Introduction
Chronic myelogenous leukemia (CML) is a cancer of
the white blood cells characterized by a balanced genetic
translocation, t (9;22) (q34;q11.2), which leads to a fusion of the
Abelson oncogene (ABL) from chromosome 9q34 with the
breakpoint cluster region (BCR) gene on chromosome 22q11.2.
This chromosomal translocation is known as the Philadelphia
chromosome. At a molecular level, the rearrangement results in the
formation of the BCR-ABL fusion oncogene, which translates
into a BCR-ABL oncoprotein (1).
The US Food and Drug Administration has approved
three tyrosine kinase inhibitors (TKIs), imatinib, nilotinib and
dasatinib, as first-line treatments for patients diagnosed with CML
in the chronic phase (CML-CP) (2–5).
Imatinib mesylate, otherwise known as Gleevec® (Novartis
Pharmaceuticals Corp., East Hanover, NJ, USA), was the first of the
TKIs to receive approval; however, 20–40% of patients receiving
imatinib as a first-line therapy are likely to eventually require
an alternative treatment, due to intolerance or resistance to
imatinib (5). It is recommended
that, upon failure of imatinib treatment, patients with CML should
be assessed for BCR-ABL kinase domain mutations, as this can
indicate which TKI should be selected for continued therapy.
Dasatinib and nilotinib have been demonstrated to retain efficacy
against several of the mutations known to confer resistance to
imatinib (6). Notably, a number of
distinct mutations leading to decreased sensitivity to dasatinib
and nilotinib have been found in in vitro and in vivo
studies (7,8). Dasatinib is favored when patients have
Y253H, E255K/V or F359C/V mutations in BCR-ABL. By contrast,
nilotinib is more effective when V299L or F317L mutations are
present (2). Despite this evidence,
it remains unclear how the M244V mutation to the BCR-ABL fusion
protein should affect the choice of treatment. The present study
describes the effect of nilotinib therapy in a patient with
imatinib-resistant CML.
Case report
This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Changzhi Medical College (Changzhi, China). Written informed
consent was obtained from the patient.
The patient was a 43-year-old female. Three months
prior to diagnosis, the patient began sweating at night, but did
not receive any special treatment. One month prior to diagnosis,
the patient developed a rash on her face and neck, accompanied by
itching. This was diagnosed as allergic dermatitis by her doctors,
and was treated with oral tablets, including cetirizine. Despite
treatment, there was no notable relief of her symptoms. Further
examination found that her white blood cell (WBC) count was
significantly higher than normal. The patient came under the care
of Heping Hospital of Changzhi Medical College as of August 22,
2008.
Physical examination of the patient revealed
scattered red papules on her face and neck, superficial swelling of
the lymph nodes and sternum tenderness. No yellowing of the skin or
mucous membranes or formation of hemorrhagic spots were observed.
The patient additionally underwent a cardiopulmonary examination,
as well as an examination of the liver and spleen under the ribs
and the abdomen. Laboratory studies revealed the patient had a WBC
count of 112.7×109 cells/l, a red blood cell (RBC) count
of 3.72×1012 cells/l, hemoglobin (Hb) levels of 116 g/l
and a platelet (PLT) count of 445×109 cells/l.
Peripheral blood smears consisted of granulocytes (1%),
neutrophilic myelocytes (20%), neutrophilic metamyelocytes (17%),
banded neutrophils (13%), neutral lobocytes (39%) and
leukomonocytes (10%), with approximately normal mature erythrocyte
levels and a normal distribution of PLTs. A significant reduction
in neutrophil alkaline phosphatase was observed, as assessed by
staining. Routine tests of the urine and of liver and renal
function were normal. Furthermore, chest X-rays revealed no evident
abnormalities, and the echocardiogram (ECG) was normal. Abdominal
ultrasound indicated that there was a 0.9×0.9-cm swollen lymph node
in the first hepatic portal. Additionally, the distance between the
spleen and rib was 4.3 cm. Level I bone marrow hyperplasia was
observed, with the cells of the immune system accounting for 87%.
The following cells were present: Original granulocytes (5%),
neutrophilic myelocytes (18%), neutrophilic metamyelocytes (31%),
banded neutrophils (16%), neutral lobocytes (25%) and eosinophilic
polymorphonuclear cells (2%). Erythroid proliferation was inhibited
(3%), of which rubricytes composed 1% and metarubricytes composed
2% (granulocytes versus erythrocytes, 97:3). The entire staining
smear contained 219 megakaryocytes, of which 210 had no PLTs and
nine contained PLTs.
Chromosome karyotype was assessed by Giemsa-banding
(G-banding). G-banding analysis revealed two subsets of cells:
Those with 46 total chromosomes (XX) containing a t (9;22)
translocation or those with 46 total chromosomes (XX) with no
translocation (2,9). Quantitative polymerase chain reaction
(qPCR) analysis was used to assess the ratio of the copy numbers of
BCR-ABL and ABL (BCR-ABL copy
number/ABL copy number). The patient exhibited an initial
ratio of 101,993/665,053 (15.3%).
During the CML-CP, the patient was prescribed
hydroxyurea (1.0 g, three times per day) and allopurinol (0.1 g,
three times per day) for one week. Beginning in September 2008,
imatinib (0.4 g) was administered once daily. The response to the
imatinib treatment was assessed via peripheral blood cell counts
and classification of peripheral blood once a week until complete
hematological remission (CRH) was achieved. Following CRH, these
assays were performed once per month, and bone marrow cytogenetic
analysis and/or fluorescence in situ hybridization (FISH)
was performed once every 3–6 months, until complete cytogenetic
remission (CCyR) was confirmed. To detect the BCR-ABL fusion
gene, qPCR was performed once every three months until CRH was
achieved. Following CRH, qPCR was performed once every 3–6 months.
Biochemical tests, liver and kidney function and ECG were evaluated
once a month.
Following three months of treatment with imatinib,
the WBC count was 6.1×109 cells/l, RBC count was
3.8×1012 cells/l, Hb levels were 117 g/l and PLT count
was 175×109 cells/l. The peripheral blood smear
contained 2% banded neutrophils, 54% neutral lobocytes, 40%
lymphocytes and 4% monocytes. Mature erythrocyte levels were
approximately normal, and the distribution of PLTs was normal. The
copy number ratio of BCR-ABL to ABL was 9,740/124,247
(7.8%).
After six months of treatment, the
BCR-ABL/ABL copy number ratio was reduced to 2,383/73,403
(3.2%). Analysis of 300 interphase cells by FISH revealed that 70
visibly expressed BCR-ABL. The remaining 230 cells did not
visibly contain the BCR-ABL fusion.
After nine months of imatinib treatment, G-banding
analysis indicated that the karyotype of the cells was 46
chromosomes, XX. FISH analysis of 300 interphase cells revealed
that eight contained the BCR-ABL fusion, while the remaining
292 did not contain the BCR-ABL fusion. The
BCR-ABL/ABL copy number ratio was 3,355/88,250 (3.8%).
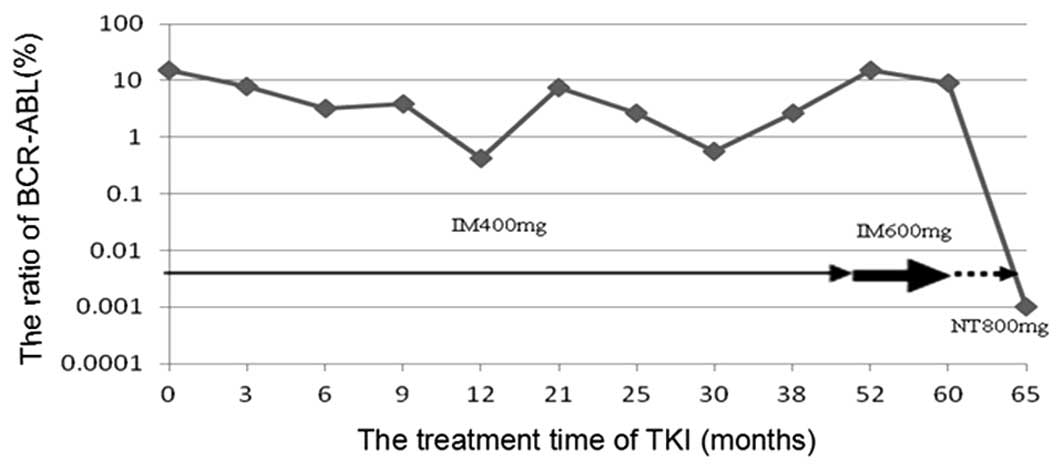
Following twelve months of imatinib treatment, the
BCR-ABL/ABL copy number ratio was 414/98,693 (0.42%). After
52 months of imatinib treatment (0.6 g, once daily), the
BCR-ABL/ABL copy number ratio was 1,002/6,557 (15.3%). At 60
months of treatment, the BCR-ABL/ABL copy number ratio was
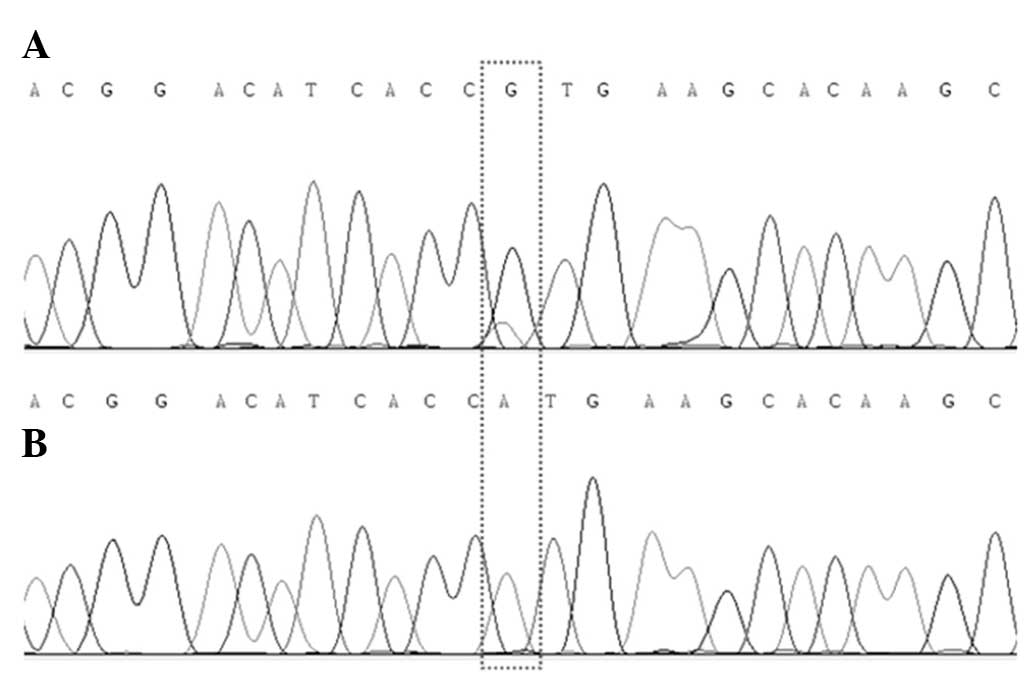
7,103/77,370 (9.2%). PCR sequencing of the ABL kinase region
of BCR-ABL revealed a mutation at nucleotide 730 (A to G),
resulting in the point mutation M244V (Fig. 1A).
Sixty-two months after the diagnosis, the patient
began receiving nilotinib (Tasigna®; Novartis Pharmaceuticals
Corp.) at a dose of 0.4 g twice per day. Following two months of
nilotinib therapy (64 months post-diagnosis), the
BCR-ABL/ABL copy number ratio was 0/7,710 (0%). PCR
sequencing detected no BCR-ABL or ABL kinase region
mutations (Fig. 1B). The application
of TKIs, such as imatinib and nilotinib, was correlated with the
BCR-ABL/ABL copy number ratio (Fig. 2). Over the course of the imatinib
therapy, the patient experienced mild edema of the face, with no
other obvious side effects. The patient suffered one urinary tract
infection over the course of the disease, which was treated with
antibiotics. Sixty-one months after the diagnosis of CML, the
patient was diagnosed with type 2 diabetes and was prescribed
insulin to regulate her blood sugar levels. Over the course of the
nilotinib treatment, the patient also experienced mild edema of the
face with headache and rash, which disappeared following
symptomatic treatment.
Discussion
Resistance to TKIs in patients with CML is often the
result of mutation to the tyrosine kinase domain of the BCR-ABL
protein. To date, there have been 11 reports of the M244V mutation
to the BCR-ABL fusion in the PubMed database (9–19).
There are only five references to imatinib
resistance following BCR-ABL mutations in patients with CML
(9–13). Among the 362 reported cases of
resistance to imatinib in patients with CML, genetic mutations to
the BCR-ABL fusion were observed in 192 cases (53%). Of
these 192 cases, 26 (13.5%) had the M2344V mutation (Table I). Furthermore, three groups have
shown that the first genetic mutation to BCR-ABL is M244V
(10,11,13). Qin
et al (10) found that
genetic mutations to BCR-ABL occurred in 74 out of 127 cases
(58%) of imatinib resistance in patients with CML. Of these, the
M244V mutation occurred in 12 cases (16%). Additionally, one
patient exhibited E355G and Y253H mutations (7). Ernst et al (11) analyzed 95 cases of imatinib
resistance in patients with CML and identified 53 cases (56%) with
BCR-ABL mutations, including six cases (11%) of M244V
mutations. Finally, Bagadi et al (13) analyzed 24 cases of imatinib
resistance in patients with CML and found 14 cases (58%) of
BCR-ABL mutations, including four cases (29%) with M244V
mutations. These data suggest that M244V may play an important role
in imatinib resistance in patients with CML.
 | Table I.M244V BCR-ABL genetic mutations
among patients with imatinib-resistant chronic myelogenous
leukemia. |
Table I.
M244V BCR-ABL genetic mutations
among patients with imatinib-resistant chronic myelogenous
leukemia.
| First author
(reference) | Cases, n | Cases with
BCR-ABL genetic mutations, n/total n (%) | Cases with M244V
mutations, n/total n (%) |
|---|
| Kim (9) | 55 | 32/55 (58) | 3/32 (9) |
| Qin (10) | 127 | 74/127 (58) | 12/74 (16) |
| Ernst (11) | 95 | 53/95 (56) | 6/53 (11) |
| Strhakova (12) | 61 | 19/61 (31) | 1/19 (5) |
| Bagadi (13) | 24 | 14/24 (58) | 4/14 (29) |
| Total | 362 | 192/362 (53) | 26/192 (14) |
To analyze the response of patients with the M244V
mutation to imatinib, Anand et al (20) reviewed the cases of six patients.
Among these patients, increasing doses of imatinib (600–1,000
mg/day) resulted in three patients achieving CCyR. Of these, two
patients were M244V-negative following treatment; however, ~1% of
genetic transcripts contained the BCR-ABL fusion gene.
Furthermore, imatinib was ineffective in the other three cases.
This suggests that the M244V mutation gives rise to imatinib
resistance. Kim et al (9)
observed 55 cases of imatinib resistance in patients with CML, of
which 32 cases (58%) had mutations to the BCR-ABL gene and
three cases (9%) had the M244V mutation. The first patient had both
the M244V and G250E mutations. These mutations disappeared
following treatment with dasatinib for six months. The second case
was a patient with the M244V mutation only. Treatment with
nilotinib for nine months had no effect on the M244V mutation;
however, it did give rise to a T315I mutation. The third case was a
patient that had only the M244V mutation, which had not disappeared
after nine months. No additional mutations were observed in this
patient. Awidi et al (14)
reported 185 cases of CML initially treated with imatinib, of which
21 cases had mutations to BCR-ABL. Of these 21 cases, two
(10%) had the M244V mutation. In one case, the imatinib treatment
was invalid. In the second case, in which both the M244V and G250E
mutations were present, the M244V status was negative following
treatment with nilotinib. The present study described the case of a
patient with imatinib-resistant CML who, after two months of
treatment with nilotinib, no longer had detectable BCR-ABL
fusion genes or M244V mutations. This suggests that nilotinib may
be effective for treating CML cases in which the BCR-ABL
fusion has a M244V mutation; however, the mechanism underlying the
action of nilotinib requires further study.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shanxi Province (grant no. 2013011056-3); the
Science and Technology Development Project of colleges and
universities of Shanxi Province (grant no. 20121013); and the
Scientific Research Subject of the Health Department of Shanxi
Province (grant no. 201202008).
References
|
1
|
Rowley JD: Letter: A new consistent
chromosomal abnormality in chronic myelogenous leukaemia identified
by quinacrine fluorescence and Giemsa staining. Nature.
243:290–293. 1973. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2014 update on diagnosis, monitoring and
management. Am J Hematol. 89:547–556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kantarjian H, Shah NP, Hochhaus A, et al:
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic
myeloid leukemia. N Engl J Med. 362:2260–2270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saglio G, Kim DW, Issaragrisil S, et al:
ENESTnd Investigators: Nilotinib versus imatinib for newly
diagnosed chronic myeloid leukemia. N Engl J Med. 362:2251–2259.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Druker BJ, Guilhot F, O'Brien SG, et al:
IRIS Investigators: Five-year follow-up of patients receiving
imatinib for chronic myeloid leukemia. N Engl J Med. 355:2408–2417.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osborn M and Hughes T: Managing imatinib
resistance in chronic myeloid leukaemia. Curr Opin Hematol.
17:97–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jabbour E, Branford S, Saglio G, et al:
Practical advice for determining the role of BCR-ABL mutations in
guiding tyrosine kinase inhibitor therapy in patients with chronic
myeloid leukemia. Cancer. 117:1800–1811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hochhaus A, La Rosée P, Müller MC, et al:
Impact of BCR-ABL mutations on patients with chronic myeloid
leukemia. Cell Cycle. 10:250–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim WS, Kim D, Kim DW, et al: Dynamic
change of T315I BCR-ABL kinase domain mutation in Korean chronic
myeloid leukaemia patients during treatment with Abl tyrosine
kinase inhibitors. Hematol Oncol. 28:82–88. 2010.PubMed/NCBI
|
|
10
|
Qin Y, Chen S, Jiang B, et al:
Characteristics of BCR-ABL kinase domain point mutations in Chinese
imatinib-resistant chronic myeloid leukemia patients. Ann Hematol.
90:47–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ernst T, Erben P, Müller MC, et al:
Dynamics of BCR-ABL mutated clones prior to hematologic or
cytogenetic resistance to imatinib. Haematologica. 93:186–192.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strhakova L, Bujalkova MG, Hojsikova I, et
al: Use of direct sequencing for detection of mutations in the
BCR-ABL kinase domain in Slovak patients with chronic myeloid
leukemia. Neoplasma. 58:548–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagadi S, Saikia T, Pany A and Das B:
Analysis of ABL kinase domain mutations conferring resistance to
tyrosine kinase inhibitors in chronic myeloid leukemia cases from
India. Clin Lab. 57:619–623. 2011.PubMed/NCBI
|
|
14
|
Awidi A, Ababneh N, Magablah A, et al: ABL
kinase domain mutations in patients with chronic myeloid leukemia
in Jordan. Genet Test Mol Biomarkers. 16:1317–1321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Q, Du X, Zhang QX and Zhuo JC:
Establishment of real-time fluorescent quantitative polymerase
chain reaction for rapid detection of M244V mutation in kinase
domain of BCR-ABL fusion gene. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
22:1728–1734. 2014.(In Chinese). PubMed/NCBI
|
|
16
|
Tanneeru K and Guruprasad L: Ponatinib is
a pan-BCR-ABL kinase inhibitor: MD simulations and SIE study. PLoS
One. 8:e785562013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Preuner S, Mitterbauer G, Mannhalter C, et
al: Quantitative monitoring of BCR/ABL1 mutants for surveillance of
subclone-evolution, -expansion and -depletion in chronic myeloid
leukaemia. Eur J Cancer. 48:233–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Florek I, Sacha T, Zawada M, et al:
Implementation of direct sequencing as a method of ABL gene
mutations analysis in patients with chronic myeloid leukemia
treated with tyrosine kinase inhibitor. Przegl Lek. 67:1292–1297.
2010.(In Polish). PubMed/NCBI
|
|
19
|
Soverini S, Colarossi S, Gnani A, et al:
GIMEMA Working Party on Chronic Myeloid Leukemia: Contribution of
ABL kinase domain mutations to imatinib resistance in different
subsets of Philadelphia-positive patients: by the GIMEMA Working
Party on Chronic Myeloid Leukemia. Clin Cancer Res. 15:7374–7379.
2006. View Article : Google Scholar
|
|
20
|
Anand M, Khorashad J, Marin D, Apperley
JF, Goldman JM and Kaeda JS: Varying response to escalating the
dose of imatinib in patients with CML who ‘acquire’ a BCR-ABL M244V
mutant allele. Blood. 108:2881–2882. 2006. View Article : Google Scholar : PubMed/NCBI
|