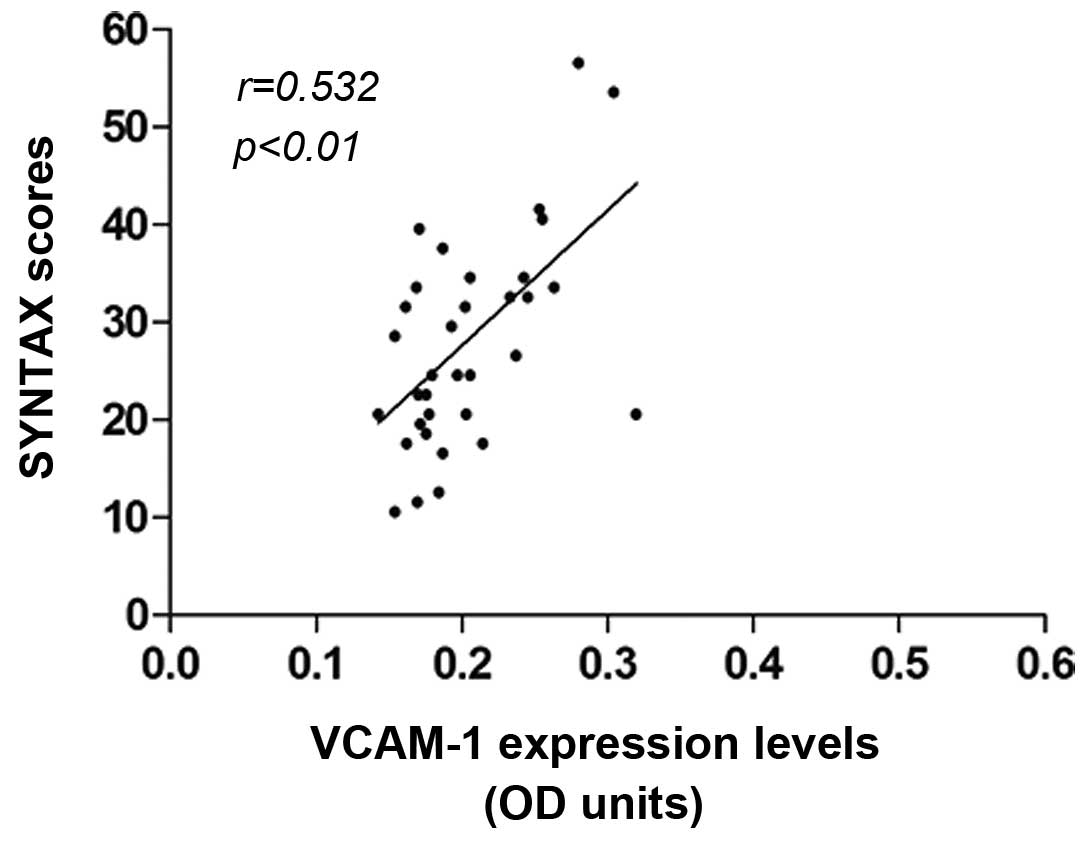
The correlation between the aortic VCAM-1 expression
and the SYNTAX scores was investigated. The average SYNTAX score of
the atherosclerotic patients was 29.35±13.26. The results indicated
that higher SYNTAX scores were accompanied by higher VCAM-1
expression levels. Correlation analysis showed that the SYNTAX
score (i.e., the lesion severity) was positively correlated with
the VCAM-1 expression in atherosclerosis (r=0.532, P<0.01)
(Fig. 2). These results suggested
that the aortic VCAM-1 expression was linked with the severity of
the coronary heart disease.
Association between aortic VCAM-1
expression and cardiovascular risk factors
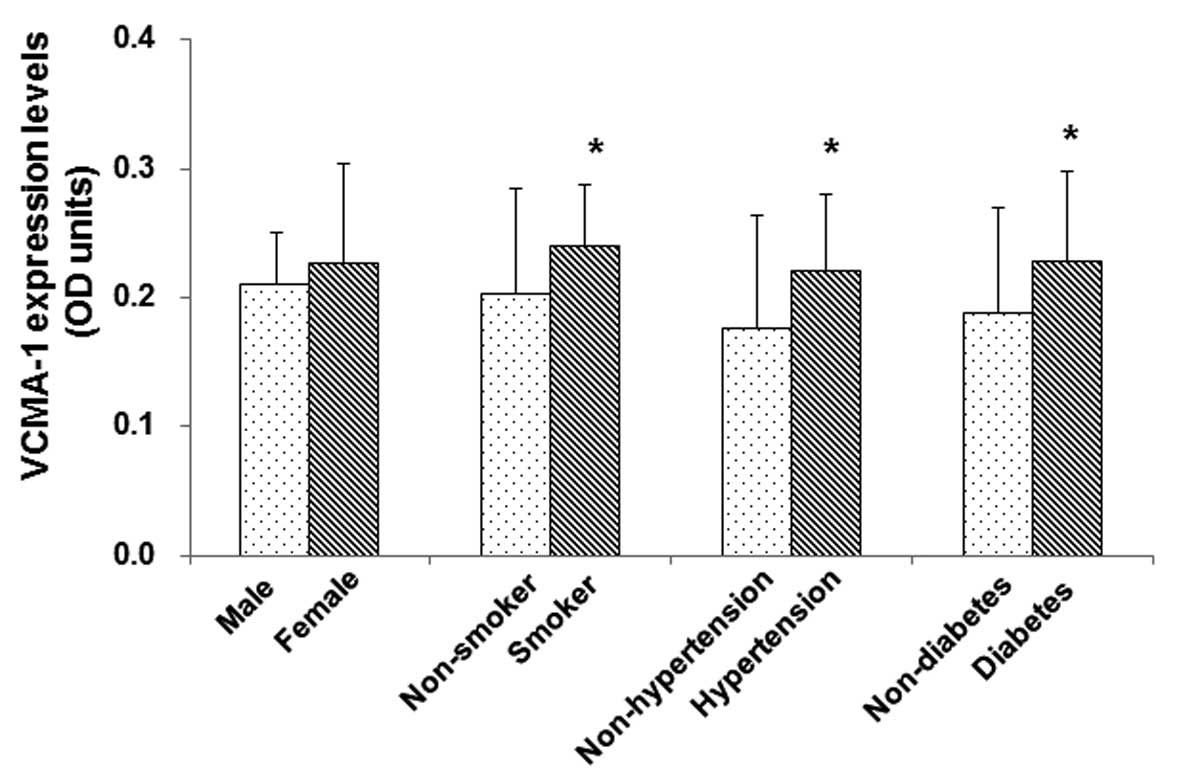
The aim of the final investigation was to determine
whether the expression of VCAM-1 in the atherosclerotic patients
would be affected by certain cardiovascular risk factors. Subgroup
analyses were performed based on gender, smoking, hypertension and
diabetes mellitus, and the results showed that there were no
significant differences in the aortic VCAM-1 expression levels
between male (n=26) and female (n=8) atherosclerotic patients
(0.21±0.04 vs. 0.22±0.07 OD units, P>0.05); however, the aortic
VCAM-1 expression levels in smokers were significantly higher
(n=18, 0.24±0.05 OD units) than those in non-smokers (n=16,
0.20±0.08 OD units) (P<0.05). Furthermore, the aortic VCAM-1
expression levels were significantly higher in atherosclerotic
patients with either hypertension (n=20, 0.22±0.06 OD units) or
diabetes mellitus (n=11, 0.23±0.07 OD units), compared with those
in non-hypertensive (n=14, 0.18±0.09 OD units) and non-diabetic
(n=23, 0.19±0.08 OD units) patients, respectively (P<0.05)
(Fig. 3). These results indicated
that major cardiovascular risk factors, such as smoking,
hypertension and diabetes mellitus, are associated with the
elevated aortic VCAM-1 expression levels in patients with
atherosclerosis.
Discussion
Cardiovascular and cerebrovascular diseases caused
by atherosclerosis have become the leading cause of mortality in
humans. Although the mechanism underlying the development of
atherosclerosis is not yet fully understood, considerable evidence
shows that inflammation is involved in the occurrence and
pathogenesis of the disease, including inflammatory cell adhesion
and migration and smooth muscle cell proliferation (4). Studies have shown that, with the
stimulation of inflammatory cytokines, the VCAM-1 expression levels
in the endothelial cells can be significantly elevated (11,12).
VCAM-1 is expressed in vascular endothelial cells,
and this expression promotes the adhesion of leukocytes to the
endothelial cells. VCAM-1 accelerates the migration of adherent
leukocytes along the endothelial surface, and promotes the
proliferation of smooth muscle cells; therefore, it is speculated
that VCAM-1 may be involved in the pathogenesis of atherosclerosis
(13,14). Using animal models, Cybulsky and
Gimbrone (15) demonstrated that LDL
could promote the expression of VCAM-1 in endothelial cells and
that hypercholesterolemia could cause atherosclerosis-related
pathophysiological changes in the arteries. In the present study,
whole human arterial wall samples were obtained, and the expression
of VCAM-1 in the tissue was detected. It was found that the aortic
VCAM-1 expression level was upregulated in atherosclerotic
patients.
VCAM-1 molecules on the surface of endothelial cells
are usually shed into the blood to form soluble proteins (sVCAM-1)
(16,17). Due to the difficulty in evaluating
VCAM-1 in vascular endothelial tissues, the detection of sVCAM-1 in
the blood has typically been used as an indirect indicator for
VCAM-1 expression levels (18,19).
Hackman et al (20) found
that the level of sVCAM-1 in the blood was elevated in patients
with higher levels of TG. Saidi et al (21) additionally showed that, in
atherosclerotic patients, an elevated sVCAM-1 level was associated
with the activation and damage of endothelial cells; however, these
findings would have been of greater significance if they related to
the expression of VCAM-1 within the arterial tissues, rather than
to the level of the protein in its soluble form. Studies have shown
that abnormal lipid metabolism is closely associated with the
occurrence and development of atherosclerosis (22–24);
therefore, the blood lipid levels and their possible association
with VCAM-1 expression were examined in the present study. The
results showed that the serum levels of TG, TC, LDL-C, Lp (a) and
Apo-B were significantly elevated, whereas the serum HDL-C and
Apo-AI levels were significantly decreased, compared with the
reference values. Furthermore, the aortic VCAM-1 expression levels
were positively correlated with the levels of LDL-C, Lp (a), TC,
Apo-B and TG, while a negative correlation was observed between the
VCAM-1 and HDL-C levels.
The SYNTAX score is a widely accepted and highly
reproducible scoring system that grades the severity and complexity
of coronary artery disease (25).
Retrospective analyses suggest that the severity of coronary artery
disease indicated by the SYNTAX score may be helpful in the
selection of revascularization strategies (26–28). The
present results showed that there was a significant correlation
between the aortic VCAM-1 expression and the SYNTAX score,
suggesting that VCAM-1 may participate in the occurrence and
development of atherosclerosis. In addition, smoking, diabetes
mellitus and hypertension have been recognized as independent risk
factors for atherosclerosis (29–31). In
the present study it was found that the expression levels of VCAM-1
in the aortic tissues were increased in patients with smoking
habits, diabetes mellitus or hypertension, compared with the
subjects without these risk factors. These results suggest that
cardiovascular risk factors increase the VCAM-1 expression in the
arterial tissues, contributing to the occurrence and development of
the disease; however, further studies are required to clarify the
specific mechanisms.
A number of studies have used animal models to
investigate the association between VCAM-1 and the pathogenesis of
atherosclerosis (12,32). The establishment of animal models of
atherosclerosis is considerably different from the natural
pathological process in humans, in terms of disease etiology and
pathophysiology. In fact, it is difficult to extrapolate the
findings obtained from atherosclerosis animal models to humans. In
the present study, the specimens were whole arterial walls,
consisting of tunica intima, media and externa. These arterial
tissues were used for the investigation of the expression levels of
certain inflammatory factors, including nuclear factor-κB,
Toll-like receptor 4 and high-mobility group protein B1, in
atherosclerotic patients (33–35).
Given the fact that aortic tissues were not available from healthy
subjects, renal artery tissues from kidney transplant cases were
instead used as a control. Despite the fact that the control
subjects did not present with any symptoms of atherosclerosis, the
possibility of subclinical atherosclerosis could not be ruled out.
Another limitation of this study was the small number of
atherosclerotic patients. In addition, the SYNTAX scoring system
that was used was based on the coronary angiography results, which
did not reflect the actual volume of the atherosclerotic plaque.
More accurate diagnostic methods, such as intravascular ultrasound
and angioscopy, may be more suitable for the evaluation of plaque
volume in future studies.
In conclusion, the results of the present study have
shown that the expression levels of VCAM-1 in aortic tissues are
significantly elevated in atherosclerotic patients and are
correlated with blood lipid levels. An upregulated VCAM-1
expression is associated with a higher coronary SYNTAX score,
indicating severe coronary artery stenosis. Furthermore,
cardiovascular risk factors have also been found to influence the
aortic VCAM-1 expression levels.
Acknowledgements
This study was supported by the Shandong Provincial
Science and Technology Development Project (grant no.
2006GG2202014).
References
|
1
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolf D, Stachon P, Bode C and Zirlik A:
Inflammatory mechanisms in atherosclerosis. Hamostaseologie.
34:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakashima Y, Raines EW, Plump AS, Breslow
JL and Ross R: Upregulation of VCAM-1 and ICAM-1 at
atherosclerosis-prone sites on the endothelium in the
ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 18:842–851.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sitia S, Tomasoni L, Atzeni F, et al: From
endothelial dysfunction to atherosclerosis. Autoimmun Rev.
9:830–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu B, Wang J, Cheng L and Liang J: Role
of JNK and NF-κB pathways in Porphyromonas gingivalis
LPS-induced vascular cell adhesion molecule-1 expression in human
aortic endothelial cells. Mol Med Rep. 8:1594–1600. 2013.PubMed/NCBI
|
|
6
|
De Caterina R, Basta G, Lazzerini G, et
al: Soluble vascular cell adhesion molecule-1 as a biohumoral
correlate of atherosclerosis. Arterioscler Thromb Vasc Biol.
17:2646–2654. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeitler H, Ko Y, Zimmermann C, et al:
Elevated serum concentrations of soluble adhesion molecules in
coronary artery disease and acute myocardial infarction. Eur J Med
Res. 2:389–394. 1997.PubMed/NCBI
|
|
8
|
Lee MS, Applegate B, Rao SV, Kirtane AJ,
Seto A and Stone GW: Minimizing femoral artery access complications
during percutaneous coronary intervention: A comprehensive review.
Catheter Cardiovasc Interv. 84:62–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sianos G, Morel MA, Kappetein AP, et al:
The SYNTAX Score: An angiographic tool grading the complexity of
coronary artery disease. EuroIntervention. 1:219–227.
2005.PubMed/NCBI
|
|
10
|
Tan HW, Xing SS, Bi XP, et al: Felodipine
attenuates vascular inflammation in a fructose-induced rat model of
metabolic syndrome via the inhibition of NF-kappaB activation. Acta
Pharmacol Sin. 29:1051–1059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeVerse JS, Sandhu AS, Mendoza N, Edwards
CM, Sun C, Simon SI and Passerini AG: Shear stress modulates VCAM-1
expression in response to TNF-α and dietary lipids via interferon
regulatory factor-1 in cultured endothelium. Am J Physiol Heart
Circ Physiol. 305:H1149–H1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fotis L, Agrogiannis G, Vlachos IS, et al:
Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion
molecule (VCAM)-1 at the early stages of atherosclerosis in a rat
model. In Vivo. 26:243–250. 2012.PubMed/NCBI
|
|
13
|
Cook-Mills JM: VCAM-1 signals during
lymphocyte migration: Role of reactive oxygen species. Mol Immunol.
39:499–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petersen EJ, Miyoshi T, Yuan Z, Hirohata
S, Li JZ, Shi W and Angle JF: siRNA silencing reveals role of
vascular cell adhesion molecule-1 in vascular smooth muscle cell
migration. Atherosclerosis. 198:301–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cybulsky MI and Gimbrone MA Jr:
Endothelial expression of a mononuclear leukocyte adhesion molecule
during atherogenesis. Science. 251:788–791. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu HH, Sheng ZQ, Wang Y and Zhang L:
Levels of soluble adhesion molecules in patients with various
clinical presentations of coronary atherosclerosis. Chin Med J
(Engl). 123:3123–3126. 2010.PubMed/NCBI
|
|
17
|
Karasek D, Vaverkova H, Frysak Z, Halenka
M, Jackuliakova D, Novotny D and Lukes J: Soluble intercellular
cell adhesion molecule-1 and vascular cell adhesion molecule-1 in
asymptomatic dyslipidemic subjects. Int Angiol. 30:441–450.
2011.PubMed/NCBI
|
|
18
|
Nakai K, Itoh C, Kawazoe K, et al:
Concentration of soluble vascular cell adhesion molecule-1 (VCAM-1)
correlated with expression of VCAM-1 mRNA in the human
atherosclerotic aorta. Coron Artery Dis. 6:497–502. 1995.PubMed/NCBI
|
|
19
|
Leca G, Mansur SE and Bensussan A:
Expression of VCAM-1 (CD106) by a subset of TCR gamma delta-bearing
lymphocyte clones. Involvement of a metalloprotease in the specific
hydrolytic release of the soluble isoform. J Immunol.
154:1069–1077. 1995.PubMed/NCBI
|
|
20
|
Hackman A, Abe Y, Insull W Jr, et al:
Levels of soluble cell adhesion molecules in patients with
dyslipidemia. Circulation. 93:1334–1338. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saidi H, Vakilian M, Noori GH, Ghafouri HB
and Abazarian N: Alterations in circulating adhesion molecules in
acute myocardial infarction before and after thrombolysis with
streptokinase. J Cardiovasc Thorac Res. 5:139–141. 2013.PubMed/NCBI
|
|
22
|
Steinberg D and Witztum JL: Oxidized
low-density lipoprotein and atherosclerosis. Arterioscler Thromb
Vasc Biol. 30:2311–2316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ross R and Harker L: Hyperlipidemia and
atherosclerosis. Science. 193:1094–1100. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feig JE, Rong JX, Shamir R, et al: HDL
promotes rapid atherosclerosis regression in mice and alters
inflammatory properties of plaque monocyte-derived cells. Proc Natl
Acad Sci USA. 108:7166–7171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashfaq F, Goel PK, Moorthy N, Sethi R,
Khan MI and Idris MZ: Lipoprotein(a) and SYNTAX score association
with severity of coronary artery atherosclerosis in North India.
Sultan Qaboos Univ Med J. 12:465–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Serruys PW, Morice MC, Kappetein AP, et
al: SYNTAX Investigators: Percutaneous coronary intervention versus
coronary-artery bypass grafting for severe coronary artery disease.
N Engl J Med. 360:961–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wykrzykowska JJ, Garg S, Girasis C, et al:
Value of the SYNTAX score for risk assessment in the all-comers
population of the randomized multicenter LEADERS (Limus Eluted from
A Durable versus ERodable Stent coating) trial. J Am Coll Cardiol.
56:272–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chakravarty T, Buch MH, Naik H, et al:
Predictive accuracy of SYNTAX score for predicting long-term
outcomes of unprotected left main coronary artery
revascularization. Am J Cardiol. 107:360–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Edirisinghe I and Rahman I: Cigarette
smoke-mediated oxidative stress, shear stress, and endothelial
dysfunction: Role of VEGFR2. Ann NY Acad Sci. 1203:66–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turnbull F, Kengne AP and MacMahon S:
Blood pressure and cardiovascular disease: Tracing the steps from
Framingham. Prog Cardiovasc Dis. 53:39–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Opie LH: Acute myocardial infarction and
diabetes. Lancet. 370:634–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JG, Ryu SY, Jung IH, et al:
Evaluation of VCAM-1 antibodies as therapeutic agent for
atherosclerosis in apolipoprotein E-deficient mice.
Atherosclerosis. 226:356–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Xing SS, Sun XL and Xing QC:
Overexpression of activated nuclear factor-kappa B in aorta of
patients with coronary atherosclerosis. Clin Cardiol. 32:E42–E47.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong YL, Xing SS, Zhang W and Xing QC:
Relationship between Toll-like receptor 4 protein and
atherosclerosis. Shan Dong Da Xue Xue (Yi Xue Ban). 48:127–130.
2010.(In Chinese).
|
|
35
|
Xing SS, Gong ZS, Mu W, Wang D, Gong YL
and Xing QC: Expression of human living aortic high mobility group
protein box1 and its relationship with the pathogenesis of
atherosclerosis. Shan Dong Da Xue Xue (Yi Xue Ban). 51:62–65.
2013.(In Chinese).
|