Introduction
Methotrexate (MTX) is an antineoplastic agent that
is associated with folic acid metabolism. MTX inhibits the
synthesis of DNA, RNA, thymidylate and proteins. As a result of
this activity, MTX is commonly used in cancer treatment, in
addition to the treatment of non-neoplastic diseases, including
rheumatoid arthritis and psoriasis (1). Previous studies have demonstrated that
liver, kidney, brain and lung toxicities are potential side effects
of MTX use (2). The predominant
manifestation of MTX-induced lung toxicity is pulmonary fibrosis
(3). In addition to pulmonary
fibrosis, MTX treatment has been associated with acute interstitial
pneumonitis (4–8).
Silibinin
(C25H22O10; molecular weight,
482.44 g/mol) is isolated from the seeds of Silybum marianum
L. (9). The compound is known to
exhibit antioxidative activity, which has been previously reported
to result in a number of biologically protective effects, including
anti-inflammatory, antitumor and hepatoprotective effects (10–13).
Silibinin has been demonstrated to be a potent antioxidant,
supporting the capacity of cellular antioxidative agents, such as
glutathione (GSH) and superoxide dismutase (SOD), to target
reactive oxygen species. This activity may partially explain the
effectiveness of silibinin in the prevention of hepatic injury,
whether this injury is as a result of disease or exposure to
toxins, since this antioxidative activity may mitigate the
oxidative stress associated with hepatic injury, subsequently
preventing the induction of lipid peroxidation (14). Furthermore, silibinin has been widely
investigated for anticancer efficacy in a broad range of cancer
models. As a consequence of the general anticancer properties
associated with flavonoids collectively, there has been significant
interest in the possibility of using silibinin as a chemopreventive
agent (11,15). Silibinin has been traditionally used
in folk medicine, and acute and chronic doses of silibinin
administration in animals and humans have resulted in no
significant toxicity. However, to the best of our knowledge, an
LD50 for silibinin has not been reported in rodent
studies. Silibinin intake has been demonstrated to be safe due to
its wide usage as a dietary supplement, with sufficient
tolerability and minimal toxicity (15). Therefore, the aim of the present
study was to assess the possible protective effects of silibinin
against MTX-induced pulmonary toxicity in rats.
Materials and methods
Animals
Animal experiments were approved by the Animal
Ethical Committee of Suleyman Demirel University
(B.30.2.SDÜ.0.05.06.00–196, 2012; Isparta, Turkey), and the study
was conducted in accordance with the National Institutes of Health
Guidelines for the Care and Use of Laboratory Animals (8th Edition,
2011). In total, 32 female albino Wistar rats (age, 8–10 weeks)
were obtained from the Experimental Research Centre of Suleyman
Demirel University and housed in an environmentally controlled room
at 21±1°C and 75±5% humidity, under a 12-h light/dark cycle. The
animals were acclimatized for 1 week prior to the study, and had
free access to standard laboratory feed and water.
Experimental protocol
Rats were divided into four groups. Since silibinin
was solubilized in dimethyl sulfoxide (DMSO), an equal amount of
DMSO (10%v/v) was included in the injections administered to the
control group rats. The silibinin group rats received 100 mg/kg/day
silibinin (Sigma-Aldrich, St. Louis, MO, USA), which was
administered via intraperitoneal (i.p.) injection for 10 days
(16). The MTX group rats were
administered 10 mg/kg/day MTX (i.p.) on days 7–9, the commercially
available form of the drug was used, it was taken from pharmacy
with our own means (17). Finally,
the MTX + silibinin group rats were cotreated with 100 mg/kg/day
silibinin for 10 days and 10 mg/kg/day MTX for 3 days. In all the
groups on day 14, anesthesia was induced by a single i.p. injection
of 50 mg/kg ketamine (Ketalar®; Pfizer, Inc., Istanbul, Turkey) and
5 mg/kg xylasine (Rompun®; Bayer, Istanbul, Turkey). Blood samples
were collected via cardiac puncture for serum analyses and the
lungs were harvested for histological and immunohistochemical
analysis.
Biochemical evaluation
All the biochemical analyses were performed in the
Department of Biochemistry at Mugla Sıtkı Kocman University (Mugla,
Turkey).
Determination of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST)
activity levels
Serum activity levels of AST and ALT were calculated
spectrophotometrically using Beckman Coulter kits and a UniCel DxC
800 Synchron autoanalyzer (Beckman Coulter, Inc., Brea, CA,
USA).
Determination of SOD activity
Tissue samples were homogenized at 4,200 × g on ice
in 5–10 ml cold buffer [20 mM HEPES buffer (pH 7.2) containing 1 mM
EGTA, 210 mM mannitol and 70 mM sucrose per gram tissue].
Subsequently, the samples were centrifuged at 1,500 × g for 5 min
at 4°C, and the supernatant was removed. In addition, the blood
samples were centrifuged at 2,000 × g for 15 min at 4°C, after
which the top yellow serum layer was pipetted off, without
disturbing the white buffy coat. The serum was diluted 1:5 with
sample buffer. The SOD activity was measured in the supernatant and
serum using a SOD assay kit (Cayman Chemical Company, Inc., Ann
Arbor, MI, USA) with an ELx-800 absorbance reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). The assay was based on the
detection of superoxide radicals generated by xanthine oxidase and
hypoxanthine. One unit of SOD was defined as the quantity of enzyme
required to induce 50% dismutation of the superoxide radical. The
results are expressed in U/mg protein tissue for the liver tissue
and U/ml for the serum.
Determination of glutathione
peroxidase (GPx) activity
Tissue samples were homogenized in 5–10 ml cold
buffer [50 mM Tris-HCl (pH 7.5), 5 mM EDTA and 1 mM DTT], and
centrifuged at 10,000 × g for 15 min at 4°C, after which the
supernatant was removed. In addition, the blood samples were
centrifuged at 700–1,000 × g for 10 min at 4°C, and the plasma was
removed. GPx activity was measured in the liver tissue and plasma
samples using a GPx assay kit (Cayman Chemical Company, Inc.) with
an ELx-50 microplate strip washer. GPx activity was measured
indirectly by a coupled reaction with glutathione reductase, where
the oxidized glutathione was produced upon the reduction of
hydroperoxide by GPx.
Determination of nitric oxide (NO)
levels
Tissue samples were homogenized in
phosphate-buffered saline (pH 7.4) and centrifuged at 10,000 × g
for 20 min to isolate the supernatant. A total NO assay was
performed via spectrophotometry at 540 nm using a nitrate/nitrite
colorimetric assay kit (Cayman Chemical Company, Inc.) with an
ELx-50 microplate strip washer. The assay was based on nitrate and
nitrite determinations, in which nitrate and nitrite were the
stable end products of the reaction of NO with molecular oxygen.
The total accumulation of nitrate and nitrite in the serum and
liver tissue samples was evaluated, and expressed in
µM/protein.
Determination of myeloperoxidase (MPO)
activity
Quantitative detection of MPO activity was conducted
using an enzyme-linked immunosorbent assay kit (MPO Instant ELISA;
eBioscience, Inc., Vienna, Austria) in an ELx-50 microplate strip
washer. The results are expressed in pg/ml/protein.
Histopathological analysis of the lung
tissue
Histopathological analyses were performed in the
Department of Pathology at Mugla Sıtkı Kocman University. Rat lung
samples from the different groups were fixed in 10% neutral
buffered formalin for 24 h. After washing with tap water, serial
dilutions of alcohol (methyl, ethyl and absolute ethyl) were
applied for dehydration. The specimens were cleared in xylene, and
embedded in paraffin at 56°C in an oven for 24 h. Paraffin bees wax
tissue blocks were prepared for sectioning at 5 µm thickness using
a microtome. Lung tissues were stained with hematoxylin and eosin
and observed under a BX46 light microscope (Olympus Corporation,
Tokyo, Japan) for histopathological evaluation. Pulmonary damage
was evaluated using six parameters, which included interstitial
lymphocytic inflammation, interstitial fibrosis, type 2 pneumocyte
infiltration, intraalveolar/interstitial macrophage existence,
eosinophil existence and granuloma existence (18). Each parameter was scored
semiquantitatively with regard to the severity using the following
system: 0 (absent), no interstitial lymphocytic inflammation,
fibrosis, type 2 pneumocyte infiltration, macrophages, eosinophils
or granulomas; 1 (low), limited number of lymphocytes, type 2
pneumocytes, macrophages, eosinophils and granulomas, with minimal
fibrosis; 2 (moderate), a variety of features in between a score of
1 and 3; and 3 (severe), diffuse infiltration of an excessive
number of lymphocytes, type 2 pneumocytes, macrophages,
eosinophils, granulomas and excessive, diffuse fibrosis (6–12).
Statistical analysis
SPSS software, version 21.0 (IBM SPSS, Armonk, NY,
USA) and PAST software (http://folk.uio.no/ohammer/past/) were used for data
analysis. For comparisons among multiple independent groups one-way
analysis of variance (ANOVA) was used. If significance was
identified in the test we used the Least Significant Difference
(LSD) test for post-hoc analysis. Parametric methods were used for
the analysis of the data with a normal distribution, while
non-parametric methods were used for the analysis of the variations
without a normal distribution. For comparisons among multiple
independent groups, ANOVA (Robust test: Brown-Forsythe), one of the
parametric methods was used, while for post-hoc analysis, Fisher's
LSD test was used. For the comparison of categorical data,
Pearson's χ2 test was conducted using the Monte Carlo
simulation technique. Quantitative data are expressed as the mean ±
standard deviation, and categorical data are expressed as a number
and percentage. Data were analyzed in the 95% confidence level,
where P<0.05 was considered to indicate a statistically
significant difference.
Results
Biochemical analysis
Serum levels of ALT and AST were significantly
increased in the MTX group when compared with the control and
silibinin groups (P<0.05; Table
I). In the MTX + silibinin group, the ALT and AST levels in
serum decreased significantly compared with the MTX group
(P<0.05). Furthermore, in the MTX group, the SOD levels
decreased significantly when compared with the control group
(P<0.05). The levels of GPx and SOD increased in the MTX +
silibinin group when compared with the MTX group, while the MPO
values decreased significantly (P<0.05).
 | Table I.Effect of silibinin on the biochemical
parameters associated with MTX-induced pulmonary toxicity. |
Table I.
Effect of silibinin on the biochemical
parameters associated with MTX-induced pulmonary toxicity.
| Group | ALT (U/L) | AST (U/L) | SOD (U/ml) | GPx (U/ml) | NO (µm/g) | MPO (ng/ml) |
|---|
| Control |
20.86±3.34 |
62.75±6.24 |
0.89±0.14 |
0.88±0.14 |
2.25±0.36 |
2.63±0.76 |
| Silibinin |
16.25±2.60 |
49.38±7.90 |
3.54±0.57 |
0.24±0.04 |
3.05±0.49 |
2.70±0.53 |
| MTX |
55.38±8.86a,b |
86.88±13.90a,b |
0.48±0.08a |
0.19±0.03a |
4.49±0.40a |
4.3±0.56a |
| MTX + silibinin |
31.25±5.01c |
60.25±9.64c |
5.40±0.86c |
0.63±0.10c |
1.06±0.17c |
2.33±0.37c |
Histopathological alterations
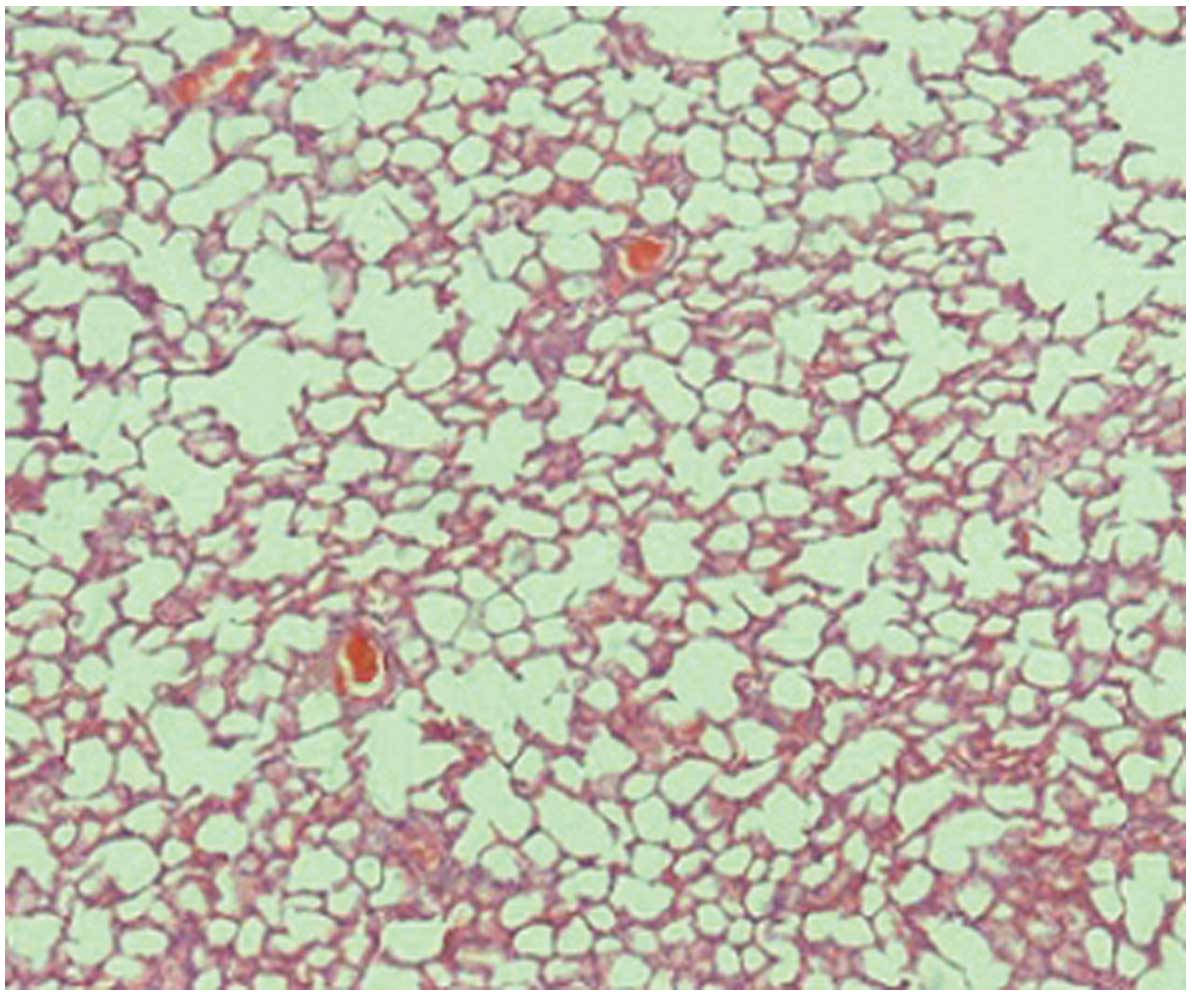
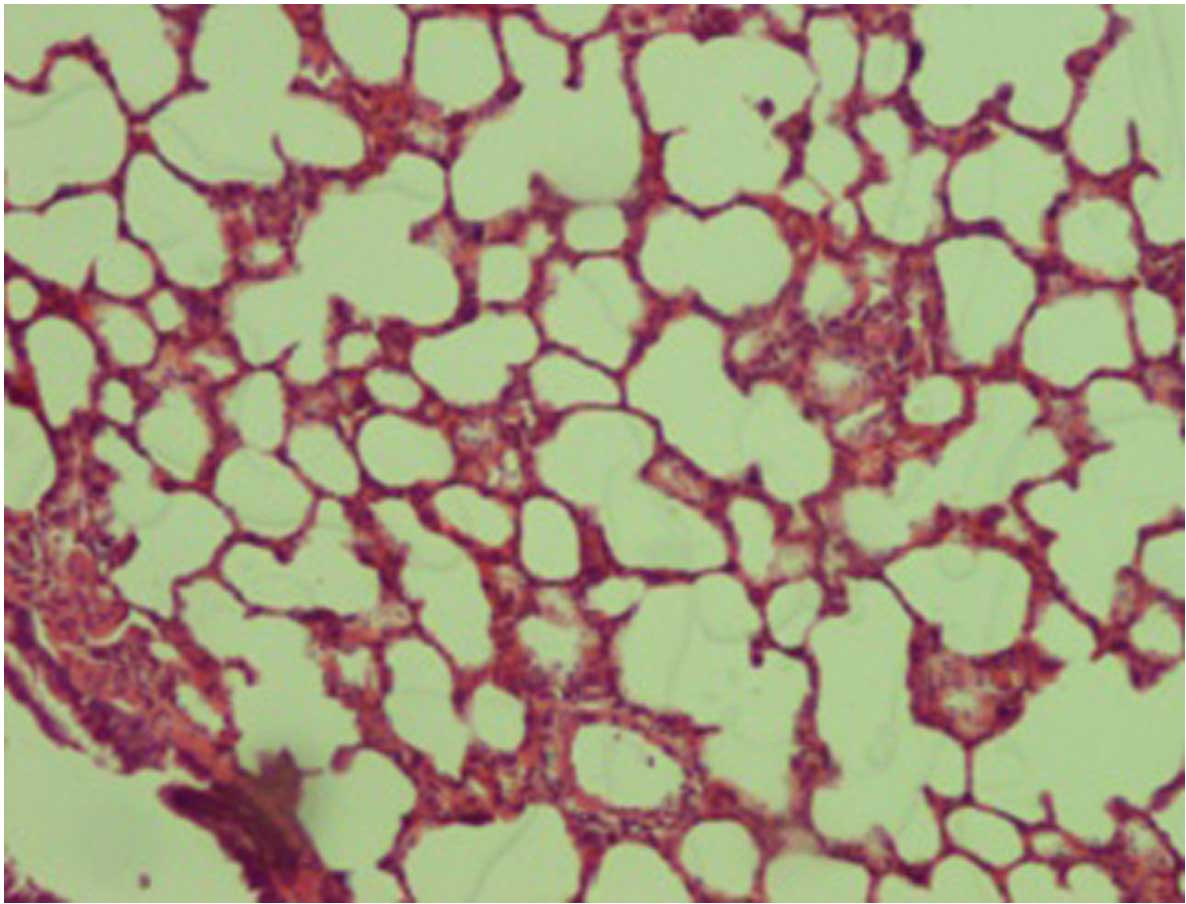
When compared with the control (Fig. 1) and silibinin groups (Fig. 2), the pulmonary damage appeared to be
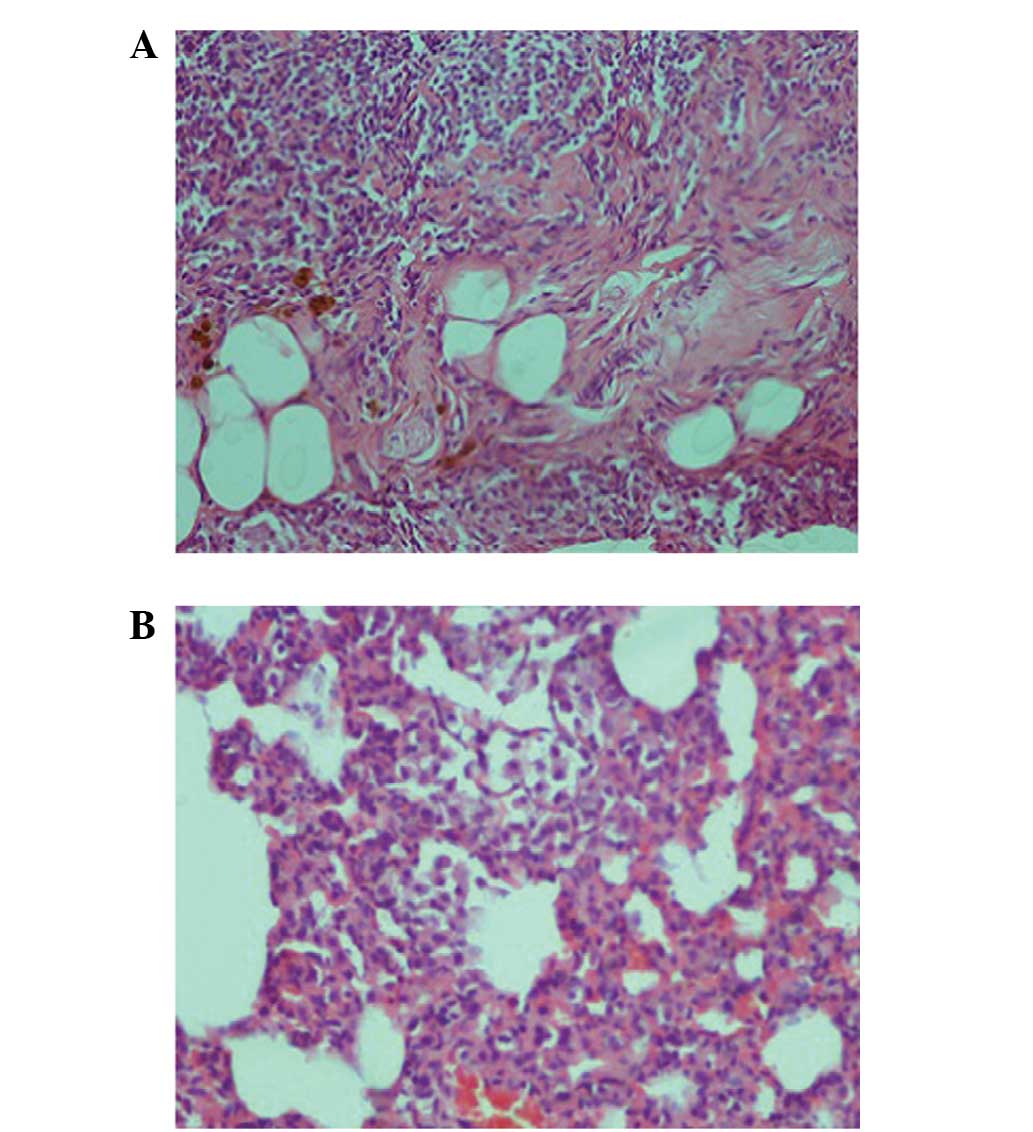
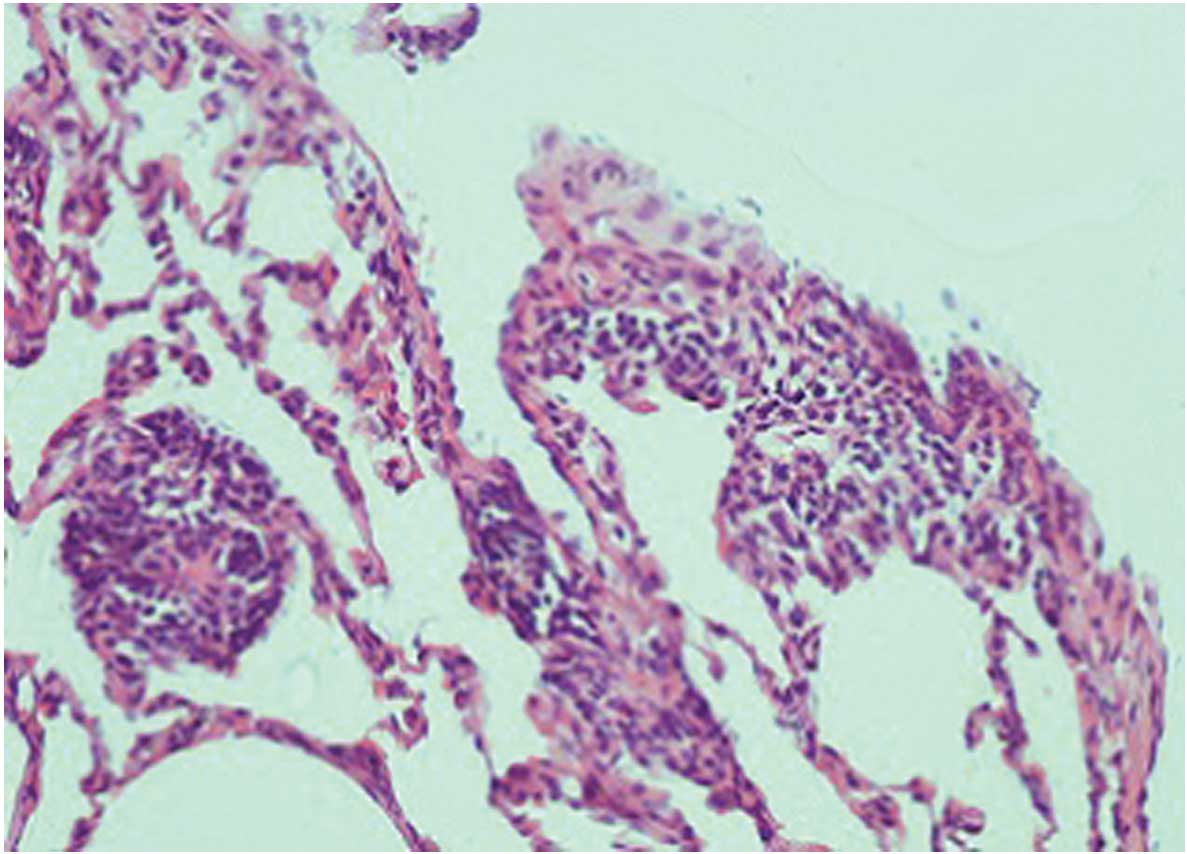
significantly increased in the MTX group (Fig. 3). Similarly, when the MTX + silibinin
group was compared with the MTX group, the damage was observed to
significantly decrease (Fig. 4 and
Table II). MTX administration was
shown to induce interstitial lymphocytic inflammation, interstitial
fibrosis, type 2 pneumocyte hyperplasia and eosinophil infiltration
in the pulmonary tissue. However, the MTX + silibinin group
exhibited significantly decreased scores for interstitial
lymphocytic inflammation, interstitial fibrosis and type 2
pneumocyte hyperplasia when compared with the MTX group, indicating
that silibinin exerted beneficial effects (Table II).
 | Table II.Histopathological damage parameters
detected in the rat pulmonary tissue samples. |
Table II.
Histopathological damage parameters
detected in the rat pulmonary tissue samples.
| Histopathological
parameter | Control | Silibinin | MTX | MTX + silibinin |
|---|
| Interstitial
lymphocytic inflammation | 0.125 | 0.375 | 1.25a,b | 0.25c |
| Interstitial
fibrosis | 0 | 0 | 0.875a,b | 0c |
| Type 2 pneumocyte
infiltration | 0 | 0 | 0.5a,b | 0c |
|
Intraalveolar/interstitial macrophage
existence | 0 | 0.375 | 0.5 | 0.125 |
| Eosinophil
existence | 0 | 0.5 | 0.875a | 0.125c |
| Granuloma
existence | 0 | 0 | 0.25 | 0 |
Discussion
The present study evaluated the interactions between
silibinin and MTX, and their effects on lung tissue. The results
indicate that treatment with silibinin ameliorated MTX-induced
alterations in the serum ALT and AST levels. In addition, silibinin
significantly mitigated the oxidation in the serum induced by MTX,
as manifested by the decreased MPO and NO levels, accompanied by
enhanced SOD and GPx activity. Furthermore, histopathological
analysis demonstrated that administration of silibinin mitigated a
number of the histopathological changes induced by MTX.
A previous study indicated that MTX exposure
activates components of the mitogen-activated protein kinase (MAPK)
signaling pathway (19). Activation
of the MAPK and Akt pathways may in turn activate the transcription
factors, activator protein-1 and nuclear factor-κB, which are
crucial for the regulation of inflammation (20). Inflammatory cells that infiltrate
into the tissue are known to cause tissue damage through activating
oxidation systems, such as MPO. Furthermore, inflammatory cells are
known to induce cell death by generating DNA damage via oxidation
systems (21). In the present study,
an increased rate of inflammatory cell infiltration was observed in
the pulmonary tissue of the MTX group rats, in addition to elevated
oxidation enzyme activity levels (Tables
I and II). These observations
are consistent with those of previous studies (2).
Silibinin possesses marked antioxidative,
anticancer, anti-inflammatory and cancer chemopreventive properties
(22,23). Previous studies have reported that
silibinin targets multiple signaling pathways, including those
associated with oxidative stress and inflammation, to subsequently
prevent tissue injuries and cancer by genotoxicity and other
agents, which are similar to the pathways triggered following
vesicant exposure (22,24,25).
Therefore, silibinin was hypothesized to exhibit notable efficacy
in attenuating MTX-induced lung injury, since MTX is known to
trigger oxidative stress. The results of a previous study by
Tewari-Singh et al (21)
indicated that vesicant drugs exert a protective effect on the
skin, combined with the anti-inflammatory and antioxidative effects
of silibinin via the MAPK pathway. In addition, the results of the
present study indicated that silibinin administration in
combination with MTX decreased the activation of serum oxidation
enzyme systems, while increasing antioxidant enzyme levels
(Table II). Furthermore, silibinin
appeared to reduce inflammatory cell infiltration and MTX-induced
changes in the lung tissue (Table
II). In accordance with previous literature (14), the current results indicated that
silibinin is able to decrease pulmonary oxidative stress, possibly
via the MAPK pathway.
The potential for hepatic toxicity remains a concern
for physicians who are increasingly using MTX (26). The preventive effect of silibinin
against liver damage has been demonstrated in a previous study
(10). In the present study,
cotreatment with MTX and silibinin was demonstrated to result in
significantly decreased serum levels of AST and ALT when compared
with MTX treatment alone (P<0.05; Table I). These findings indicate that
silibinin decreases MTX-associated tissue damage, which may provide
an advantage for clinicians regarding the use of this drug.
In conclusion, silibinin was demonstrated to protect
the lung tissue against MTX-induced pulmonary toxicity in rats. The
antioxidant activity of silibinin may be the primary factor
responsible for such pulmonary protective effects. Therefore,
silibinin represents a potential candidate agent for the prevention
of lung injury, which is a major and dose-limiting side effect of
MTX therapy.
Acknowledgements
The authors thank Mugla Sıtkı Kocman University
Hospital and School of Medicine.
References
|
1
|
Beckmann-Knopp S, Rietbrock S, Weyhenmeyer
R, Böcker RH, Beckurts KT, Lang W, Hunz M and Fuhr U: Inhibitory
effects of silibinin on cytochrome P-450 enzymes in human liver
microsomes. Pharmacol Toxicol. 86:250–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abelson HT, Fosburg MT, Beardsley GP,
Goorin AM, Gorka C, Link M and Link D: Methotrexate-induced renal
impairment: Clinical studies and rescue from systemic toxicity with
high-dose leucovorin and thymidine. J Clin Oncol. 1:208–216.
1983.PubMed/NCBI
|
|
3
|
Ohbayashi M, Kubota S, Kawase A, Kohyama
N, Kobayashi Y and Yamamoto T: Involvement of
epithelial-mesenchymal transition in methotrexate-induced pulmonary
fibrosis. J Toxicol Sci. 39:319–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oktem F, Yilmaz HR, Ozguner F, Olgar S,
Ayata A, Uzare E and Uz E: Metothrexate-induced renal oxidative
stres in rats: The role of a novel antioxidant caffeic acid
phenethyl ester. Toxicol Ind Health. 22:241–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jahovic N, Cevik H, Sehirli AO, Yegen BC
and Sener G: Melatonin prevents methotrexate-induced hepatorenal
oxidative injury in rats. J Pineal Res. 34:282–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kose E, Sapmaz HI, Sarihan E, Vardi N,
Turkoz N and Ekinci N: Beneficial effects of montelukast against
methotrexate-induced liver toxicity: A biochemical and histological
study. ScientificWorldJournal. 2012:9875082012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozkan E, Yardimci S, Dulundu E, Topaloğlu
U, Sehirli O, Ercan F, Velioglu-Ogunc A and Sener G: Protective
potential of montelukast against hepatic ischemia/reperfusion
injury in rats. J Surg Res. 159:588–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vardi N, Parlakpinar H, Cetin A, Erdogan A
and Ozturk IC: Protective effect of carotene on
methotrexate-induced oxidative liver damage. Toxicol Pathol.
38:592–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen PN, Hsieh YS, Chiou HL and Chu SC:
Silibinin inhibits cell invasion through inactivation of both
PI3K-Akt and MAPK signaling pathways. Chem Biol Interact.
156:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schümann J, Prockl J, Kiemer AK, Vollmar
AM, Bang R and Tiegs G: Silibinin protects mice from T
cell-dependent liver injury. J Hepatol. 39:333–340. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim R, Morwood CJ, Barker G and Lappas M:
Effect of silibinin in reducing inflammatory pathways in in
vitro and in vivo models of infection-induced preterm
birth. PLoS One. 9:e925052014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El Hafny B, Cano N, Piciotti M, Regina A,
Scherrmann JM and Roux F: Role of P-glycoprotein in colchicine and
vinblastine cellular kinetics in an immortalized rat brain
microvessel endothelial cell line. Biochem Pharmacol. 53:1735–1742.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ting H, Deep G and Agarwal R: Molecular
mechanisms of silibinin mediated cancer chemoprevention with major
emphasis on prostate cancer. AAPS J. 15:707–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ligeret H, Brault A, Vallerand D, Haddad Y
and Haddad PS: Antioxidant and mitochondrial protective effects of
silibinin in cold preservation-warm reperfusion liver injury. J
Ethnopharmacol. 115:507–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mateen S, Raina K and Agarwal R:
Chemopreventive and anti-cancer efficacy of silibinin against
growth and progression of lung cancer. Nutr Cancer. 65 (Suppl
1):3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nassuato G, Iemmolo RM, Strazzabosco M,
Lirussi F, Deana R, Francesconi MA, Muraca M, Passera D, Fragasso A
and Orlando R: Effect of Silibinin on biliary lipid composition.
Experimental and clinical study. J Hepatol. 12:290–295. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sekeroğlu ZA and Sekeroğlu V: Effects of
Viscum album L. extract and quercetin on
methotrexate-induced cyto-genotoxicity in mouse bone-marrow cells.
Mutat Res. 746:56–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imokawa S, Colby TV, Leslie KO and Helmers
RA: Methotrexate pneumonitis: Review of the literature and
histopathological findings in nine patients. Eur Respir J.
15:373–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YJ, Song M and Ryu JC: Inflammation in
methotrexate-induced pulmonary toxicity occurs via the p38 MAPK
pathway. Toxicology. 256:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pal A, Tewari-Singh N, Gu M, Agarwal C,
Huang J, Day BJ, White CW and Agarwal R: Sulfur mustard analog
induces oxidative stress and activates signaling cascades in the
skin of SKH-1 hairless mice. Free Radic Biol Med. 47:1640–1651.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tewari-Singh N, Jain AK, Inturi S, Agarwal
C, White CW and Agarwal R: Silibinin attenuates sulfur mustard
analog-induced skin injury by targeting multiple pathways
connecting oxidative stress and inflammation. PLoS One.
7:e461492012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh RP and Agarwal R: Mechanisms and
preclinical efficacy of silibinin in preventing skin cancer. Eur J
Cancer. 41:1969–1979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deep G and Agarwal R: Antimetastatic
efficacy of silibinin: Molecular mechanisms and therapeutic
potential against cancer. Cancer Metastasis Rev. 29:447–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhanalakshmi S, Agarwal C, Singh RP and
Agarwal R: Silibinin up-regulates DNA-protein kinase dependent p53
activation to enhance UVB-induced apoptosis in mouse epithelial JB6
cells. J Biol Chem. 280:20375–20383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mallikarjuna G, Dhanalakshmi S, Singh RP,
Agarwal C and Agarwal R: Silibinin protects against
photocarcinogenesis via modulation of cell cycle regulators,
mitogen-activated protein kinases and Akt signaling. Cancer Res.
64:6349–6356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hassan W: Methotrexate and liver toxicity:
Role of surveillance liver biopsy. Conflict between guidelines for
rheumatologists and dermatologists. Ann Rheum Dis. 55:273–275.
1996. View Article : Google Scholar : PubMed/NCBI
|