Introduction
Salivary gland malignant neoplasms (SGMNs) are a
group of malignant solid tumors that present favorable features
with regard to locoregional invasion and metastasis (1). SGMNs account for 3–6% of all head and
neck cancers (2), with an estimated
global morbidity rate ranging between 0.4 and 2.6 cases per 100,000
individuals (3). In modern
pathology, SGMNs are one of the major diagnostic challenges due to
their heterogeneity in cellular make-up, which poses difficulty for
the immunohistochemical confirmation of their cytological features
(4). However, the SGMN
classification system continues to be based on their
histopathological features, biological behavior and histogenesis
(5). Previous studies have
demonstrated that salivary gland carcinomas exhibit overexpression
of cyclin-dependent kinase inhibitor 2A (CDKN2A),
TP53 and epidermal growth factor (EGFR) (6–8). In
addition, the frequency of EGFR-positive mucoepidermoid carcinomas
was 82–91% in SGMN samples (7,9). These
observations indicate that aberrant expression of cell cycle
factors and EGFR in a subfraction of tumor cells refers to
deregulated cell homeostasis.
The pituitary tumor transforming gene (PTTG) has
been identified as an oncogene, inducing cellular transformation
in vitro and tumor formation in nude mice (10). PTTG encodes human securin, which
participates in the mitotic spindle checkpoint pathway and inhibits
sister chromatid separation to ensure chromosomal stability
(11,12). Although PTTG expression is restricted
in normal tissue, PTTG has been shown to be abundantly expressed in
SGMNs (13) and other
non-endocrine-associated cancers, such as colon (13), gastric (14), lung (15) and esophagus cancer (16). In particular, PTTG has been
recognized as one of the key ‘signature genes’ to predict
metastasis in primary adenocarcinomas of the lung, breast and
prostate, as well as medulloblastomas (17). Although PTTG overexpression
correlates with metastasis and poor overall survival times in these
cancer types (18), whether the
oncogenic molecule contributes to the tumorigenesis of SGMNs
remains unclear.
In the present study, PTTG overexpression was
evaluated in human mucoepidermoid carcinoma specimens from the
submaxillary salivary gland using immunohistochemical analysis and
western blot analysis. In addition, to investigate the oncogenic
role of PTTG in SGMNs, a cell line was constructed that
overexpressed PTTG and coexpressed an enhanced green fluorescence
protein (EGFP) marker. The A-253 cell line was an epidermoid
carcinoma cell line originating from the human submaxillary
salivary gland. Subsequently, the influence of PTTG on the
proliferation and migration rates of A-253 cells was investigated.
The role of PTTG in SGMN cell migration was investigated with the
aim of assessing the potential of PTTG as a target for anticancer
therapy.
Materials and methods
Submaxillary SGMN specimens, cell
culture and construction of PTTG-overexpressing A-253 cells
The study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Inner Mongolia Medical
University (Hohhot, China). In total, 19 human mucoepidermoid
carcinoma specimens of the submaxillary salivary gland and 18
control submaxillary salivary gland specimens were obtained by
surgical resection prior to the administration of radiotherapy or
chemotherapy for the SGMN patients or pleomorphic adenoma patients.
Informed consent and agreement were provided by all the patients.
Clinicopathological data of the patients were recorded
prospectively, including the age at diagnosis, tumor size, axillary
lymph node metastasis and histological grade. All fresh tumor
specimens were immediately frozen in liquid nitrogen and stored at
−70°C following resection. An epidermoid carcinoma cell line
originating from the human submaxillary salivary gland (A-253 cell
line) was purchased from the American Type Culture Collection
(Rockville, MD, USA). The cells were cultured at 37°C in a
humidified atmosphere of 5% CO2 in McCoy's 5a Medium
Modified (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10%
fetal bovine serum (Gibco Life Technologies, Rockville, MD,
USA).
To generate an A-253 cell line that overexpressed
PTTG, the wild-type PTTG coding sequence was amplified and cloned
into a pcDNA3.1 (+) vector (Invitrogen Life Technologies, Carlsbad,
CA, USA). Subsequently, the PTTG-2A-EGFP-pcDNA3.1 (+) or control
CAT-pcDNA3.1 (+) vectors were transfected into the A-253 cells
using Lipofectamine 2000 (Invitrogen Life Technologies). The PTTG-
and EGFP-positive cell clones were selected under the pressure of
1.5 mg/ml G418 (Sigma-Aldrich), and maintained in medium containing
1 mg/ml G418.
Immunohistochemical staining
SGMN tissue slides were successively deparaffinized
by heating at 55°C for 30 min, and rehydrated serially in 100, 90
and 70% ethanol, and phosphate-buffered saline (PBS). Subsequently,
antigen retrieval was performed by heating for 20 min at 98°C in 10
mM sodium citrate (pH 6.0), after which the endogenous peroxidase
activity was blocked by incubation with 0.3% hydrogen peroxide for
20 min. Rabbit polyclonal antibodies targeting human PTTG (1:100;
PA5-14240) and β-actin (1:300; PA1-183; Thermo Fisher Scientific,
Waltham, MA, USA) were utilized to stain for PTTG and β-actin
expression. The samples were incubated with the primary antibodies
against PTTG and β-actin for 1 h at room temperature, followed by
incubation with a goat anti-rabbit/mouse IgG horseradish peroxidase
(HRP)-conjugated secondary antibody (1:1,000; ab6721; Abcam,
Cambridge, UK) at room temperature for 1 h. Subsequently, the HRP
substrate, 3,3′-diaminobenzidene (Abcam), was added for 1–5 min and
counterstained with Mayer's hematoxylin, after which the samples
were dehydrated and sealed with cover slips.
RNA isolation and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total cellular mRNA was isolated from the tumor
specimens or from the A-253 cells using TRIzol reagent (Invitrogen
Life Technologies), according to the manufacturer's instructions.
RT-qPCR was performed to quantify the mRNA expression levels of
PTTG using a SYBR PrimeScript RT-qPCR kit (Takara Bio, Inc., Tokyo,
Japan) and a LightCycler 2.0 (Roche Diagnostics Gmbh, Mannheim,
Germany), where β-actin was used as an internal control. The primer
sequences for PTTG were as follows: 5′-ACC TTT GCT TCT CCC ACC
TT-3′ (sense) and 5′-CAAATA CAC ACA AAC TCT GAA GCA-3′ (antisense).
The primer sequences for β-actin were as follows: 5′-TTC TAC AAT
GAG CTG CGT GTG-3′ (sense) and 5′-GGG GTG TTG AAG GTC TCA AA-3′
(antisense). All the primers were synthesized by Shanghai Sangon
Biological Engineering Technology & Services Co., Ltd.
(Shanghai, China). PTTG expression was normalized against β-actin,
and was expressed as the fold change over the control, as
calculated according to the ∆∆Ct method (19).
Western blot analysis for PTTG
expression
PTTG protein expression levels in the tumor
specimens were analyzed by western blot analysis. The tumor
specimens were homogenized prior to protein isolation. The
homogenized specimens or cultured cells were collected and lysed
with a lysis reagent (Pierce Biotechnology, Inc., Rockford, IL,
USA), according to the manufacturer's instructions, which was
supplemented with a protease inhibitor cocktail (Thermo Fisher
Scientific). Protein samples were separated by 12% SDS-PAGE and
transferred to a nitrocellulose membrane (EMD Millipore, Bedford,
MA, USA). The protein expression levels of PTTG and β-actin were
quantified via successive incubation at 37°C for 1 h with anti-PTTG
(1:300; PA5-14240) or anti-β-actin (1:1,00; PA1-183) polyclonal
rabbit primary antibodies, followed by a monoclonal
peroxidase-conjugated secondary antibody against rabbit IgG
(1:1,000; A1949; Sigma-Aldrich). An electrochemiluminescence
detection system (GE Healthcare Life Sciences, Uppsala, Sweden) was
used for visualization of the membranes, according to the
manufacturer's instructions.
Cell count assay and cell migration
assay
A cell count assay was performed as previously
described (20). Briefly,
1×103 or 1×104 A-253 PTTG (+) and A-253 PTTG
(-) cells/ml were seeded in six-well plates and incubated at 37°C
for 12, 24 or 48 h, or for 1, 2 or 4 days, after which the cells
were trypsinized. The number of viable cells was counted in a
hemocytometer (Tainuokeji, Taian, China) with the use of trypan
blue staining (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
The rate of cell migration was determined by a scratch assay. A-253
PTTG (+) or A-253 PTTG (-) cells were cultivated to 90% confluence
on six-well plates. Subsequently, cell scrapers (Corning, Inc.,
Corning, NY, USA) were utilized to scratch the confluent cells.
After 48 h, the cells that had migrated across the baseline were
observed and counted using a BX60 microscope (Olympus Optical Co.,
Ltd., Tokyo, Japan). All the experiments were repeated in
triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). All
data are expressed as the mean ± standard error of the mean. The
difference in the PTTG-positivity rate between the two groups was
evaluated using the χ2 test. Comparisons between the
expression of PTTG at an mRNA or protein level, or the cell number
in the two groups, were analyzed using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Overexpression of PTTG in the SGMN
specimens
To identify a possible oncogenic role of PTTG in
SGMNs, the PTTG expression levels in the SGMN specimens were
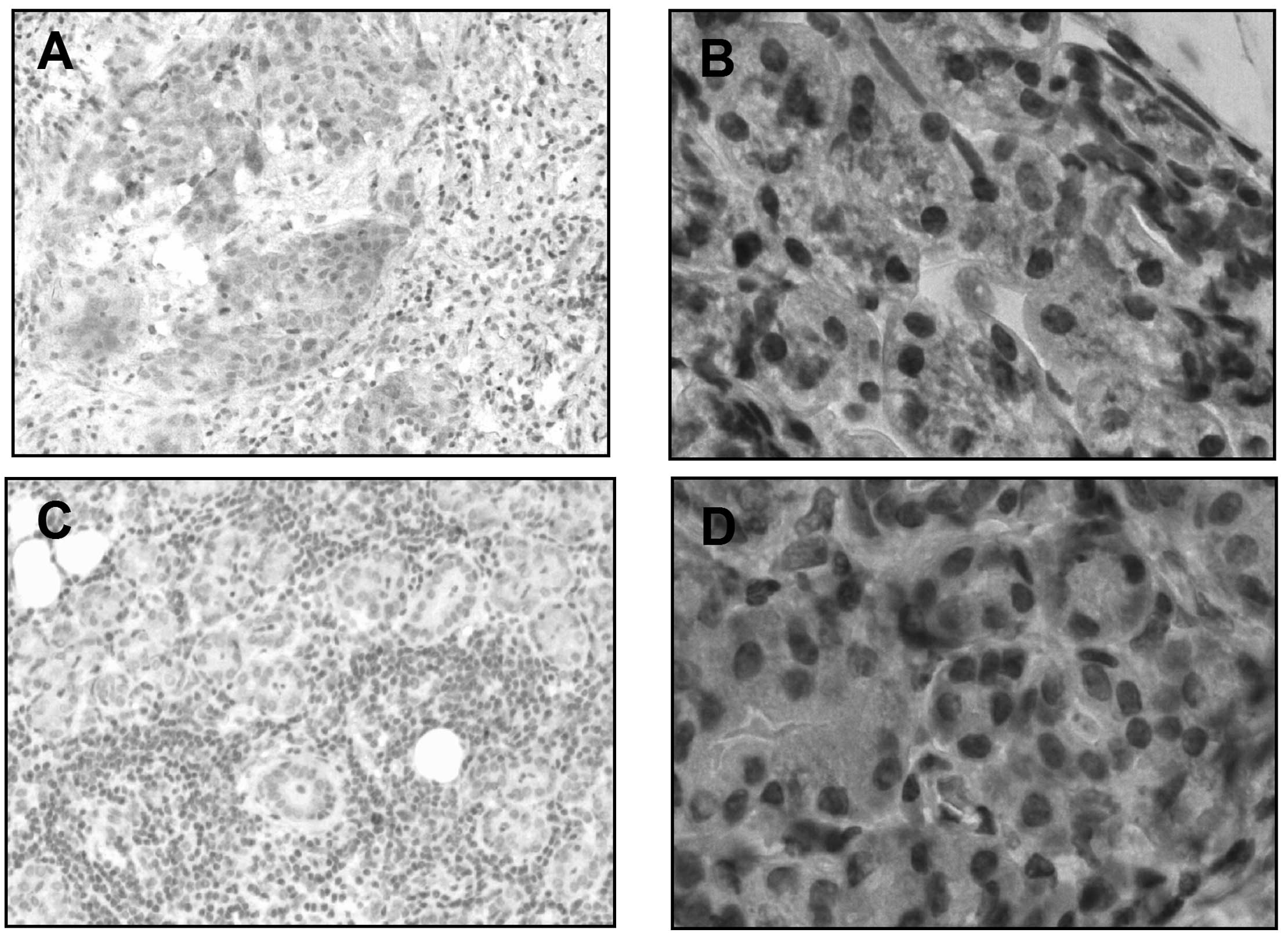
determined. Firstly, as shown in Fig. 1A
and B, there was a higher rate of PTTG-positive cells in the
SGMN tissues when compared with the control submaxillary salivary
gland tissues (14/19 vs. 7/18, P=0.0327), with a cutoff of 2
positive cells per field. To further reveal the difference in the
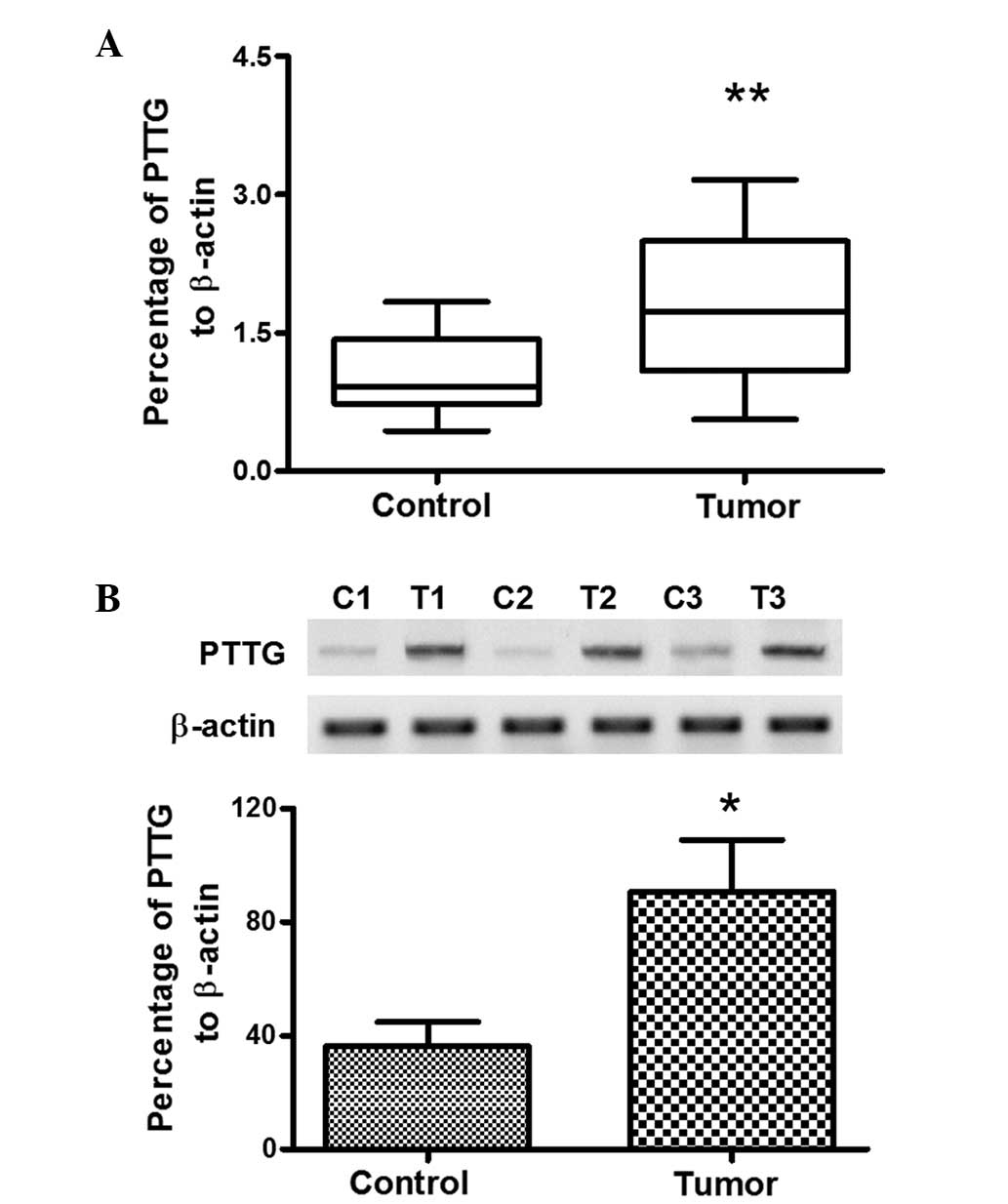
PTTG expression levels between the tumor and non-tumor groups, the
mRNA expression levels of PTTG in all the specimens from the two
groups were analyzed. The results demonstrated that the mRNA
expression level of PTTG in the SGMN specimens was 1.772±0.187
(n=19), as compared with the average level of 1.032±0.096 (n=18) in
the control group, which was a statistically significant difference
(P=0.0014; Fig. 2A). In addition,
the protein expression levels of PTTG were further analyzed using
western blot analysis in 16 SGMN specimens and 14 control specimens
that were of sufficient sample volume to facilitate the assay. As
shown in Fig. 2B, the protein
expression level of PTTG in the SGMN specimens was also
significantly higher when compared with the control specimens
(P<0.05, paired-samples t-test). Therefore, the overexpression
of PTTG in SGMN specimens was confirmed.
Construction of an A-253 stable cell
line overexpressing PTTG
To identify the oncogenic role of PTTG in SGMN
cells, a PTTG and EGFP coexpressing A-253 cell line was
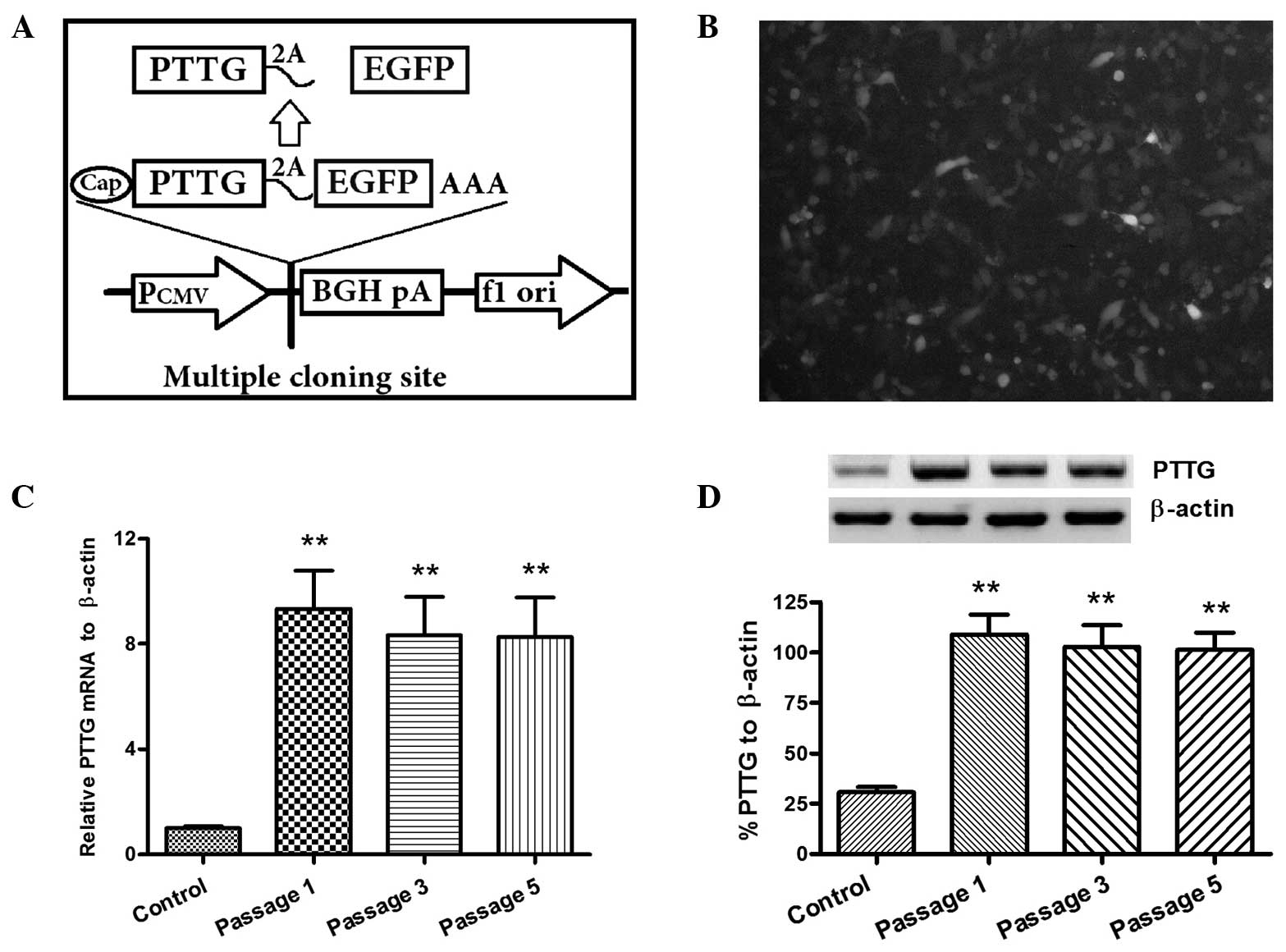
constructed. As shown in Fig. 3A,
the cDNA of PTTG and EGFP were linked by a 2A peptide coding
sequence, which guaranteed the transcription of PTTG and EGFP in
one mRNA molecule, but enabled the translation of two separate
proteins (21,22). EGFP-positive A-253 cells, which were
transfected with the recombinant PTTG-2A-EGFP-pcDAN3.1 (+) plasmid,
were selected under G418 pressure. Following serial passages with
1.5 mg/ml G418, >85% of the A-253 cells were determined to be
EGFP-positive (Fig. 3B).
Subsequently, the overexpression of PTTG was determined in the
EGFP-positive A-253 cells. Stabilized PTTG overexpression at the
mRNA level in the A-253 PTTG (+) cells was determined using a
RT-qPCR method with the PTTG specific primers. Significantly higher
expression levels of PTTG mRNA were observed in the A-253 PTTG (+)
cells at the passages of 1, 3 or 5 (P<0.01, vs. control A-253
cells, using the t-test; Fig. 3C).
In addition, PTTG overexpression at a protein level was stabilized
in the A-253 PTTG (+) cells (P<0.01, vs. control A-253 cells, as
determined with the t-test; Fig.
3D). Therefore, the A-253 PTTG (+) cells were shown to stably
express PTTG.
PTTG overexpression promotes the
growth and migration of A-253 cells
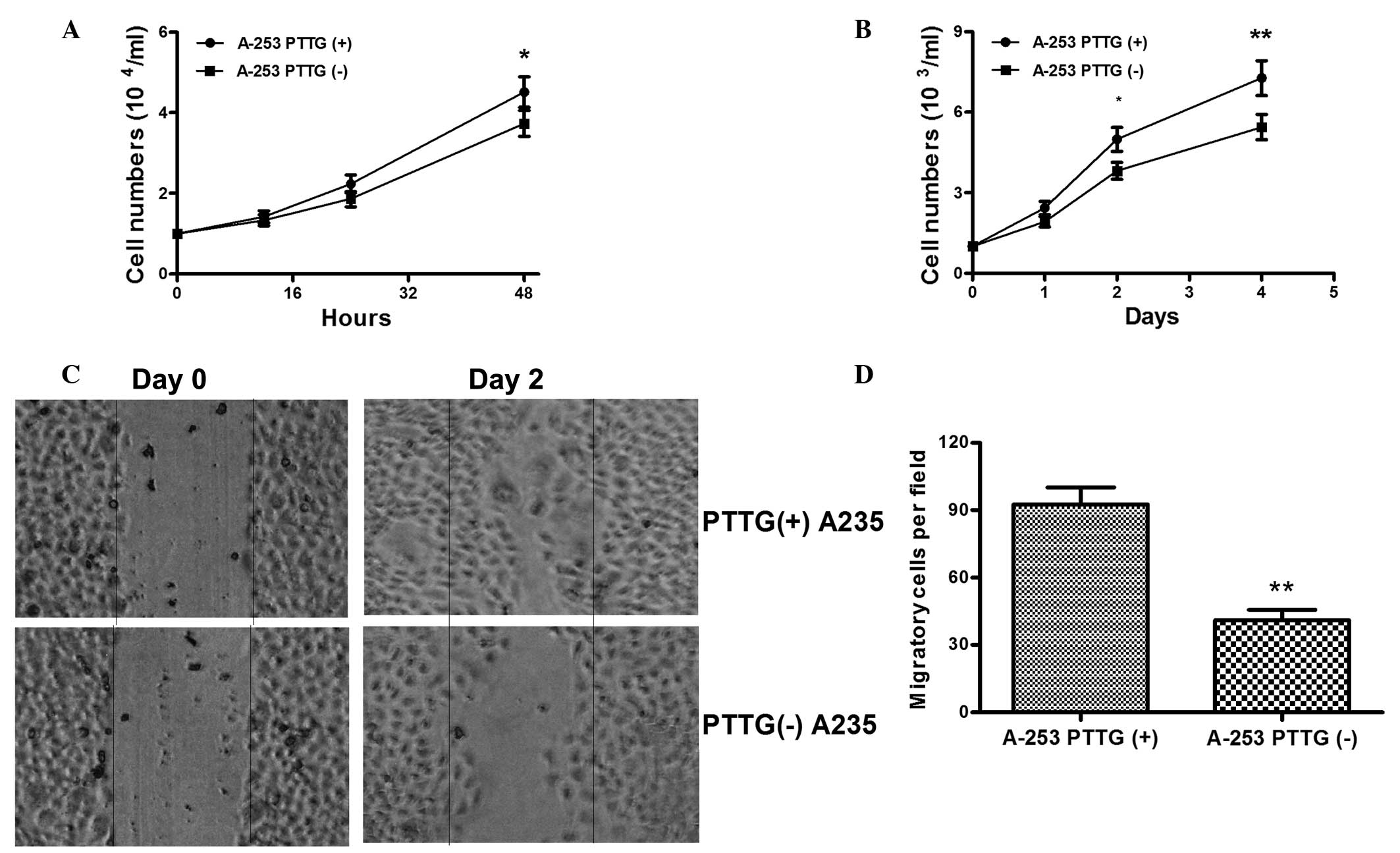
The influence of PTTG overexpression on the growth
of SGMN cells was subsequently evaluated. The growth of the A-253
PTTG (+) or A-253 PTTG (-) cells in vitro was assessed using a cell
count assay, in which cell counting was conducted at 12, 24 and 48
h after cell inoculation. As shown in Fig. 4A, when inoculated at a density of
104 cells/ml, the A-253 PTTG (+) cells grew more
efficiently compared with the A-253 PTTG (-) cells at 48 h after
cell inoculation, and the difference was statistically significant
(4.51±0.38×104/ml for A-253 PTTG (+) cells and
3.733±0.32×104/ml for A-253 PTTG (-) cells at 48 h after
inoculation; P<0.05). In addition, the difference in growth
between the two cell lines was reconfirmed following inoculation at
103 cells/ml, where the growth of the A-253 PTTG (+)
cells was also significantly more efficient compared with the A-253
PTTG (-) cells (Fig. 4B; P<0.05
for 24 h; P<0.01 for 48 h). Thus, the promotive role of PTTG in
the growth of SGMN cells was confirmed. Cell migration contributes
to the extent of tumor metastasis; thus, the migration of the A-253
PTTG (+) and A-253 PTTG (-) cells were further assessed using a
scratch assay. As shown in Fig. 4B and
C, at 48 h after the initiation of the scratch assay, a
significantly increased number of A-253 PTTG (+) cells had migrated
across the baseline when compared with the A-253 PTTG (-) cells
(92.67±7.51 vs. 41±4.62, P<0.01). Therefore, the results
indicated that overexpression of PTTG stimulated the growth and
migration of SGMN cells.
Discussion
In addition to the low incidence of SGMNs, the
heterogeneity of their cellular make-up poses difficulty for the
immunohistochemical confirmation of their cytological features
(4). In addition, SGMNs are a major
diagnostic challenge since the SGMN classification system is based
on histopathological features, biological behavior and histogenesis
(5). In previous years, a number of
studies have recognized several molecular markers, such as
CDKN2A, TP53 and EGFR, in SGMNs (6–8). The
aberrant expression of these markers has been shown to
significantly correlate with the malignancy of neoplasms in the
salivary glands. To the best of our knowledge, the present study
has demonstrated for the first time that PTTG is overexpressed in
SGMN specimens. Furthermore, the overexpression of PTTG was
repeatedly confirmed at a protein level by immunohistochemical
analysis and western blot analysis, and at an mRNA level by RT-qPCR
analysis. Therefore, PTTG overexpression is associated with
SGMNs.
PTTG is frequently upregulated in human tumors and
is known to be involved in metastasis. Increasing evidence reveals
a correlation between PTTG overexpression and metastasis in various
types of cancer, including colon (23), breast (13), gastric (24), and prostate cancer (25). However, the oncogenic role of PTTG in
SGMNs remains unclear. In the present study, an A-253 stable cell
line that overexpressed PTTG was constructed using a 2A peptide
coexpressing method (21,22). Simultaneous expression of EGFP with
PTTG guaranteed the simplicity of PTTG-positivity selection. The
EGFP-positive A-253 PTTG (+) cells were shown to stably overexpress
PTTG at an mRNA and protein level. Further investigations
demonstrated that the overexpression of PTTG significantly promoted
A-253 cell proliferation and migration, as demonstrated by the cell
count assay and cell migration assay. However, the detailed
mechanisms underlying the PTTG-induced promotion of cell
proliferation and migration remain unknown; thus, further
investigation is required.
In conclusion, the present study identified PTTG
overexpression in human mucoepidermoid carcinomas of the
submaxillary salivary gland. In addition, PTTG was confirmed to
significantly promote SGMN cell proliferation and migration in
A-253 cells overexpressing PTTG and coexpressing EGFP; a cell line
that was first constructed in this study. Therefore, the
observations of the present study indicate that PTTG plays an
important role in SGMN cell migration, and may subsequently be a
notable marker for SGMN diagnosis and a potential target for
anticancer therapy.
Acknowledgements
The study was supported by a grant from the
Affiliated Hospital of Inner Mongolia Medical University (no.
201207).
References
|
1
|
Lopes MA, Santos GC and Kowalski LP:
Multivariate survival analysis of 128 cases of oral cavity minor
salivary gland carcinomas. Head Neck. 20:699–706. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hocwald E, Korkmaz H, Yoo GH, Adsay V,
Shibuya TY, Abrams J and Jacobs JR: Prognostic factors in major
salivary gland cancer. Laryngoscope. 111:1434–1439. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Speight PM and Barrett AW: Prognostic
factors in malignant tumours of the salivary glands. Br J Oral
Maxillofac Surg. 47:587–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rapidis AD, Givalos N, Gakiopoulou H,
Stavrianos SD, Faratzis G, Lagogiannis GA, Katsilieris I and
Patsouris E: Mucoepidermoid carcinoma of the salivary glands.
Review of the literature and clinicopathological analysis of 18
patients. Oral Oncol. 43:130–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
6
|
Etges A, Nunes FD, Ribeiro KC and Araújo
VC: Immunohistochemical expression of retinoblastoma pathway
proteins in normal salivary glands and in salivary gland tumours.
Oral Oncol. 40:326–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang J, Shui Y, Sheng L, Wang K, Hu Q and
Wei Q: Epidermal growth factor receptor and human epidermal growth
receptor 2 expression in parotid mucoepidermoid carcinoma: Possible
implications for targeted therapy. Oncol Rep. 19:435–440.
2008.PubMed/NCBI
|
|
8
|
Ettl T, Schwarz S, Kleinsasser N, Hartmann
A, Reichert TE and Driemel O: Overexpression of EGFR and absence of
C-KIT expression correlate with poor prognosis in salivary gland
carcinomas. Histopathology. 53:567–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senft E, Lemound J, Stucki-Koch A,
Gellrich NC, Kreipe H and Hussein K: Expression of cyclin-dependent
kinase inhibitor 2A 16, tumour protein 53 and epidermal growth
factor receptor in salivary gland carcinomas is not associated with
oncogenic virus infection. Int J Oral Sci. 7:18–22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pei L and Melmed S: Isolation and
characterization of a pituitary tumor-transforming gene (PTTG). Mol
Endocrinol. 11:433–441. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou H, McGarry TJ, Bernal T and Kirschner
MW: Identification of a vertebrate sister-chromatid separation
inhibitor involved in transformation and tumorigenesis. Science.
285:418–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jallepalli PV, Waizenegger IC, Bunz F,
Langer S, Speicher MR, Peters JM, Kinzler KW, Vogelstein B and
Lengauer C: Securin is required for chromosomal stability in human
cells. Cell. 105:445–457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Solbach C, Roller M, Fellbaum C, Nicoletti
M and Kaufmann M: PTTG mRNA expression in primary breast cancer: A
prognostic marker for lymph node invasion and tumor recurrence.
Breast. 13:80–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen CY, Nakayama T, Wang AP, Nakashima M,
Ding YT, Ito M, Ishibashi H, Matsuu M, Shichijo K and Sekine I:
Expression of pituitary tumor transforming gene in human gastric
carcinoma. World J Gastroenterol. 10:481–483. 2004.PubMed/NCBI
|
|
15
|
Honda S, Hayashi M, Kobayashi Y, Ishikawa
Y, Nakagawa K and Tsuchiya E: A role for the pituitary
tumor-transforming gene in the genesis and progression of non-small
cell lung carcinomas. Anticancer Res. 23:3775–3782. 2003.PubMed/NCBI
|
|
16
|
Zhou C, Liu S, Zhou X, Xue L, Quan L, Lu
N, Zhang G, Bai J, Wang Y, Liu Z, et al: Overexpression of human
pituitary tumor transforming gene (hPTTG), is regulated by
beta-catenin/TCF pathway in human esophageal squamous cell
carcinoma. Int J Cancer. 113:891–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibata Y, Haruki N, Kuwabara Y, Nishiwaki
T, Kato J, Shinoda N, Sato A, Kimura M, Koyama H, Toyama T, et al:
Expression of PTTG (pituitary tumor transforming gene) in
esophageal cancer. Jpn J Clin Oncol. 32:233–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang
L, Li X, Li L, Ma W, Wu J, et al: Six1 promotes proliferation of
pancreatic cancer cells via upregulation of cyclin D1 expression.
PLoS One. 8:e592032013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szymczak-Workman AL, Vignali KM and
Vignali DA: Verification of 2A peptide cleavage. Cold Spring Harb
Protoc. 2012:255–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szymczak AL, Workman CJ, Wang Y, Vignali
KM, Dilioglou S, Vanin EF and Vignali DA: Correction of multi-gene
deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based
retroviral vector. Nat Biotechnol. 22:589–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heaney AP, Singson R, McCabe CJ, Nelson V,
Nakashima M and Melmed S: Expression of pituitary-tumour
transforming gene in colorectal tumours. Lancet. 355:716–719. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen CY, Nakayama T, Wang AP, et al:
Expression of pituitary tumor transforming gene in human gastric
carcinoma. World J Gastroenterol. 10:481–483. 2004.PubMed/NCBI
|
|
25
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar : PubMed/NCBI
|