Introduction
BK polyomavirus (BKV), which belongs to the genus
Polyomavirus of the family Polyomaviridae, is a
ubiquitous double-stranded DNA virus prevalent in humans. It
remains latent in the urogenital tract or mononuclear cells
following a primary infection during childhood. Its serum positive
rate reaches up to 90% in adults (1–3).
BKV rarely activates in immune-competent
individuals, while it reactivates frequently in renal transplant
recipients under immunosuppressive conditions. At an early stage,
10–60% of transplant recipients exhibit asymptomatic viruria.
Viruria can also be observed at a late stage (4). As BKV DNA is detected in peripheral
blood, it reflects the continuous replication of BKV and the
destruction of targeted cells. A high number of copies of BKV DNA
in the blood is associated with an increased incidence of
BKV-associated nephropathy (BKVN), ureteral stenosis and
hemorrhagic cystitis. Among these, BKVN is the most common. It
mainly manifests as renal tubular necrosis or interstitial
nephritis. Eventually, it results in the loss of graft (5–7).
The BKV genome is a double-stranded DNA that
comprises ~5 kb. It is generally divided functionally into three
parts: An early encoding region that encodes small and large
T-antigens, a late encoding region that encodes the structural
proteins VP1–3, as well as one that encodes agnoprotein (8,9). BKV can
be genotyped by molecular phylogenetic analysis and serological
typing. Based on restriction fragment length polymorphism and
comparisons of partial VP1 sequences, a classification system
comprising four subtypes I–IV has been introduced (10). This system corresponds to a previous
serological typing scheme (11).
According to mutation of the VP1 sequence, subtypes I and IV are
divided further into four subgroups (I/a, I/b-1, I/b-2, I/c) and
six subgroups (IV/a-1, IV/a-2, IV/b-1, IV/b-2, IV/c-1, IV/c-2),
respectively (12,13). BKV subtypes show a specific
geographic distribution worldwide. It is reported that subtype I is
prevalent throughout the world, while subtype IV is prevalent
mostly in East Asia (14). There are
numerous mutation sites in the BKV genome, and the most polymorphic
coding region in the viral genome is VP1. Significant variation is
also present in the large T-antigen gene, wherein polymorphisms are
found in 11.39% of all nucleotide sites (15). BKV polymorphism loci can also be
obtained through comparisons of non-coding control region (NCCR)
regions (16). Studies focusing on
the polymorphism of the VP1 gene and mutations in amino acid
residues encoded by the VP1 gene have developed quickly (17). In the majority of cases, researchers
have opted to reconstruct a phylogenetic tree on the basis of the
VP1 gene (18,19). However, investigation of the BKV VP2
gene has been limited. In 2009, Luo et al conducted the
first genotyping of BKV using the BKV VP2 gene (20). However, considering the variable
genetic background of BKV in that study, whether the conclusions
can be applied to the Chinese population merits investigation.
Studies of single nucleotide polymorphisms (SNPs) and BKV
prevalence in the Chinese population are scarce, and the BKV
genotyping and polymorphism analysis of Chinese samples has rarely
been reported. The principal aims of the present study were to
subtype BKV from a phylogenetic standpoint, identify the conserved
sequence, and determine SNPs in the VP2 gene. Additionally, SNP and
genotyping analysis were conducted specifically on samples from a
Chinese population.
Materials and methods
Patients and samples
A total of 135 adult renal transplant recipients (69
males and 66 females) from the Transplantation Center of the First
Affiliated Hospital of Wenzhou Medical University (Wenzhou, China)
between April 2010 and November 2012 were included. The clinical
samples consisted of 95 urine samples, 23 plasma samples and 17
other samples. They were collected after explaining the purpose of
the research and obtaining written informed consent from the
patients. None of the patients developed nephropathy or renal
transplantation dysfunction during the study. This research was
approved by the Ethical Decision Committee of the Research
Administration at Wenzhou Medical University.
DNA extraction
BKV DNA was extracted from samples using the DNA
Fast 2000 DNA Extraction kit (Shanghai Fastagen Biotechnology Co.,
Ltd., Shanghai, China) according to the manufacturer's
specifications. Centrifugation at 800 × g for 10 min was conducted
to separate 100 µl urinary sediment from 10 ml urine. In addition,
100 µl plasma with leukocytes was extracted from 2 ml whole blood
after standing for 5–15 min. A 100-µl volume was used for other
samples. DNA was then extracted from pretreated samples following
the respective instructions.
Nested polymerase chain reaction (PCR)
and sequencing
Amplification of the VP2 gene fragment was conducted
by nested PCR, using TransStart FastPfu DNA Polymerase (TransGen
Biotech, Beijing, China). Two pairs of specific primers were
designed using Primer Premier 5 software (Premier Biosoft
International, Palo Alto, CA, USA) to select a highly conserved
region of the BKV (BK polyomavirus DNA, complete
genome, isolate: TW-2, GenBank accession number: AB213487.1). The
pair of outer primers was as follows: Upstream primer,
5′-TGGGACCTAGTTGCCAGTGTATC-3′ (676–697) and downstream primer,
5′-AAGAGCAGGTGTTACAGTCCC-3′ (1557–1577). The pair of inner primers
was as follows: Upstream primer, 5′-AAGTTCAAATTGCATCCCT-3′
(766–784) and downstream primer, 5′-TTCTTTGATTAGCACCTCCTG-3′
(1495–1515). The specificity of these primers was tested using the
Primer-BLAST tool provided by the National Center for Biotechnology
Information (NCBI: http://www.ncbi.nlm.nih.gov/), using default
parameters. DNA extracted from clinical specimens was amplified by
nested PCR with the aforementioned primers. Agarose gel
electrophoresis was performed for the relatively qualitative
screening of BKV-positive samples and to judge the
credibility of the PCR product. BKV-positive PCR products
were sequenced by Sun Biotech Co. Ltd. (Beijing, China). The
polymorphic sites of a 684-bp sequence in the VP2 gene were
selected for analysis. The sequence analysis utilized BioEdit
version 7.0.2 (Ibis Biosciences, Carlsbad, CA, USA). DNAMAN version
5.2.10 (Lynnon Biosoft, San Ramon, CA, USA) and DNAStar
bioinformatics software version 7.1 (DNASTAR Inc., Madison, WI,
USA).
Extracted reference sequence of BKV
strains for phylogenetic analysis
Twenty-eight BKV whole-genome sequences, the
majority of which were from China, were obtained from GenBank
(http://www.ncbi.nlm.nih.gov/genbank/). The subtypes
and accession numbers of the 28 virus strains used for the sequence
analysis are shown in Table I.
Corresponding fragments of reference strains in the VP2 gene were
sorted using DNAMAN software.
 | Table I.Reference BKV strains in the
phylogenetic analysis. |
Table I.
Reference BKV strains in the
phylogenetic analysis.
| Virus isolate | Subtype/subgroup | Geographical
origin | GenBank
accession |
|---|
| DUN | I/a | USA | NC_001538 |
| WW | I/b-1 | South Africa | AB211371 |
| DIK | I/b-1 | Netherlands | AB211369 |
| FNL-12 | I/b-2 | Finland | AB263918 |
| JL | I/b-2 | Netherlands | AB211370 |
| SHA-62 | I/c | China | AB365175 |
| SHA-70 | I/c | China | AB365176 |
| FUJ-4 | I/c | China | AB369095 |
| FUJ-6 | I/c | China | AB269826 |
| GBR-12 | II | England | AB263920 |
| ETH-3 | II | Ethiopia | AB263916 |
| AS | III | England | M23122 |
| SEC-3 | IV/a-1 | China | AB269860 |
| SHA-43 | IV/a-1 | China | AB365171 |
| RYU-3 | IV/a-2 | Japan | AB211389 |
| FUJ-13 | IV/a-2 | China | AB269826 |
| THK-8 | IV/b-1 | Japan | AB211390 |
| TW-3 | IV/b-1 | Japan | AB211391 |
| KOM-2 | IV/b-2 | Japan | AB211387 |
| JPN-15 | IV/b-2 | Japan | AB269834 |
| FUJ-32 | IV/c-1 | China | AB269828 |
| SHA-78 | IV/c-1 | China | AB365178 |
| NEC-4 | IV/c-1 | China | AB269854 |
| NWC-8 | IV/c-1 | China | AB269858 |
| NWC-14 | IV/c-1 | China | AB269855 |
| SWC-1 | IV/c-1 | China | AB269863 |
| SWC-2 | IV/c-1 | China | AB269864 |
| SWC-4 | IV/c-1 | China | AB269865 |
Phylogenetic analysis
Using phylogenetic analysis, VP2 sequences of BKV
from the 135 specimens were aligned with the 28 recently published
sequences of reference strains using NCBI BLAST, DNAMAN and
ClustalX version 2.1 software (University College Dublin, Dublin,
Ireland). Following the alignment, sequence analysis was performed
with ClustalX software. Neighbor joining (NJ) and an un-weighted
pair-group method with arithmetic means (UPGMA) trees were
constructed using MEGA software, version −5.03 (Mega Software,
Philadelphia, PA, USA). To estimate the confidence level of the
branching patterns of the tree, bootstrap resampling tests were
performed 1,000 times.
SNP analysis of urine and plasma
samples in a Chinese population
For SNP analysis, 36 reference strains isolated from
a population in China were extracted from GenBank. They included 18
sequences of subtype I and 18 sequences of subtype IV. The 118
specimen strains from plasma or urine (samples from other sources
were excluded) together with 36 reference strains were analyzed to
determine the SNP positions using DNAMAN software. These positions
can be used to distinguish between subtypes I and IV in China. The
subtypes and accession numbers of the 36 reference strains are
shown in Table II.
 | Table II.Extracted reference BKV sequences
from China. |
Table II.
Extracted reference BKV sequences
from China.
| Strain | Subtype | Sample source | GenBank
accession |
|---|
| FUJ-13 | IV | Urine | AB269826 |
| NEC-15 | IV | Urine | AB269856 |
| NEC-7 | I | Urine | AB365144 |
| NEC-12 | I | Urine | AB263930 |
| NWC-7 | IV | Urine | AB269857 |
| NWC-14 | IV | Urine | AB269855 |
| SEC-3 | IV | Urine | AB269860 |
| SEC-21 | IV | Urine | AB269862 |
| SHA-7 | I | Urine | AB365159 |
| SHA-10 | I | Urine | AB365161 |
| SHA-22 | I | Urine | AB365164 |
| SHA-25 | I | Urine | AB365166 |
| SHA-30 | IV | Urine | AB365168 |
| SHA-41 | I | Urine | AB365170 |
| SHA-47 | IV | Urine | AB365172 |
| SHA-56 | I | Urine | AB365174 |
| SHA-72 | I | Urine | AB365177 |
| SWC-2 | IV | Urine | AB269864 |
| NEC-4 | I | Urine | AB269854 |
| NEC-22 | IV | Urine | AB365146 |
| NEC-8 | I | Urine | AB263931 |
| NEC-14 | IV | Urine | AB269852 |
| NWC-8 | IV | Urine | AB269858 |
| NWC-15 | IV | Urine | AB269856 |
| SEC-6 | IV | Urine | AB269861 |
| SHA-4 | I | Urine | AB365158 |
| SHA-13 | I | Urine | AB365162 |
| SHA-19 | I | Urine | AB365163 |
| SHA-23 | I | Urine | AB365165 |
| SHA-28 | IV | Urine | AB365167 |
| SHA-40 | I | Urine | AB365169 |
| SHA-43 | IV | Urine | AB365171 |
| SHA-55 | IV | Urine | AB365173 |
| SHA-62 | I | Urine | AB365175 |
| SWC-1 | IV | Urine | AB269863 |
| SWC-4 | IV | Urine | AB269865 |
Statistical analysis
All statistical analyses were conducted using SPSS
software, version 18.0 (IBM SPSS, Armonk, NY, USA). Differences in
the viral subtypes obtained from plasma and urine samples were
compared using Fisher's exact test. P<0.05 was considered to
indicate a statistically significant result.
Results
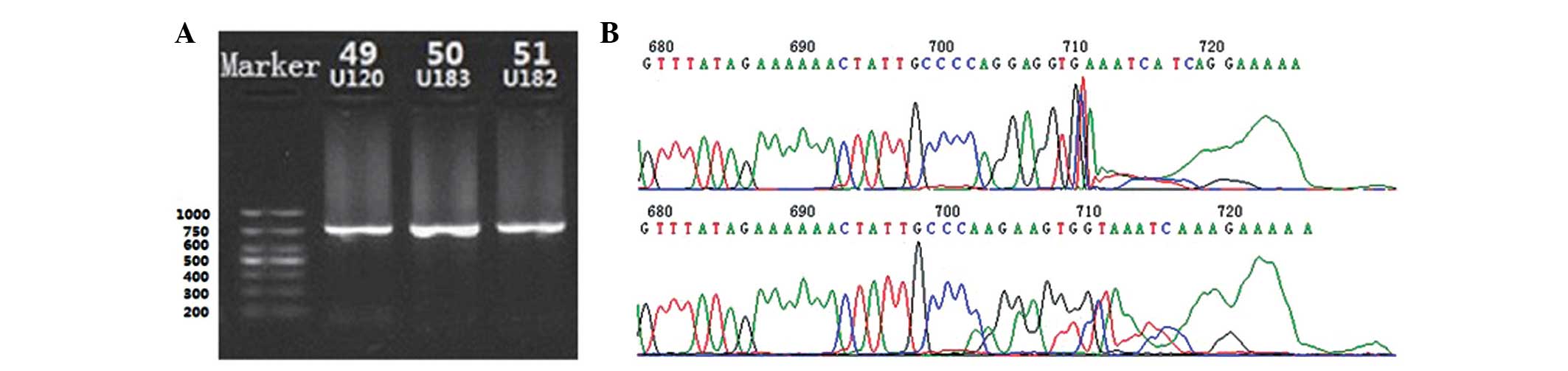
Amplification using nested PCR
Following amplification by nested PCR with an inner
and outer primer, corresponding BKV VP2 gene products with a total
length of 750 bp were obtained. The products were verified by
agarose gel electrophoresis (Fig.
1A). DNA sequencing results confirmed that the products were
the target gene (Fig. 1B).
Phylogenetic analysis
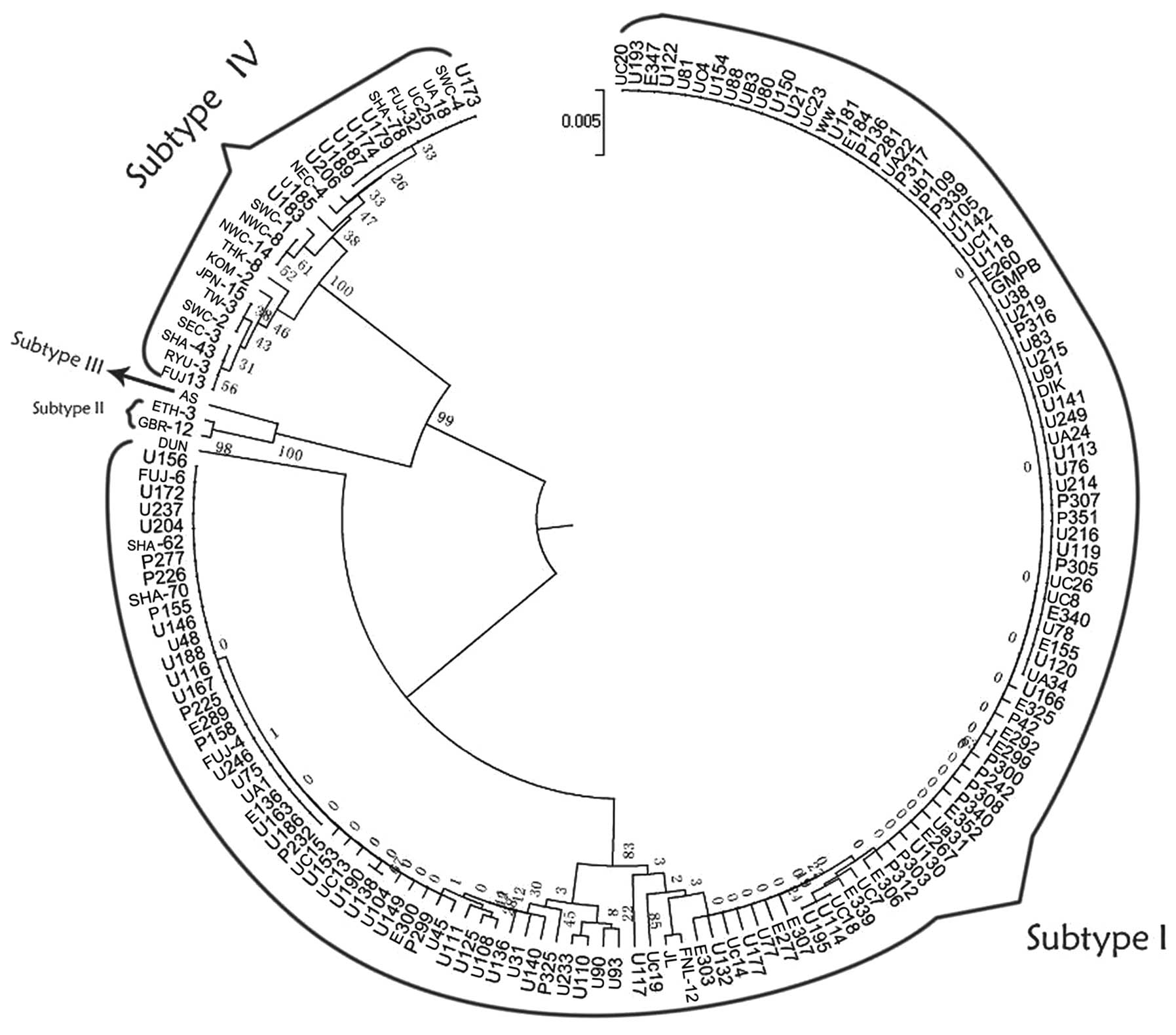
UPMGA method
A UPGMA phylogenetic tree (Fig. 2) was constructed for partial segments
of the VP2 gene and the 163 strains (135 sample strains and 28
reference strains) were grouped into three major branches. Two
primary branches had high bootstrap probabilities (BPs), both
reaching 99%. Based on the locations of reference strains, the
majority of the samples belonged to the largest branch on the
phylogenetic tree (subtype I), whereas the rest were ascribed to
the other branch (subtype IV). The results were confirmed to have
high reliability and were consistent with serology and the
genotyping consequences of other gene segments.
The largest branch included the reference strains
DIK, WW, JL, FNL-12, FUJ-4, FUJ-6, SHA-62, SHA-70 and DUN, which
have conventionally been classified as subtype I. DUN belongs to
subgroup Ia, as reported previously (19). Subtype Ia was distant from the other
clusters at the evolutionary distance shown in Fig. 2. These strains could then be
subdivided into corresponding subgroups, for example, DIK and WW
belonging to subgroup Ib-1. However, certain clinical isolated
strains, for example U93 and U90, could not be classified within
any subgroup; the BP values of certain strains, such as UC20 and
U193, were as low as 0. These strains indicate the poor credibility
of the subgroups when divided.
Another large branch contained reference strains
such as FUJ-13 and RYU-3, which were attributed to subtype IV. The
bootstrap value of each clade ranged from 25 to 61%, and typing
credibility was low. SHA-78 and SWC-4 (IV/c-1) were subdivided into
one group, but with a low BP value (32%). By analyzing tiny
arborizations of this branch, it was found that it was not possible
to precisely classify many of the identified reference strains into
correct clades. For example, SEC-3 (IV/a-1), SHA-43 (IV/a-1), RYU-3
(IV/a-2) and FUJ-13 (IV/a-2) clustered in one clade. NEC-4 and
NWC-14 (IV/c-1) did not cluster together although they belonged to
the same subgroup.
The small cluster in the subtype IV branch included
two more detailed clades: One comprised strains GBR-13 and ETH-3,
and the other was AS. The former belonged to subtype II. The latter
belonged to subtype III. According to the constructed phylogenetic
tree, there is a tight evolutionary link between subtype II and
III. There were, however, no clinical isolated strains in the
cluster.
In the genotyping results, all 23 plasma samples
belonged to subtype I. There were 82 samples of subtype I and 13 of
subtype IV in 95 urine samples. Thus, there were certain
statistical differences between the subtype distribution in the
blood and urine samples (P=0.082).
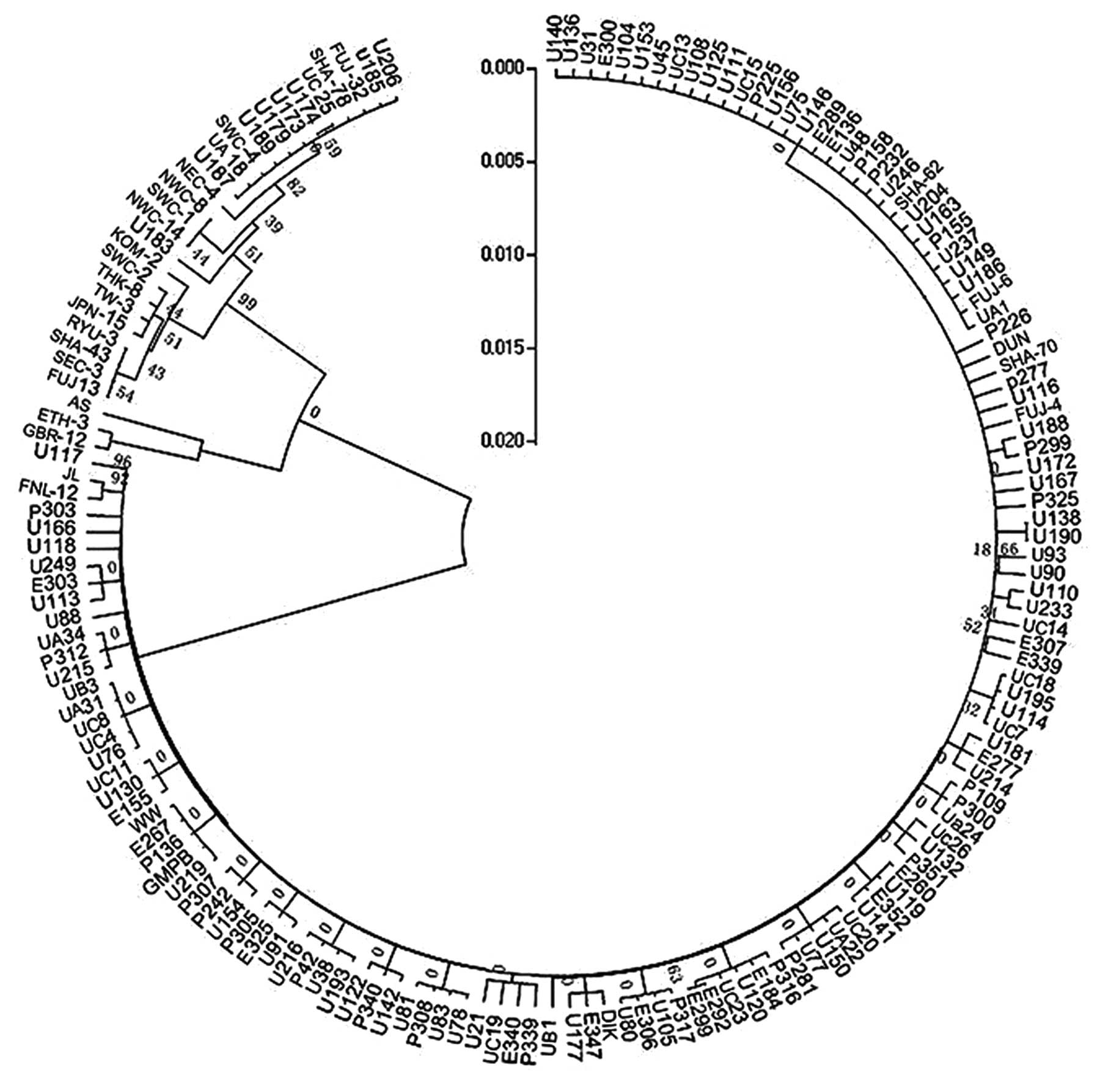
NJ method
A phylogenetic tree (Fig.
3) was constructed with the NJ method for partial segments of
the VP2 gene. Isolates that were analyzed could be divided into two
main clades by this tree. However, the BPs values of major branches
ranged from 0 to 17%. This indicated the low reliability of the
construction results. The confidence of subgroups in the first
branch was verified. The bootstrap value was 0. Among three
subclusters from the second branch, the third bootstrap value was
99%. Other grouped isolates shared BP values from 7 to 82%. In
addition, reference strains of the same subgroup could not be
distributed into the same branch, whereas different ones were
distributed into the same cluster.
Application of SNP in specimen strains
derived from plasma and urine in genotyping
SNP sites and ubiquitous mutations in Chinese
strains
BKV sequences from plasma and urine samples were
aligned with 36 reported viral strains obtained from Chinese
sources, and 92 SNP sites were identified in total (Table III). In addition, there were 11
SNPs (Table IV) existing in all of
them as hot spots. This indicates that these sites may be prevalent
in a Chinese population. Among these common SNP sites, G889C,
A922G, T1146G, C1272G, G930A and G931A could lead to changes in
amino acid residues. The specific transformations are as follows:
Serine to threonine (TAG→TAC), lysine to arginine (AAG→AGG), serine
to alanine (TCT→GCT), glutamine to glutamic acid (CAA→GAA), and
aspartic acid to serine (GAT→AGT). All the aforementioned changes
are sense mutations. A905G, G1160A, C1023T, T1049A and G1169A are
synonymous mutations.
 | Table III.Nucleotide polymorphisms of the VP2
gene segment. |
Table III.
Nucleotide polymorphisms of the VP2
gene segment.
| Position | Subtype I | Subtype IV |
|---|
|
790 | A | G/A |
|
795 | A | T/A |
|
811 | T | C/T |
|
848 | T | A/T |
|
856 | T | G/T |
|
889 | G | C |
|
894 | A | G/A |
|
899 | T | G/T |
|
902 | C | T/C |
|
905 | A | G |
|
908 | T | C/T |
|
922 | A | G |
|
926 | C | T/C |
|
930 | G | A |
|
931 | A | G |
|
935 | T | C/T |
|
965 | C | G/T/C |
|
970 | A | G/A |
|
975 | T | C/T |
|
986 | T | A/T |
|
989 | G | A/G |
|
990 | G | A/G |
|
996 | T | G/T |
| 1015 | A | G/A |
| 1023 | C | T |
| 1027 | T | C/T |
| 1043 | T | A/T |
| 1049 | T | A |
| 1067 | T | A/T |
| 1068 | G | A/G |
| 1078 | A | G/A |
| 1079 | T | G/A |
| 1083 | G | A/G |
| 1091 | T | C/T |
| 1146 | T | G |
| 1154 | C | A/T/C |
| 1160 | G | A |
| 1166 | G | A/G |
| 1169 | G | G |
| 1174 | G | A/G |
| 1181 | A | G/A |
| 1182 | A | T/A |
| 1187 | T | C/T |
| 1189 | T | C/T |
| 1191 | A | G/A |
| 1200 | T | C/T |
| 1208 | A | G/A |
| 1217 | G | A/G |
| 1226 | C | T/C |
| 1248 | A | G/A |
| 1271 | A | G/A |
| 1272 | C | G |
| 1274 | A | T/A |
| 1284 | G | A/G |
| 1287 | C | T/C |
| 1289 | T | G/T |
| 1295 | T | C/T |
| 1300 | G | A/G |
| 1304 | C | T/C |
| 1316 | A | G/A |
| 1322 | A | G/T/A |
| 1337 | T | A/ |
| 1342 | G | A/G |
| 1343 | T | G/T |
| 1345 | T | C/T |
| 1347 | C | A/C |
| 1361 | T | C/T |
| 1364 | T | C/T |
| 1367 | T | A/T |
| 1385 | T | G/T |
| 1388 | A | G/A |
| 1389 | G | C/G |
| 1392 | G | A/G |
| 1400 | A | C/A |
| 1407 | A | T/A |
| 1408 | T | G/T |
| 1412 | C | G/C |
| 1422 | C | A/C |
| 1425 | C | G/C |
| 1427 | A | G/A |
| 1429 | G | A/C/G |
| 1431 | G | A/G |
| 1440 | G | T/G |
| 1441 | G | C/G |
| 1446 | T | A/T |
| 1449 | A | T/A |
| 1450 | T | A/C/T |
| 1451 | A | G/A |
| 1452 | G | A/G |
| 1457 | A | G/A |
| 1458 | A | G/A |
| 1465 | C | T/C |
 | Table IV.Prevalent SNP sites in Chinese viral
strains. |
Table IV.
Prevalent SNP sites in Chinese viral
strains.
| Position | 889 | 905 | 922 | 930 | 931 | 1023 | 1049 | 1146 | 1160 | 1169 | 1272 |
|---|
| Standard
strain | G | A | A | G | A | C | T | T | G | G | C |
| Specimen | C | G | G | A | G | T | A | G | A | A | G |
SNP sites for distinguishing subtype I and
IV
On the basis of the phylogenetic tree, alignment on
a partial fragment of the VP2 gene at the nucleotide level was
conducted with the aforementioned 154 strains. Thirty SNP sites
reflected the difference between subtype I and IV in a Chinese
population. These sites are clearly able to distinguish BKV
subtypes I and IV (Table V).
 | Table V.Thirty sites to distinguish subtypes
I and IV. |
Table V.
Thirty sites to distinguish subtypes
I and IV.
| Position | Subtype I | Subtype IV |
|---|
|
848 | T | C |
|
902 | C | T |
|
965 | C | T |
|
986 | T | A |
| 1067 | A | A |
| 1154 | C | A |
| 1181 | A | G |
| 1187 | T | C |
| 1217 | G | A |
| 1248 | A | G |
| 1274 | A | T |
| 1284 | G | A |
| 1287 | C | T |
| 1316 | C | T |
| 1304 | A | G |
| 1322 | G | T |
| 1337 | A/G | A |
| 1342 | T | A |
| 1343 | G | G |
| 1347 | T | A |
| 1361 | C | C |
| 1364 | T | C |
| 1367 | T | A |
| 1389 | G | C |
| 1400 | A | C |
| 1412 | C | G |
| 1422 | C | A |
| 1425 | C | G |
| 1427 | A | G |
| 1429 | G/C | A |
Discussion
BKV is the only polyomavirus reported to be divided
into four subtypes on the basis of serological and genotyping
methods. Four subgroups within subtype I (I/a, I/b-1, I/b-2, I/c)
and six subgroups within subtype IV can be further distinguished
with nucleotide variation (10,21).
Previous studies describe basically four genotyping schemes
associated with the phylogenetic construction, where the genotyping
is: i) based on the whole BKV genome; ii) based on the complete BKV
VP1 gene; iii) based on a part of the BKV VP1 gene; and iv) based
on the complete BKV large T antigen (LTA) gene (16,22,23).
However, the contributory role of the VP2 gene in genotyping has
not been well examined. According to the phylogenetic tree in the
present study, the VP2 gene was able to resolve the subtypes, but
not the subgroups.
The 940-bp VP2 gene belongs to the late coding
region. It encodes viral capsid protein VP2 (9). A complete VP2 gene was first used for
genotyping by Luo et al in 2009 (20), and was demonstrated to enable the
four subtypes of BKV to be classified, but not their subdivision
into subgroups. The results of the present study, using a VP2 gene
fragment, are in accordance with those of the study by Luo et
al (20). Similar phylogenetic
analysis on other regions beyond the VP2 gene or genetic
polymorphism analysis is required to differentiate subgroups from
subtypes. In the process of phylogenetic analysis, the present
study found that the NJ method was not able to produce a proper
phylogenetic tree, and indicates that the UPMGA method is better
for genotyping. The NJ method is commonly adopted for the VP1 gene
(18,19). Therefore, the analysis conducted in
the present study indicates that various means of construction
should be put into use according to the target gene. It is notable
that genotyping based on the VP1 gene yields a clearer definition
of subgroups and shorter gene fragments (~300 bp) (22,24).
This indicates that VP1 carries more information than the VP2 gene.
The VP2 gene failed to subdivide strains into subgroups in the
present study of Chinese samples, restricting its further
application in BKV genotyping.
Numerous researchers have shed light on the
association between the distribution of BKV subtypes and patient
populations (14,25,26). The
results of these studies have indicated that subtype I predominates
in all geographical regions, while subtype IV occurs at lower rates
and mostly in East Asia, and subtypes II and III rarely occur.
These results are consistent with the observations of the present
study. The distribution pattern of BKV subtypes clarified in a
study conducted by Zhong et al showed that the frequency of
subtype IV was variable among populations, and in northeast USA,
Finland, Japan and China, and its frequency ranged between 17 and
36% (27). In the present study,
subtype IV accounted for only 7% of the samples, and the study
included a greater number of clinical samples than most previous
research. The small proportion of subtype IV identified in the
present study is inconsistent with the co-migration hypothesis of
Zhong et al which suggests that BKV migrates with human race
and the proportion of subtype IV in East Asia is relatively
large.
With the evident regional characteristics of BKV
gene polymorphism, its patterns of mutations differ from place to
place. In order to reveal the unique patterns at the nucleic acid
level in China, a large-sample investigation among the Chinese
population would be of tremendous significance. Therefore, to
further this effort, using the phylogenetic tree with UPGMA method,
the present polymorphism study targeting BKV strains in China was
performed.
A total of 11 SNP sites that are prevalent in BKV
isolated from Chinese individuals were identified. Only four sites,
1023, 1146, 1169 and 1272, correspond with those in the global
range outcomes reported by Luo et al (20). Specific SNPs for the identification
of subtype I or IV in a Chinese population were also analyzed. The
specific SNPs that were identified include many previously reported
SNPs, but not all of them. Based on this finding, it is notable
that BKV in the Chinese population may evolve and mutate in a
particular pattern. With an appropriately chosen SNP as a target,
defining subgroups would not require sequencing of the entire gene,
and would be more simple and rapid, providing an alternative to BKV
genotyping. Ultimately, the reliability of phylogenetic
reconstruction depends on the accuracy of the algorithm, and no
matter what method is adopted, deviation exists (28). The application of SNP genotyping can
avoid this problem. The present study of the features and
genotyping of BKV in China included a greater number of clinical
samples than were included in the majority of studies in this
field, and so should be reliable. However, limitations also exist,
as the samples were collected from a localized area. Additional
sequences from other areas in China are required to confirm the
results.
Precise and accurate detection of BKV offers
technical support for the monitoring of BKV infection status and
the prophylaxis of BKVN in renal transplant recipients. With regard
to mutations of the BKV genome, nucleotide-based testing would not
detect all BKV DNA. The gold standard of quantitative PCR and a
standard substance remain unavailable. The separated conserved
region of the VP2 gene may be useful as a new target for PCR or a
specific probe assay.
Since the discovery of BKV in 1971 (29), its pathogenicity, virulence, and the
mechanism of reactivation have been actively studied. The initial
manifestation of BKV reactivation following renal transplantation
is asymptomatic viruria. The subsequent viremia and overt
nephropathy may culminate in graft loss (30). In earlier studies, researchers
conjectured that the severity of infection and viral spread had a
close correlation with certain mutants in the amino acid residues
of the protein (31). In the present
study, it was found that with the exception of a few positions, the
majority of the SNPs did not result in variations in amino acids.
Whether these mutants influence the expression level of the VP2
protein and cause the change in pathogenicity remains to be
elucidated in further research. In the present study, the BKV
isolated from blood samples was all of subtype I, while subtype IV
was only extracted from urine. It is conceivable that the imbalance
in subtypes may have a close affinity to viral virulence or can be
accounted for by different stages of infection. Another theory
suggests that the intensity of immunosuppression and genetic
susceptibility of the host immune system are the principal
determinants of BKV-mediated tissue injury in the kidney (32). To verify the validity of these
assumptions, an innovative experimental design and new experimental
methods are urgently required.
In summary, the construction of a phylogenetic tree
based on the VP2 gene of BKV was able to divide BKV into four
subtypes, but not into subgroups. Certain prevalent SNPs of BKV in
a Chinese population were isolated. The specific positions at which
SNPs occur could be used for the identification of subtypes I and
IV. A conserved sequence within the VP2 gene was identified.
Further studies involving novel genotyping methods may lead to
accurate identification of BKV subtypes and elucidate the genetic
diversity of BKV.
Acknowledgements
The authors thank Hui-Ying Xu for editing the
manuscript. The study was supported by a grant for surgical
nutrition from the Top Key Discipline and Technology Foundation of
Zhejiang Province (No. 2013C37046). The study was also supported by
the Zhejiang Provincial Undergraduate Scientific and Technological
Innovation Project and Fresh Talent Program. (No. 2014R413014).
References
|
1
|
Martini F, Iaccheri L, Lazzarin L, et al:
SV40 early region and large T antigen in human brain tumors,
peripheral blood cells and sperm fluids from healthy individuals.
Cancer Res. 56:4820–4825. 1996.PubMed/NCBI
|
|
2
|
De Mattei M, Martini F, Corallini A, et
al: High incidence of bk virus large-T-antigen-coding sequences in
normal human tissues and tumors of different histotypes. Int J
Cancer. 61:756–760. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bechert CJ, Schnadig VJ, Payne DA and Dong
J: Monitoring of BK viral load in renal allograft recipients by
real-time PCR assays. Am J Clin Pathol. 133:242–250. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Randhawa P and Brennan D: BK virus
infection in transplant recipients: an overview and update. Am J
Transplant. 6:2000–2005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drew RJ, Walsh A, Laoi Ní B, Conneally E
and Crowley B: BK virus (BKV) plasma dynamics in patients with
BKV-associated hemorrhagic cystitis following allogeneic stem cell
transplantation. Transpl Infect Dis. 15:276–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirsch HH, Brennan DC, Drachenberg CB, et
al: Polyomavirus-associated nephropathy in renal transplantation:
Interdisciplinary analyses and recommendations. Transplantation.
79:1277–1286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moens U and Rekvig OP: Molecular biology
of BK virus and clinical and basic aspects of BK virus renal
infectionHuman polyomaviruses: molecular and clinical perspectives.
Khalili K and Stoner GL: John Wiley & Sons; New York, NY: pp.
359–408. 2001, View Article : Google Scholar
|
|
8
|
Cole CN and Conzen SD: Polyomaviridae: The
viruses and their replicationFields Virology. Knipe DM and Howley
PM: 4th. (Lippincott Williams and Wilkins). Philadelphia, PA: pp.
2141–2174. 2001
|
|
9
|
Seif I, Khoury G and Dhar R: The genome of
human papovavirus BKV. Cell. 18:963–977. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin L: Rapid genomic typing of BK virus
directly from clinical specimens. Mol Cell Probes. 7:331–334. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knowles WA, Gibson PE and Gardner SD:
Serological typing scheme for BK-like isolates of human
polyomavirus. J Med Virol. 28:118–123. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stoner GL, Alappan R, Jobes DV,
Ryschkewitsch CF and Landry ML: BK virus regulatory region
rearrangements in brain and cerebrospinal fluid from a leukemia
patient with tubulointerstitial nephritis and meningoencephalitis.
Am J Kidney Dis. 39:1102–1112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng HY, Nishimoto Y, Chen Q, et al:
Relationships between BK virus lineages and human populations.
Microbes Infect. 9:204–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishimoto Y, Zheng HY, Zhong S, et al: An
Asian origin for subtype IV BK virus based on phylogenetic
analysis. J Mol Evol. 65:103–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma PM, Gupta G, Vats A, Shapiro R and
Randhawa P: Phylogenetic analysis of polyomavirus BK sequences. J
Virol. 80:8869–8879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drew RJ, Walsh A, Laoi BN and Crowley B:
Phylogenetic analysis of the complete genome of 11 BKV isolates
obtained from allogenic stem cell transplant recipients in Ireland.
J Med Virol. 84:1037–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo C, Hirsch HH, Kant J and Randhawa P:
VP-1 quasispecies in human infection with polyomavirus BK. J Med
Virol. 84:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boukoum H, Nahdi I, Foulongne V, et al:
Distribution of BK polyomavirus genotypes in Tunisian renal
transplant recipients. J Med Virol. 83:725–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slavov S, Tsekov I and Kalvatchev Z:
Sequence variations of the VP1 gene of Polyomavirus hominis 1 among
Bulgarians. J Med Virol. 82:325–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo C, Bueno M, Kant J, Martinson J and
Randhawa P: Genotyping schemes for polyomavirus BK, using
gene-specific phylogenetic trees and single nucleotide polymorphism
analysis. J Virol. 83:2285–2297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikegaya H, Saukko PJ, Tertti R, et al:
Identification of a genomic subgroup of BK polyomavirus spread in
European populations. J Gen Virol. 87:3201–3208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin L and Gibson PE: Genomic function and
variation of human polyomavirus BK (BKV). Rev Med Virol. 6:201–214.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith RD, Galla JH, Skahan K, et al:
Tubulointerstitial nephritis due to a mutant polyomavirus BK virus
strain, BKV (Cin), causing end-stage renal disease. J Clin
Microbiol. 36:1660–1665. 1998.PubMed/NCBI
|
|
24
|
Takasaka T, Goya N, Tokumoto T, et al:
Subtypes of BK virus prevalent in Japan and variation in their
transcriptional control region. J Gen Virol. 85:2821–2827. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledesma J, Bouza E, González-Nicolás M, de
Viedma DG, Rodríguez-Sánchez B and Muñoz P: BK polyomavirus
genotyping at inter-and intra-patient level in Spain. J Med Virol.
85:1402–1408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yogo Y, Zhong S, Xu Y, et al: Conserved
archetypal configuration of the transcriptional control region
during the course of BK polyomavirus evolution. J Gen Virol.
89:1849–1856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong S, Randhawa PS, Ikegaya H, et al:
Distribution patterns of BK polyomavirus (BKV) subtypes and
subgroups in American, European and Asian populations suggest
co-migration of BKV and the human race. J Gen Virol. 90:144–152.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WH: Simple method for constructing
phylogenetic trees from distance matricesProc Natl Acad Sci. USA:
78. pp. 1085–1089. 1981, View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gardner SD, Field AM, Coleman DV and Hulme
B: New human papovavirus (BK) isolated from urine after renal
transplantation. Lancet. 1:1253–1257. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chehadeh W and Nampoory MR: Genotypic
diversity of polyomaviruses circulating among kidney transplant
recipients in Kuwait. J Med Virol. 85:1624–1631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Randhawa P, Kant J, Shapiro R, Tan H, Basu
A and Luo C: Impact of genomic sequence variability on quantitative
PCR assays for diagnosis of polyomavirus BK infection. J Clin
Microbiol. 49:4072–4076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carr MJ, McCormack GP, Mutton KJ and
Crowley B: Unique BK virus non-coding control region (NCCR)
variants in hematopoietic stem cell transplant recipients with and
without hemorrhagic cystitis. J Med Virol. 78:485–493. 2006.
View Article : Google Scholar : PubMed/NCBI
|