Introduction
Post-stroke depression (PSD) is one of the most
frequent neuropsychiatric diseases following a stroke (1). PSD occurs subsequent to the obvious
symptoms of stroke. Patients diagnosed with PSD require the
following two conditions: Evident clinical symptoms of stroke, and
symptoms of depression following stroke (a patient must meet the
diagnostic criteria for depression). The incidence of PSD is ~33%
in all post-stroke patients (2). PSD
has serious negative effects on individuals, family and society so
its treatment is of great importance.
Pioglitazone, an agonist of peroxisome
proliferator-activated receptor (PPAR) γ, is an antidiabetic drug
of the thiazolidinedione class. PPARs mainly exist in
insulin-targeted tissues, such as liver, fat and muscle tissues.
They ameliorate blood glucose by reducing insulin resistance
(3). A number of studies have
reported that pioglitazone is effective in the treatment of
depression (4–6); however, whether pioglitazone can
ameliorate depressive symptoms in patients with PSD remains
unknown. In the present study, pioglitazone was administered to
patients with PSD and type 2 diabetes mellitus to examine whether
pioglitazone can ameliorate the depressive symptoms in such
patients.
Materials and methods
Ethics and consent
This study was approved by the Medical Ethics
Committee of Binzhou Medical University Affiliated Hospital
(Binzhou, China), and performed in accordance with the Declaration
of Helsinki. Enrolled patients provided informed consent.
Subjects
Between April 2012 and January 2014, consecutive
patients with stroke who attended the stroke ward of Binzhou
Medical University Affiliated Hospital were interviewed to assess
PSD 3 months after the stroke. Patients were selected for inclusion
in the present prospective, randomized controlled study if: i) They
were aged ≥18 years; ii) brain magnetic resonance imaging +
diffusion-weighted imaging revealed fresh infarctions following
admission to the stroke ward; iii) fasting blood glucose (FBG)
levels were ≥7.0 mmol/l twice following admission; iv)
6.5<glycated hemoglobin (HbA1c)≤11%; v) the body mass index was
≥27 kg/m2; vi) the diagnosis of PSD (3 months subsequent
to admission) fulfilled the criteria defined in the Diagnostic and
Statistical Manual of Mental Disorders (DSM-IV) (7); and vii) the activities of daily living
(ADL) score was >50 points. Patients were excluded if i) the FBG
level was <7.0 mmol/l following their stroke; ii) they had a
history of vascular disease during the 5 years prior to the stroke;
iii) their mini-mental state examination (MMSE) score was <24
points; iv) they had severe aphasia following their stroke; v)
there was a history of psychiatric disease; vi) they had a
medication history of the thiazolidinedione class; and vii) they
received administration of other antidiabetic drugs but no insulin
injections during the 3 months post-stroke.
Hypertension was diagnosed according to the
following values: Systolic blood pressure ≥140 mmHg and/or
diastolic blood pressure ≥90 mmHg. Patients who took
antihypertensive medications to control blood pressure were classed
as being diagnosed with hypertension.
Clinical assessment and outcomes
The experimental group was given fluoxetine [20 mg
once daily (qd)] and pioglitazone (30 mg qd) whilst the control
group was given fluoxetine (20 mg qd) and metformin (0.5 g twice
daily). The two groups were further divided into a mild and
moderate (MM) depression and a severe depression group.
Baseline data collected from the patients included
age, gender, family status, risk factors, MMSE score and National
Institutes of Health stroke scale (NIHSS) score. The following
stroke risk factors were identified: i) Hypertension; ii) total
cholesterol (TC); iii) high triglyceride levels (TG); iv) high low
density lipoprotein (LDL) levels; v) high FBG levels; vi) high
fasting insulin (FINS) levels; and vii) high HbA1c levels (8).
The patients were interviewed in order to evaluate
their Hamilton depression scale (HAMD) and ADL scores. HAMD scores
were assessed and administered by psychiatrists. Neurological
physicians were responsible for the ADL, NIHSS and MMSE scores. The
follow-up interview was conducted at an average of 3 months
following the onset of stroke. The diagnosis of PSD was assessed
according to DSM-IV (7). The HAMD
scale was also used to simultaneously evaluate MM depression (23
points ≥ HAMD score ≥8 points) and severe depression (HAMD score
≥24 points) (9). ADL scores were
also assessed at the onset of this interview. The severity of
depression was evaluated by HAMD scores following 1 and 3 months of
therapy. During the 3 months of therapy, the ADL scores and the
levels of FBG and FINS were detected when the treatment was first
initiated and at 1 and 3 month time-points.
Statistical analysis
The baseline assessment scores and demographic
characteristics were analyzed by appropriate test procedures,
including the independent sample t-test and the χ2 test.
One-way analysis of variance was used to compare differences
between the MM depression and the severe depression groups.
Graphpad prism software (version 5.0; Graphpad Software Inc., La
Jolla, CA, USA) was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
General and baseline
characteristics
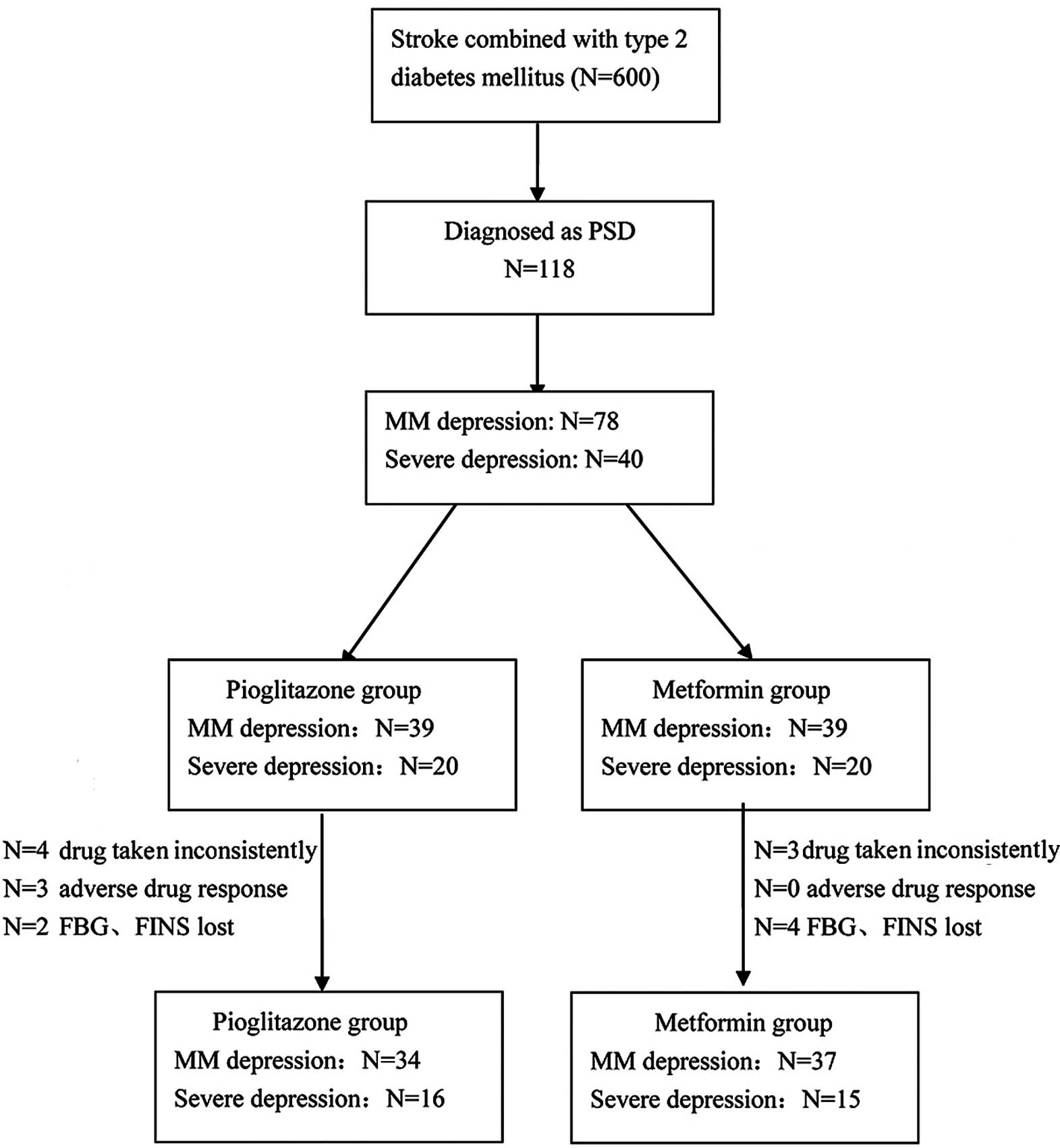
A total of 600 patients who had acute stroke
combined with type 2 diabetes were enrolled in the present study.
Of these 600 patients, 470 were excluded since they did not match
the DSM-IV criteria and 12 had received antidiabetic drugs other
than injected insulin during the 3 months following stroke onset.
The remaining 118 patients were diagnosed with PSD according to
DSM-IV. The MM depression group comprised 78 patients and the
severe depression group comprised 40 patients. These patients were
separately distributed into the pioglitazone and metformin
subgroups. Each subgroup comprised 39 patients with MM depression
and 20 patients with severe depression; however, in the
pioglitazone subgroup, 4 patients with severe depression did not
consistently take the drugs, and 3 patients with MM depression had
a mild adverse drug response during the first month of therapy.
Furthermore, the FBG and FINS levels of 2 patients with MM
depression were not measured at the correct time during the 3-month
therapy. In the metformin subgroup, 3 patients with severe
depression did not consistently take the drugs during the first
month of therapy, and the FBG and FINS data were lost for 2
patients with MM depression and 2 patients with severe depression
after 1 month of therapy (Fig.
1).
No significant differences were observed between the
pioglitazone and metformin subgroups regarding sociodemographic and
baseline variables. Nevertheless, the severe depression group had
higher NIHSS values than the MM depression group (F=8.328,
P<0.0001; Tables I and II).
 | Table I.Sociodemographic and baseline scores
of the pioglitazone and metformin groups on admission to the
program. |
Table I.
Sociodemographic and baseline scores
of the pioglitazone and metformin groups on admission to the
program.
| Variable | Pioglitazone group
(n=59) | Metformin group
(n=59) | P-value |
|---|
| Agea (years) | 63.85±6.58 | 65.36±7.89 | 0.2619 |
| Gender (female
%) | 61 | 52.5 | 0.3528 |
| Family status |
|
| 0.6591 |
|
Single | 2 | 4 |
|
|
Married | 25 | 26 |
|
|
Divorced/widowed | 32 | 29 |
|
| Hypertension (n) | 33 | 37 | 0.5742 |
| TCa (mmol/l) | 6.26±1.60 | 6.02±1.90 | 0.4613 |
| TGa (mmol/l) | 2.73±1.27 | 2.91±1.64 | 0.5075 |
| LDLa (mmol/l) | 3.92±1.19 | 3.60±1.30 | 0.1658 |
| FBGa | 9.27±1.71 | 8.85±1.72 | 0.1764 |
| FINSa | 12.20±2.69 | 11.43±2.85 | 0.1313 |
| HbA1ca (%) |
8.89±1.52 |
8.61±1.67 | 0.2676 |
 | Table II.ADL and HAMD scores of the
pioglitazone and metformin groups during 3 months of therapy. |
Table II.
ADL and HAMD scores of the
pioglitazone and metformin groups during 3 months of therapy.
|
| Pioglitazone group
(n=59) | Metformin group
(n=59) |
|
|
|---|
|
|
|
|
|
|
|---|
| Neuropsyciatric
scores | MMDa | Severe Db | MMDc | Severe Dd | F-value | P-value |
|---|
| At 0 months |
|
|
|
|
|
|
|
NIHSS |
3.54±2.33 |
6.15±2.70 |
3.56±2.40 |
5.75±2.69 |
8.328 | <0.0001 |
| MMSE |
26.31±1.45 |
26.55±1.54 |
26.79±1.64 |
26.45±1.43 |
0.6839 |
0.5636 |
| ADL |
74.10±9.38 |
62.75±8.35 |
72.44±7.68 |
60.75±7.83 |
16.95 | <0.0001 |
| HAMD |
21.13±3.64 |
44.35±6.11 |
20.46±4.06 |
46.45±8.05 |
39.46 | <0.0001 |
| At 1 month |
|
|
|
|
|
|
| ADL |
75.14±9.22 |
63.75±9.04 |
73.72±8.41 |
60.59±7.26 |
16.04 | <0.0001 |
| HAMD |
17.86±4.05 |
41.63±6.10 |
19.74±3.35 |
43.59±7.67 | 112.6 | <0.0001 |
| At 3 months |
|
|
|
|
|
|
| ADL |
77.94±8.35 |
64.06±9.87 |
75.14±10.31 |
63.00±7.97 |
14.47 | <0.0001 |
|
HAMD |
16.59±3.58 |
35.25±5.69 |
19.32±3.49 |
40.60±8.41 | 115.6 | <0.0001 |
At 1 month
In the MM depression group, the pioglitazone
subgroup (n=36) had lower HAMD scores compared with the metformin
subgroup (n=39) following 1 month of therapy (P=0.0302); however,
no significant difference was observed in HAMD scores between the
metformin subgroup (n=17) and the pioglitazone subgroup (n=16) in
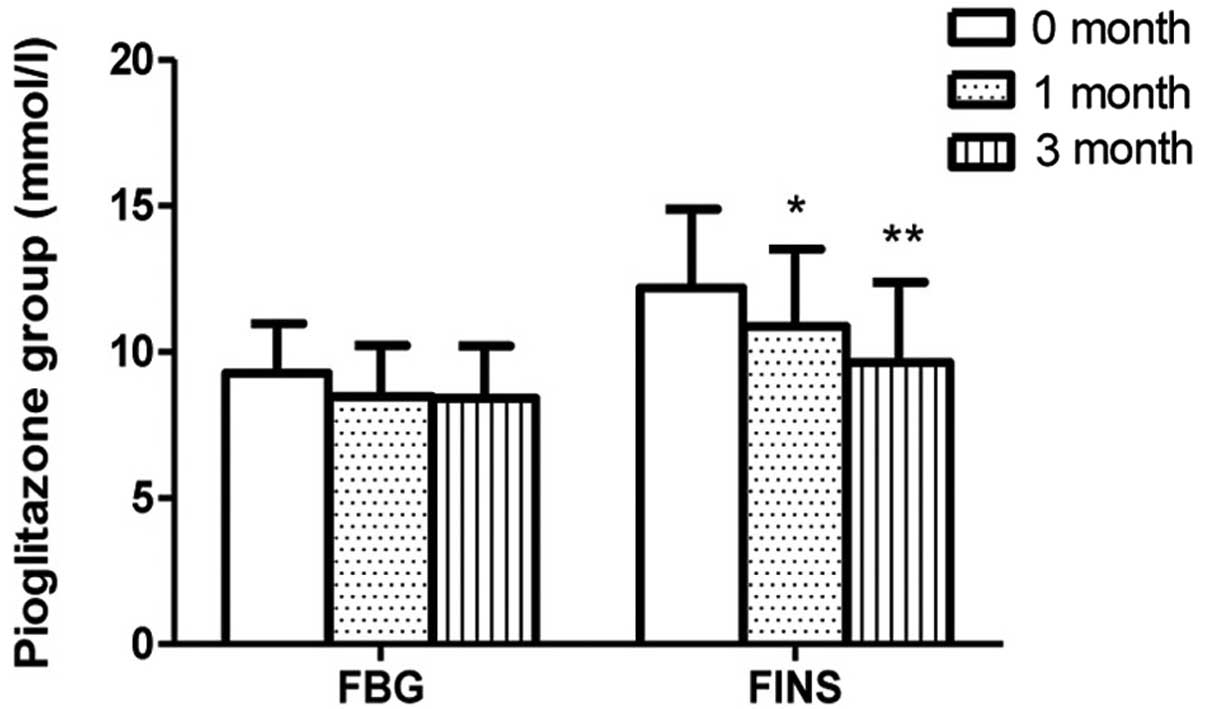
the severe depression group. The FINS levels of the pioglitazone
group at 1 month (n=52) were lower than those at onset (n=59;
P=0.0103); however, no such change in FINS levels was detected in
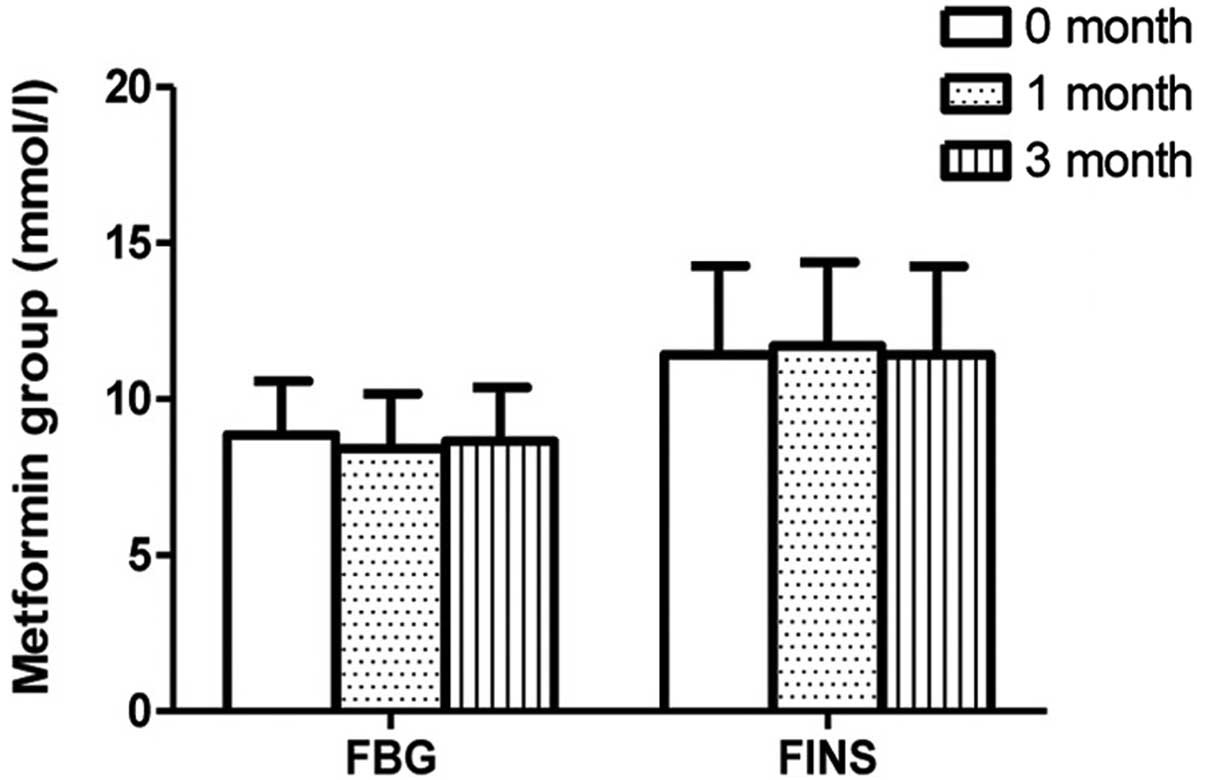
the metformin group following 1 month of therapy. The FBG levels
were observed to be stable in the pioglitazone and metformin groups
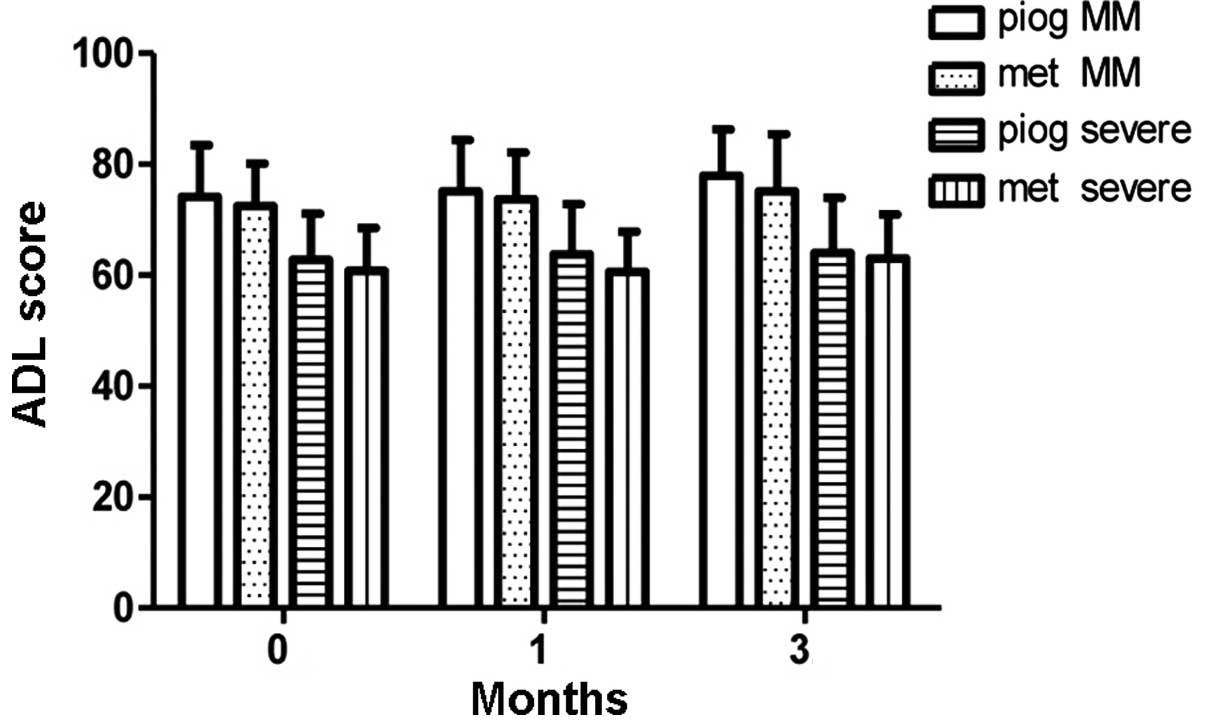
(Figs. 2 and 3). No differences in ADL scores were
observed between the pioglitazone and metformin groups, in either
the MM depression or severe depression subgroups (Fig. 4).
At 3 months
In the severe depression group, HAMD scores were
lower in the pioglitazone subgroup (n=16) than those in the
metformin group (n=15) after 3 months of therapy (P=0.0457). A
similar result was observed in the MM depression group (P=0.0017).
In the pioglitazone group, the FINS levels detected following 3
months of therapy (n=50) were lower than those detected following 1
month of therapy (n=52; P=0.0246); however, no such change was
exhibited in the metformin group. No difference in FBG level was
observed in patients between 1 month of therapy and 3 months of
therapy in the pioglitazone and metformin subgroups. ADL scores
were also higher in the MM depression group compared with those in
the severe depression group (F=14.47, P<0.0001, Table II). In the MM depression and severe
depression groups, ADL scores showed no significant difference
between the pioglitazone group and the metformin group after the 3
months therapy (Figs. 2–5).
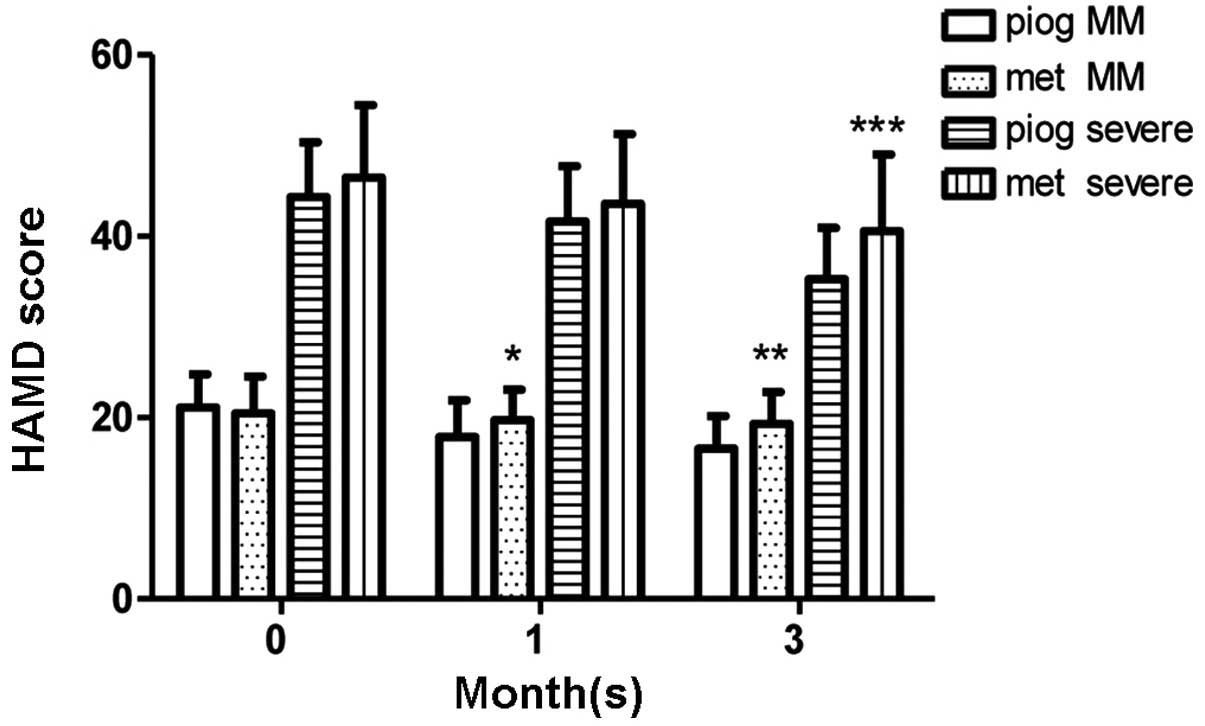 | Figure 5.HAMD scores during the 3-month therapy
period. In the MM depression group, the pioglitazone subgroup
(n=36) had lower HAMD scores than the metformin subgroup (n=39)
following 1 month of therapy (*P=0.0302). In the MM depression
(**P=0.0017) and severe depression groups (***P=0.0457), the
pioglitazone subgroups (MM depression, n=34; severe depression,
n=16) presented lower HAMD scores than the metformin subgroups (MM
depression, n=37; severe depression, n=15) after 3 months of
therapy. Data are presented as mean ± standard deviation. MM, mild
to moderate depression; HAMD, Hamilton depression scale; piog,
pioglitazone; met, metformin. |
Discussion
The present study was, to the best of our knowledge,
the first to examine the efficacy of pioglitazone using the HAMD
scores of patients with PSD combined with type 2 diabetes mellitus.
The antidepressive effects of pioglitazone were observed after 3
months of therapy. Pioglitazone ameliorated the depressive symptoms
and decreased FINS levels whereas metformin did not; however,
pioglitazone was useful for only MM depression following 1 month of
treatment. After 3 months of treatment, pioglitazone was also
effective against severe depression. The results suggest that
pioglitazone may be useful for the treatment of patients with PSD
combined with type 2 diabetes.
In the current study the morbidity of PSD was 19.7%,
which did not correspond with a previous study describing the
prevalence of PSD as 33.3% (2). The
difference in results may be due to the fact that the patients
selected in the present study were also diagnosed with type 2
diabetes; numerous inclusion criteria could also support this
phenomenon. The present study was initiated 3 months following
acute stroke as most studies report that the prevalence of PSD is
highest during this period (10)
enabling the recruitment of more patients in this period. The
prevalence of PSD in female patients was 56.8% in this study, which
is in accordance with a systematic review (11).
Kemp et al (5)
reported a patient who had severe depression following 12 weeks of
pioglitazone treatment with an initial dose of 15 mg qd and an
end-point dose of 30 mg qd. In the study, a marked antidepressant
response was demonstrated and a significant improvement in insulin
resistance. In addition, Kemp et al (12) reported antidepressive effects and
improvements in insulin sensitivity among 23 severe depression
patients receiving pioglitazone therapy. Kashani et al
(4), however, found that patients
with severe depression and polycystic ovarian syndrome who took
pioglitazone drugs had lower HAMD scores than patients who took
metformin. No difference was found between the onset and end of
therapy with respect to insulin resistance. Whether the
antidepressive effect of pioglitazone is induced by the
amelioration of insulin resistance remains unclear as different
studies have reached different conclusions. Numerous studies have
reported that pioglitazone can decrease the immobility time in the
forced swimming test depression model, which cannot be explained by
the amelioration of insulin resistance (13,14).
This phenomenon was attributed to N-methyl-D-aspartate receptor
signaling. In the present study, FINS levels gradually decreased in
the pioglitazone subgroup, which demonstrates that pioglitazone
decreased insulin resistance (homeostasis model
assessment-estimated insulin resistance was calculated using the
formula fasting plasma insulin (IU/l) × fasting plasma glucose
(mmol/l)/22.5) (15). Depression
could dysregulate the hypothalamic-pituitary-adrenal axis causing
the deposition of visceral fat, which could lead to insulin
resistance (16). Visceral adipose
tissue could also lead to inflammatory cytokine production, further
contributing to impaired insulin signaling (17).
Few studies have compared the antidiabetic effects
of pioglitazone and metformin. The TODAY Study Group found that
pioglitazone combined with metformin is better than metformin alone
when used on patients with type 2 diabetes mellitus (18). This combination can stabilize blood
glucose levels and improve insulin sensitivity. In the current
study, no difference was found in FBG levels between the
pioglitazone and metformin subgroups during 3 months of therapy.
Patients with severe depression had higher NIHSS scores than
patients with MM depression; thus, a patient would more easily
succumb to severe depression if they had a more severe stroke. A
similar trend was observed in ADL scores, in which the MM
depression group had higher ADL scores than the severe depression
group. Badaru et al (19)
reported that PSD may have a passive effect on functional
independence in ADL. In depressed or non-depressed patients the
intake of antidepressant drugs within the first month post-stroke
would be more likely to improve ADL scores than the intake of
antidepressant drugs following the first month post-stroke
(20). In this previous review, ADL
scores were assessed from 6 months following a stroke to 24 months
following a stroke. This observation had minimal association with
the present results. No change was observed in ADL scores for the
pioglitazone and metformin subgroups during the 3-month treatment
period. The mechanism underlying this phenomenon should be explored
in future studies.
The present study had several limitations. Firstly,
the patients were diagnosed with PSD in accordance with DSM-IV 3
months after stroke; however, several patients suffered from
depression between the first to third months following their
stroke. This occurrence delayed the optimal therapy time. Secondly,
patients with higher MMSE and ADL scores were selected in order for
this study to proceed conveniently; thus, the sample population did
not represent all patients with PSD. Thirdly, the HbA1c values of
the patients were ≤11%; certain patients with higher HbA1c were
excluded from this study. Finally, patients are recommended to be
injected with insulin to control blood glucose levels during the
acute stroke period. These patients took pioglitazone or metformin
combined with insulin at the start of this project, and the insulin
dosage may have varied from one individual to another.
Despite these limitations, the data suggest that
pioglitazone can decrease depressive symptoms and improve insulin
sensitivity compared with metformin. Although the mechanisms of
this phenomenon remain unclear, the administration of pioglitazone
to patients with PSD combined with type 2 diabetes mellitus may be
considered to decrease their HAMD scores.
Acknowledgements
This study was partly supported by the Chinese State
Natural Science Fund (No. 81301182).
References
|
1
|
Morris PL, Robinson RG and Raphael B:
Prevalence and course of depressive disorders in hospitalized
stroke patients. Int J Psychiatry Med. 20:349–364. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner A, Hambridge J, White J, et al:
Depression screening in stroke: a comparison of alternative
measures with the structured diagnostic interview for the
diagnostic and statistical manual of mental disorders, fourth
edition (major depressive episode) as criterion standard. Stroke.
43:1000–1005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith RC, Jin H, Li C, et al: Effects of
pioglitazone on metabolic abnormalities, psychopathology, and
cognitive function in schizophrenic patients treated with
antipsychotic medication: a randomized double-blind study.
Schizophr Res. 143:18–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kashani L, Omidvar T, Farazmand B, et al:
Does pioglitazone improve depression through insulin-sensitization?
Results of a randomized double-blind metformin-controlled trial in
patients with polycystic ovarian syndrome and comorbid depression.
Psychoneuroendocrinology. 38:767–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kemp DE, Ismail-Beigi F, Ganocy SJ, et al:
Use of insulin sensitizers for the treatment of major depressive
disorder: a pilot study of pioglitazone for major depression
accompanied by abdominal obesity. J Affect Disord. 136:1164–1173.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sepanjnia K, Modabbernia A, Ashrafi M,
Modabbernia MJ and Akhondzadeh S: Pioglitazone adjunctive therapy
for moderate-to-severe major depressive disorder: Randomized
double-blind placebo-controlled trial. Neuropsychopharmacology.
37:2093–2100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pettersson A, Bengtsson Boström K,
Gustavsson P and Ekselius L: Which instruments to support diagnosis
of depression have sufficient accuracy? A systematic review. Nord J
Psychiatry. March 3–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia J and Chen S: Brain vessel
diseaseNeurology. 7th. People's Medical Publishing House; Beijing,
China: pp. 1992013
|
|
9
|
Zimmerman M, Martinez JH, Young D,
Chelminski I and Dalrymple K: Severity classification on the
Hamilton Depression Rating Scale. J Affect Disord. 150:384–388.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider MA and Schneider MD: Recognizing
poststroke depression. Nursing. 42:60–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poynter B, Shuman M, Diaz-Granados N,
Kapral M, Grace SL and Stewart DE: Sex differences in the
prevalence of post-stroke depression: a systematic review.
Psychosomatics. 50:563–569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kemp DE, Ismail-Beigi F and Calabrese JR:
Antidepressant response associated with pioglitazone: support for
an overlapping pathophysiology between major depression and
metabolic syndrome. Am J Psychiatry. 166:6192009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salehi-Sadaghiani M, Javadi-Paydar M,
Gharedaghi MH, et al: NMDA receptor involvement in
antidepressant-like effect of pioglitazone in the forced swimming
test in mice. Psychopharmacology (Berl). 223:345–355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadaghiani MS, Javadi-Paydar M, Gharedaghi
MH, Fard YY and Dehpour AR: Antidepressant-like effect of
pioglitazone in the forced swimming test in mice: The role of
PPAR-gamma receptor and nitric oxide pathway. Behav Brain Res.
224:336–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamayo T, Jacobs DR Jr, Strassburger K, et
al: Race-and sex-specific associations of parental education with
insulin resistance in middle-aged participants: the CARDIA study.
Eur J Epidemiol. 27:349–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Austin AW, Gordon JL, Lavoie KL, Arsenault
A, Dasgupta K and Bacon S: Differential association of insulin
resistance with cognitive and somatic symptoms of depression.
Diabet Med. 31:994–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hardy OT, Czech MP and Corvera S: What
causes the insulin resistance underlying obesity? Curr Opin
Endocrinol Diabetes Obes. 19:81–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
TODAY Study Group, . Effects of metformin,
metformin plus rosiglitazone, and metformin plus lifestyle on
insulin sensitivity and β -cell function in TODAY. Diabetes Care.
36:1749–1757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badaru UM, Ogwumike OO, Adeniyi AF and
Olowe OO: Variation in functional independence among stroke
survivors having fatigue and depression. Neurol Res Int.
2013:8429802013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robinson RG and Spalletta G: Poststroke
depression: a review. Can J Psychiatry. 55:341–349. 2010.PubMed/NCBI
|