Introduction
Lung cancer is a global public health issue. In the
USA, cancer of the lung and bronchus represents 13.5% of all new
cancer cases. The estimated number of new cases for 2014 in the USA
is 224,210 and the estimated number of patients that will succumb
to these diseases is 159,260 (1).
The US National Cancer Institute defines targeted cancer therapies
as drugs or other substances that interfere with specific molecules
associated with cancer cell growth and survival. Targeted cancer
therapies that have been approved for use include agents that
prevent cell growth signaling, interfere with the development of
tumor-supplying blood vessels, induce cancer cell death, stimulate
the immune-mediated destruction of cancer cells and/or deliver
toxic drugs to cancer cells. These therapies are frequently
cytostatic, blocking tumor cell proliferation. At present, targeted
therapies are the focus of copious anticancer drug development
research efforts, which aim to identify of targets that are crucial
to cancer cell growth and survival (2).
The US Food and Drug Administration (FDA) approved
coumarin as an orphan drug for the treatment of renal cell
carcinoma on December 22, 1994 (3).
The name coumarin is derived from the word coumarou, an alternative
name for the tonka bean (Dipteryx odorata Willd., Fabaceae).
Chemically, coumarins have a benzopyrone structure. Umbelliferone,
esculetin and scopoletin are the most widespread coumarins in
nature (4). Coumarins have been
investigated as potential treatments for various clinical
conditions, such as high protein edema (5), chronic infections (6,7) and
cancer (8–10). The apoptogenic properties of
coumarins have attracted intense interest in recent years. The
induction of apoptosis by natural (11–18) and
synthetic (19–21) coumarins has been reported in human
leukemia cells, lung carcinoma cell lines, adipocytes, HeLa cells,
hepatocellular carcinoma, human neuroblastoma cell lines and human
prostate cancer cell lines. The induction of apoptosis occurs via
mitochondrial pathways, including the modulation of the NF-κB,
mitogen-activated protein kinase (MAPK) and p53 pathways, which
subsequently activate caspase-3 (C-3)-dependent mechanisms. The
downregulation of Rho GTPases (RhoGDIα) by a coumarin derivative
through transcriptomic and proteomic mechanisms (22) has been described. A previous study
(12) observed that the A427 lung
carcinoma cell line exhibited increases in the proportion of
Annexin-V-positive cells of 50 and 83% compared with
solvent-treated cells (estimated using flow cytometry), when
exposed to 100 µg/ml coumarin and 7-hydroxycoumarin (7-HC),
respectively, for 4 h.
The aim of the present study was to determine
whether changes in C-3 activity are induced in a single live A549
human lung carcinoma cell by treatment with 7-HC, the primary human
biotransformation product of coumarin (23), by performing the single-cell
microinjection of a C-3 substrate.
Materials and methods
Reagents
A549 lung carcinoma cells (CRM-CCL-185) were
obtained from American Type Culture Collection (Rockville, MD,
USA). Ionomycine and RPMI-1640 medium were purchased from Gibco
Life Technologies (Carlsbad, CA, USA). Fetal bovine serum (FBS) was
obtained from GE Healthcare Life Sciences (Logan, UT, USA). MTT,
5-bromo-4-chloro-3-indolyl phosphate/nitro-blue tetrazolium
chloride (BCIP/NBT), ethylene glycol-bis (β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid (EGTA) tetrasodium salt and a
caspase-3 colorimetric assay kit were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Monoclonal anti-caspase 3 clone 4–1–18
(#MA1-16843) and monoclonal anti-poly (ADP-ribose) polymerase
(PARP) clone 123 antibodies (#43600) were obtained from Zymed
Laboratories, Inc. (San Francisco, CA, USA). Rhodamine 110,
bis-(N-CBZ-L-aspartyl-L-glutamyl-L-valyl-L-aspartic acid
amide) (Z-DEVD-R110) was purchased from Invitrogen Life
Technologies (Camarillo, CA, USA). Borosilicate glass capillaries
(1B100F-4) were obtained from World Precision Instruments, Ltd.
(Sarasota, FL, USA). Dextran-Texas Red [3,000 molecular weight
(MW)], dextran-fluorescein isothiocyanate (FITC) (3,000 MW) and
Fura-2AM dyes were purchased from Molecular Probes Life
Technologies (Carlsbad, CA, USA). Tissue culture flasks (80
cm2) and 6-well multidishes were obtained from Nunc A/S
(Roskilde, Denmark). A 35×10-mm polystyrene dish was purchased from
Corning, Inc. (New York, NY, USA).
Cell culture
A549 human lung carcinoma cells were cultured at
37°C in 5% CO2 using RPMI-1640 medium, supplemented with
a 10% heat-inactivated FBS. Cells were sub-cultured through
trypsinization, seeded at 2×105 cells/well in 6-well
boxes or Petri dishes, and left to affix for 24 h prior to exposure
to ethanol or a solution of 7-HC in ethanol. In each case the final
solvent concentration was 3%.
Cell viability assay
The viability of the cell line was determined using
an MTT assay (24). Cells were
seeded at a density of 5×103 cells/100 µl RPMI-1640 in a
96-well microplate. Cells were treated with ethanol (control) or a
solution of 7-HC in ethanol (1.85 mM) for 24 h. The final
concentration of ethanol was 3% (v/v). The number of viable cells
was estimated by treatment with 20 µl/well MTT (5 mg/ml) for 4 h,
enabling the mitochondrial succinate dehydrogenase in viable cells
to reduce MTT to purple formazan crystals. The medium was aspirated
and 100 µl dimethyl sulfoxide (DMSO)/well was added. Crystals were
dissolved in DMSO and the absorbance at 570 nm was measured using
an ELx800 microplate reader (Biotek Instruments, Inc., Winooski,
VT, USA). The percentage inhibition of cell viability (%IC) was
measured using the following formula: %IC = [(1 - absorbance of
cells treated with 7-HC in ethanol/absorbance of cells in ethanol)
× 100].
Colorimetric assay to determine the
time course and concentration response of C-3 activity
To evaluate the concentration response, A549 cells
were exposed to ethanolic solutions of 7-HC (0.61, 1.23 or 1.85 mM)
for 24-h with three replicates per concentration. In order to
determine the time course, A549 cells were exposed to a 1.85-mM
ethanolic solution of 7-HC for 6, 12 or 24 h. Cell lysates were
obtained (as described below) and an endpoint colorimetric method
based on peptide hydrolysis (Ac-DEVD-pNA) was employed (Novex
Caspase-3 Colorimetric Protease Assay Kit; Thermo Fisher
Scientific, Waltham, MA, USA) and the absorbance at 405 nm was
measured using an ELx800 microplate reader. The mean value of the
solvent-treated cell absorbance was calculated for use as a basal
reference. Response-increase percentages of the different drug
exposures were determined and plotted as the C-3 enzymatic activity
index: Ratio of C-3 activity = [optical density (OD) treatment/OD
control treatment] × 100.
Protein isolation and
quantitation
Following each cell treatment, cell lysates were
obtained using a lysis buffer containing 20 mM Tris, 150 mM NaCl, 1
mM NaOH, 1 mM ethylenediaminetetraacetic acid, 1 mM EGTA and 1%
Triton X-100. The protein concentration was estimated using a Micro
BCA Protein assay kit (Pierce Biotechnology, Inc., Rockford, IL
USA).
Concentration-response assays
In 6-well multidishes 1.5×106 A549 cells
were let to affix in three RPMI-1640 replicates, then exposed to
0.3, 0.6, 0.92 or 1.85 mM 7-HC or ethanol for 24 h.
Time course assays
Next, 1.5×106 A549 cells were
triplicated, then exposed to either ethanol or 1.85 mM
ethanol-dissolved 7-HC for 6, 12, 18 or 24 h. In each case the
final solvent concentration was 3%. The procedure was completed as
described in the protein isolation and quantitation section.
Western blot analysis
Protein expression was determined by conducting
electrophoresis using a polyacrylamide gel (SDS-PAGE) at 15%.
Proteins were then transferred at 25 V/300 mA to polyvinylidene
fluoride membranes. Procaspase-3, C-3 and PARP expression levels
were determined by incubating the membranes with primary antibodies
(1.5 µg/ml) against caspase-3 (1:250) and PARP (1:1,000) overnight
at 5°C. Membranes were then stir-washed three times for 10 min in
phosphate-buffered saline (PBS). Subsequently, goat anti-mouse
IgG-biotin secondary antibodies (1:10,000; Sigma-Aldrich) for 1 h
at 37°C. ExtrAvidin-Alkaline Phosphatase (0.15 µg/ml;
Sigma-Aldrich) with BCIP/NBT solution (Roche Diagnostics, Basel,
Switzerland) was used to visualize the membranes. Western blot
assays were conducted in triplicate and analyzed using densitometry
(Molecular Imager Gel Doc and ChemiDoc systems) using Quantity One
1-D analysis software, version 4.6.9 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Observation of morphological changes
indicating apoptosis
A549 cells were exposed to an 1.85 mM solution of
7-HC in ethanol or 3% ethanol for 6, 12 or 24 h with three
replicates. Phase contrast images were captured using an Eclipse
TS100 inverted microscope and a DXM1200c camera (Nikon Corporation,
Tokyo, Japan) at a magnification of ×400.
Calcium influx measurement
In order to determine calcium influx,
3×106 A549 cells were exposed in three replicates to an
ethanolic solution of 7-HC (0.9 or 1.85 mM 7-HC) for 3, 6 and 12 h
(data for 12 h not shown as the results were not significantly
different). Cells were loaded for 40 min at 37°C with 4 µM Fura-2
AM. The cells were subsequently centrifuged at 300 × g for 5 min in
5 ml PBS. The pellet (100 µl) was immediately added to a heated
cuvette (37°C) containing 2.5 ml PBS, which was constantly stirred
with a magnetic bar. Ionomycine was used as positive control. Next,
6 mM EGTA was used to chelate the calcium. The fluorescence was
detected at 488 nm using an optical filter (Andover Corporation,
Salem, NH, USA), alternately exciting Fura-2 AM at 340/380 nm using
a monochromator purchased from Photon Technology International
(PTI; Monmouth Junction, NJ, USA). Data were acquired and digitized
at 0.83 Hz using the PTI interface.
Single-cell microinjection
Cell microinjection (25) was performed using the Nikon Eclipse
TS100 inverted microscope (Nikon Corporation, Tokyo, Japan)
equipped with a programmable IM-300 microinjector and an MHW-3
hydraulic micromanipulator (Narishige International, Ltd., London,
UK). Microinjection needles were crafted using borosilicate glass
capillaries using a P-87 device (Sutter Instrument Co., Novato, CA,
USA). Microinjection needles were loaded using Eppendorf
microloaders (Eppendorf AG, Hamburg, Germany). Microinjection time
and pressure parameters were determined using 0.1% dextran-FITC
(3,000 MW) microinjection solution. The optimal injection time was
determined as being between 200 and 400 msec, while the optimal
microinjection pressure was 6.5 psi. In order to determine the
basal activity of C-3, the specific substrate Z-DEVD-R110 was
microinjected at a concentration of 1.3 mg/ml into single cells.
Images were captured using a DXM-1200C camera (Nikon
Corporation).
For each assay, A549 cells were incubated for 3, 6,
12, 18 and 24 h with a 1.85 mM solution of 7-HC in ethanol or 3%
ethanol (data for 18 h not shown as the results were not
significantly different). Then, culture medium was withdrawn from
the Petri dish and the cells were washed twice with 1 ml PBS.
Finally, the cells were resuspended in 1 ml PBS and the
microinjection of Z-DEVD-R110 into single cells was performed.
Image analysis
Prior to microinjection, images were captured at
×400 magnification in phase contrast and green fluorescence (520
nm) to act as the negative control. Following the microinjection of
the substrate, images were captured using a red filter at 615 nm.
Immediately after this, fluorescence images were captured every 20
sec for 10 min. This method was standardized by determining the
fluorescence intensity changes in each image. Digital images were
obtained using NIS-Elements AR software, version 3 (Nikon
Corporation), which was calibrated for use with a ×40 lens. The C-3
activation dynamic was then analyzed in the various cell exposure
groups.
Statistical analysis
Plots were generated and statistical analysis was
performed using SigmaPlot for Windows, version 11.0 and SigmaStat,
version 3.5 (Systat Software, Inc., San Jose, CA, USA). Analysis of
variance tests were performed, and if a statistically significant
difference was identified to isolate the group or groups that
differ from the others then a multiple comparison versus control
group (Dunnett's method) test was used. P<0.05 was considered to
indicate a statistically significant result.
Results
Treatment with 7-HC reduces cell
viability and increases C-3 activity in a time-dependent
manner
A549 cells were incubated with 7-HC and MTT assays
were performed to determine the cell viability. A 10% reduction in
cell viability was observed following a 3-h exposure to 1.85 mM
7-HC; the cell viability further decreased by 30% after 12 h and
~40% after 24 h (data not shown).
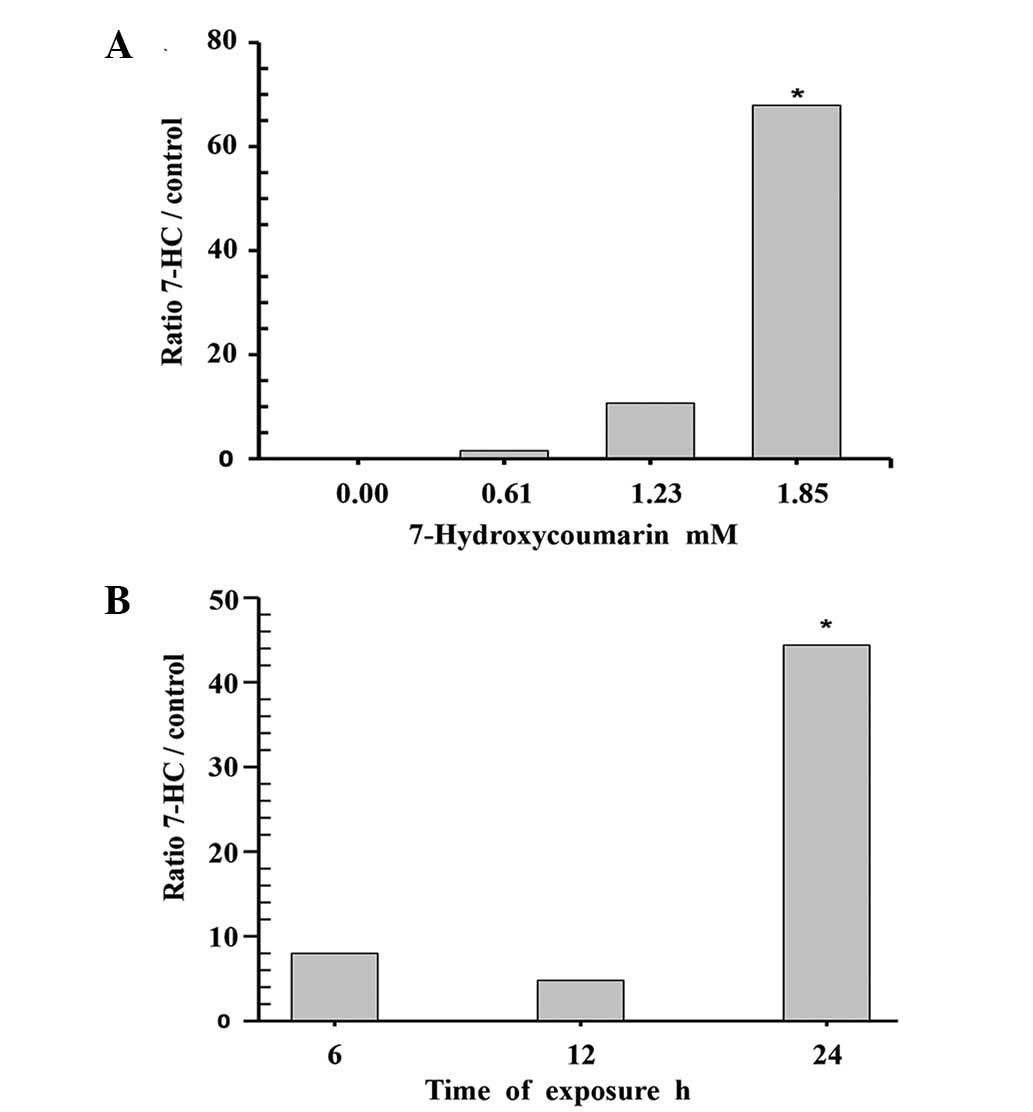
The concentration-response of C-3 to 7-HC was
obtained using endpoint colorimetric assays of the cell lysates. A
statistically significant increase (P=0.009, Dunnett's method) in
enzymatic activity (65%) was observed compared with the control
group when the cells were incubated with 1.85 mM 7-HC for 24 h. The
time course of treatment with 1.85 mM 7-HC was evaluated, with
measurements taken after 6, 12 and 24 h of exposure. The cells
exposed to 1.85 mM 7-HC for 24 h exhibited a statistically
significant difference in C-3 activity compared with the control
(P<0.05, Dunnett's method; Fig.
1).
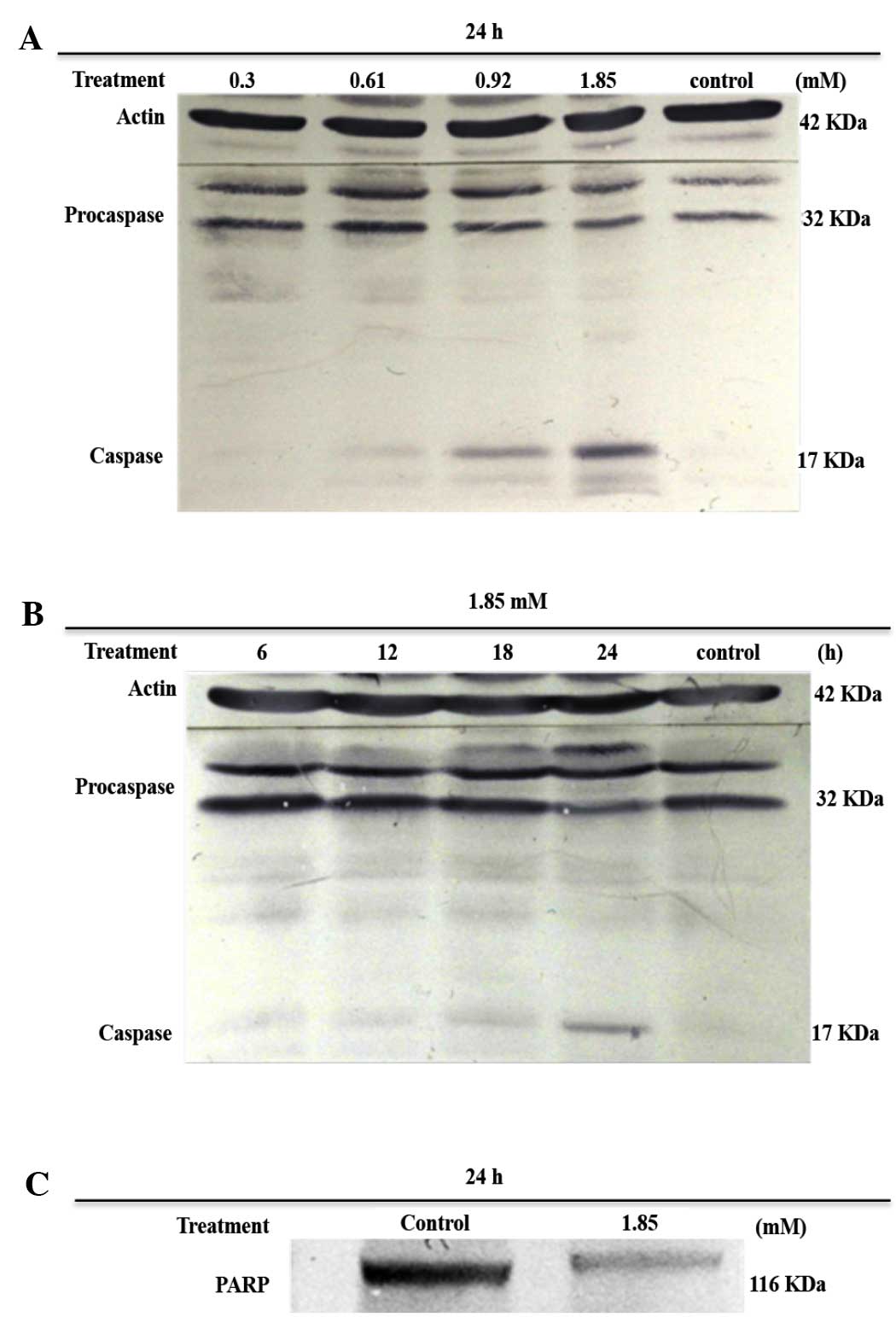
7-HC increases the cleavage of
procaspase-3 to C-3 in a time-dependent manner
After 24 h of exposure to various concentrations of
7-HC, lysates were obtained and subjected to gel electrophoresis in
denaturing conditions. Subsequently, the levels of procaspase-3,
C-3 and PARP were evaluated using western blot analysis. Bands for
procaspase-3 and cleavage product C-3 were immunodetected in the
cells that were treated with 0.92 and 1.85 mM 7-HC, but C-3 bands
were not detected for the control (ethanol-treated) cells (Fig. 2A).
Treatment with 1.85 mM 7-HC did not induce the
conversion of procaspase-3 into C-3 until the cells had been
subjected to 24 h of exposure (Fig.
2B). Furthermore, the cells exposed to 1.85 mM 7-HC exhibited
the characteristic activity of C-3 in the cleavage of PARP
(Fig. 2C).
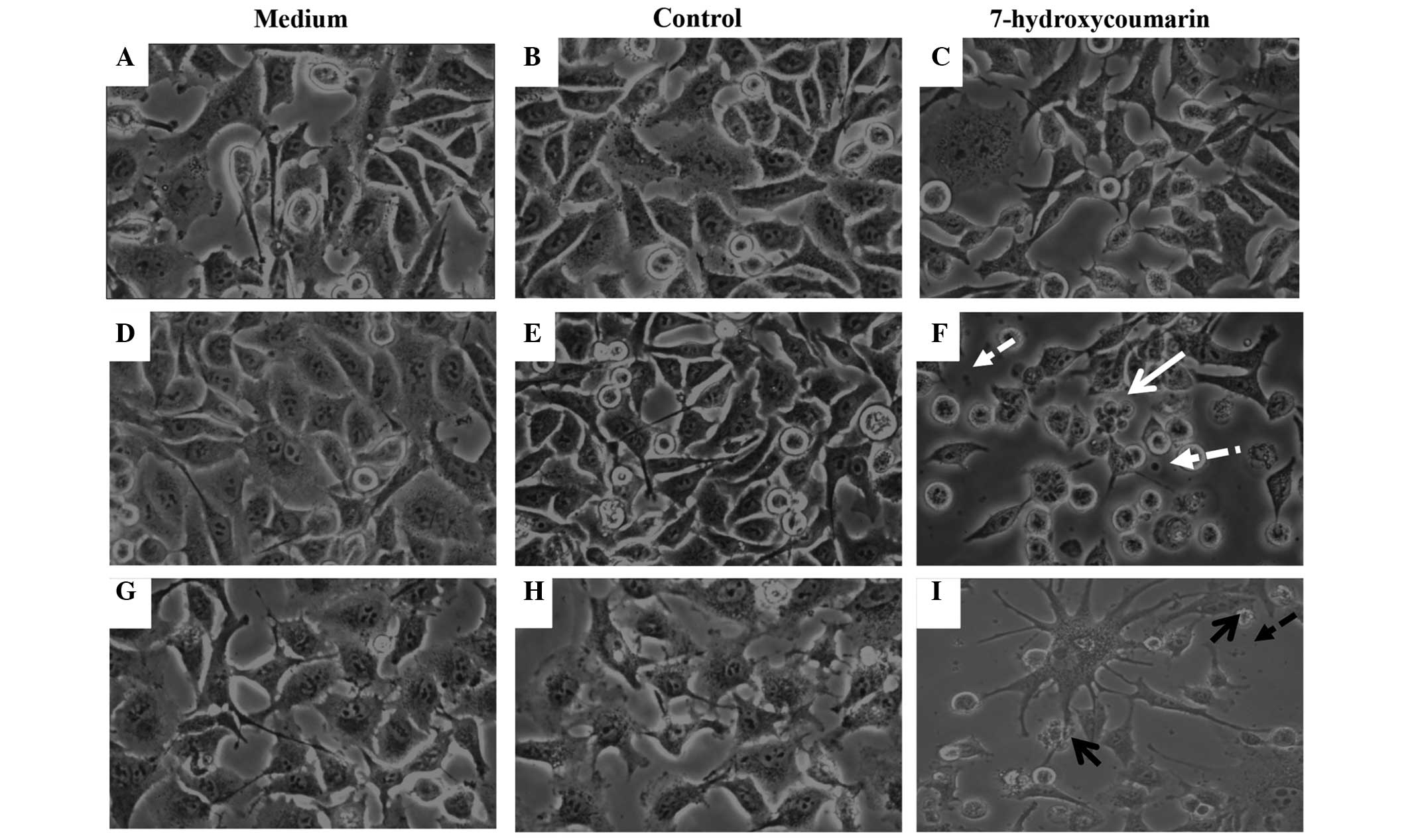
Furthermore, only 12 h of exposure to 1.85 mM 7-HC
induced characteristic apoptotic changes in the cells, such as
blebbing and shrinking, which were not observed in the control or
RPMI-1640 (untreated) cells (Fig.
3).
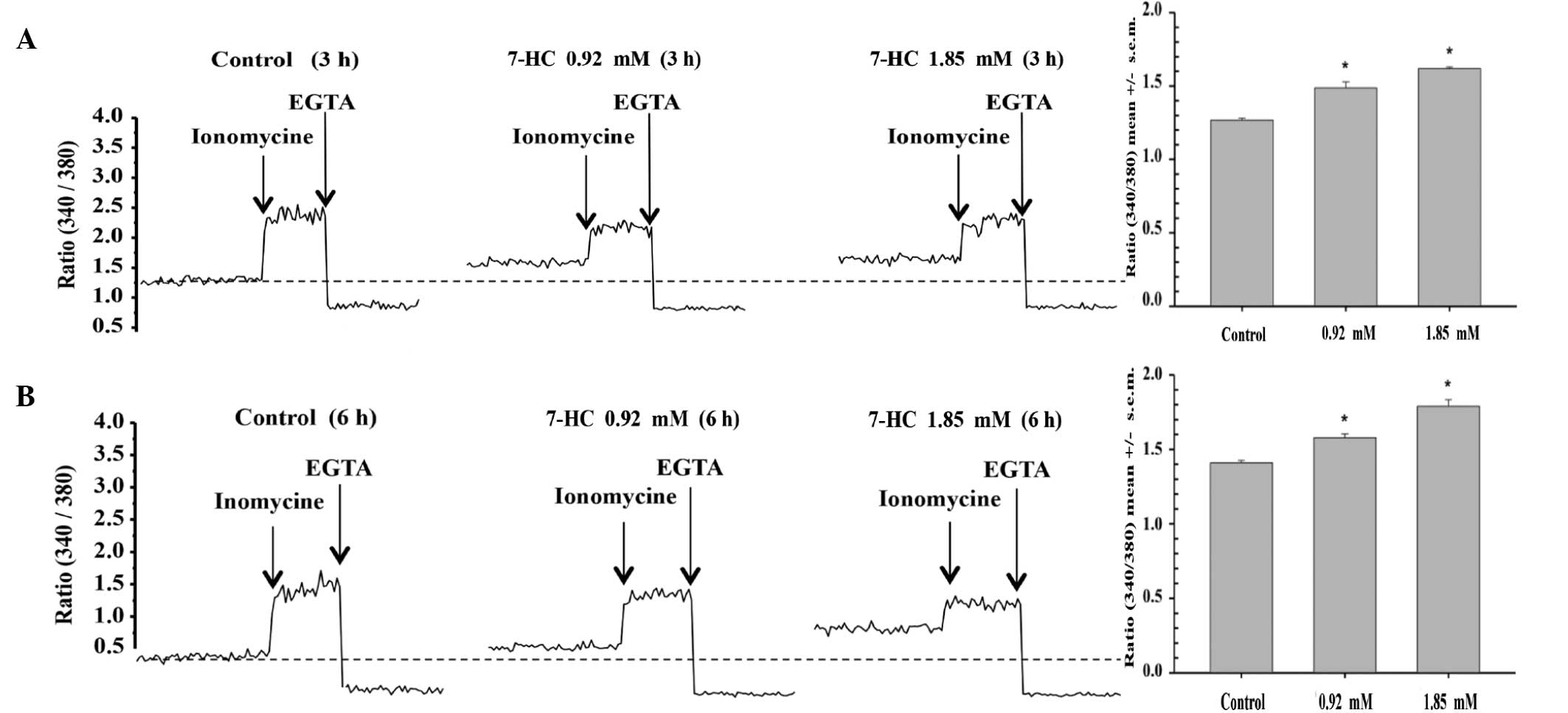
7-HC exposure increases calcium
conductance in A549 cells. The effects of 7-HC on calcium
conductance in A549 cells were investigated
It was observed that a 3-h exposure to 0.9 mM 7-HC
significantly increased calcium conductance by 17%, while exposure
to 1.85 mM 7-HC for the same time period increased conductance by
27% (P<0.05) compared with that in the control group (Fig. 4A). Similarly, a 6-h exposure to 0.9
mM 7-HC increased conductance by 12%, while exposure to 1.85 mM
7-HC for the same time period increased conductance by 27%
(P<0.05) compared with that in the control group (Fig. 4B).
7-HC exposure increases the activity
of caspase-3 in single cells
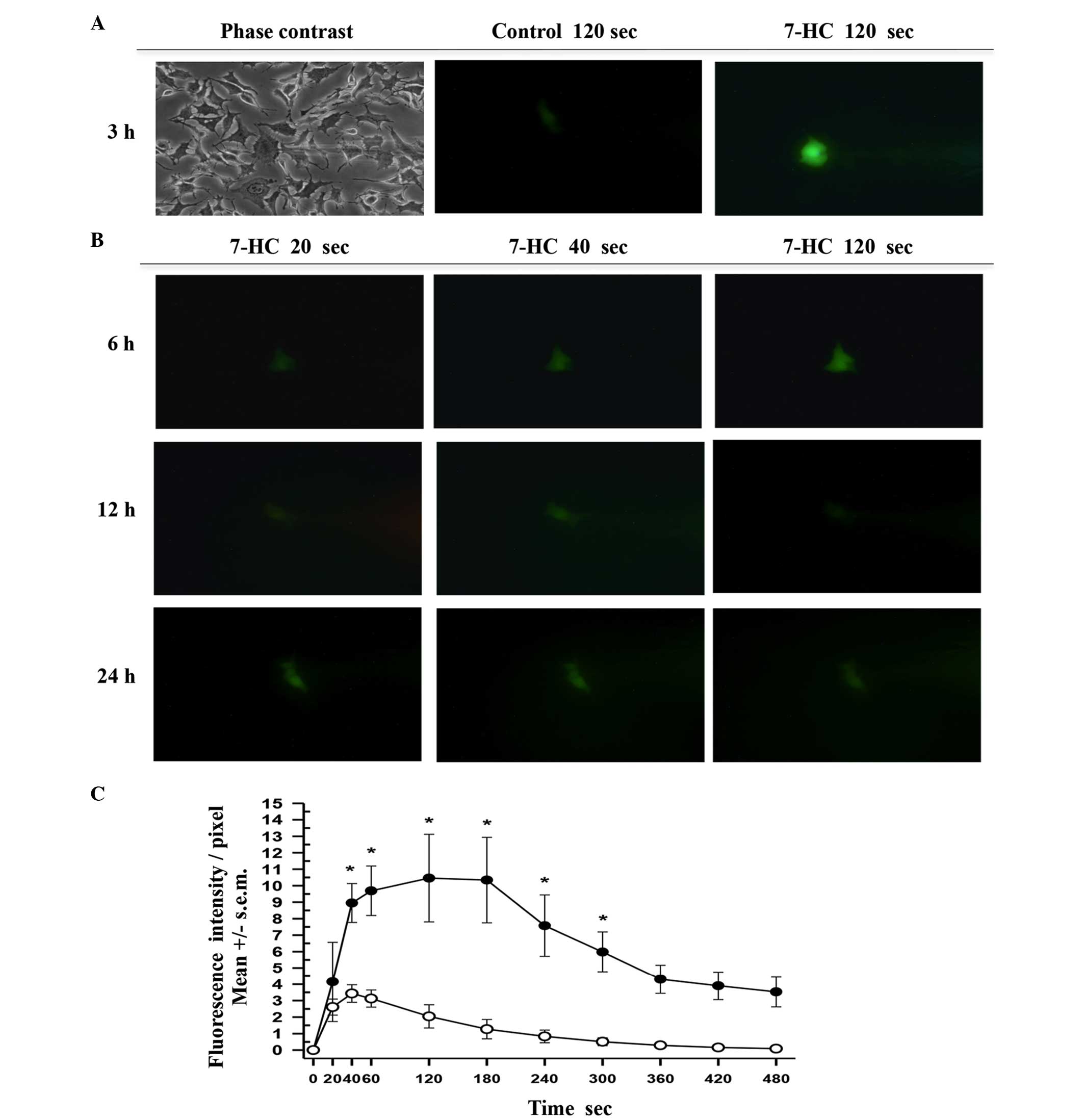
Finally, single-cell microinjection assays were
performed on cells that had been exposed to ethanol or 1.85 mM 7-HC
for 3, 6, 12, 18 and 24 h and time course curves were prepared. The
process was documented by capturing digital images every 5 sec over
a 10-min period (Fig. 5A and B).
Following the analysis of similar areas in each cell by measuring
the intensity/pixel ratio using green fluorescence, the mean values
were plotted (Fig. 5C). In each
curve a phase of exponential increase was evident, followed by a
plateau and an exponential phase of fluorescence reduction.
Comparisons of the initial velocities through linear regression
indicated that the real regression corresponds to 7-HC (slope
>0), while the control had no slope >0. Thus, the results
indicated the presence of typical first order enzymatic kinetics
corresponding to C-3.
Discussion
In a previous study, it was demonstrated using flow
cytometry that the exposure of A427 human lung adenocarcinoma cells
to 0.98 mM 7-HC for 4–6 h, produced an 83% increase of
Annexin-V-positive cells (12). In
the present study, the inductive effect of 7-HC on C-3 activity in
cellular lysates was demonstrated to occur in a
concentration-dependent manner, and the optimal exposure time to
obtain the maximum enzymatic activity was identified. Furthermore,
the results of western blot analysis demonstrated the expression of
procaspase-3 in the cells and its conversion into C-3 in a
concentration- and time-dependent manner, in addition to the
proteolytic cleavage of PARP by C-3, as described by Yang et
al (13). Morphological changes
associated with apoptosis were identified, such as blebbing and
shrinking, comparable to the apoptotic bodies reported by Chuang
et al (14) and Elinos-Báez
et al (26). Chuang et
al (14) reported a significant
increase in calcium flux in HeLa cells treated for 24 h with 25–100
µM coumarin, using flow cytometry. Through fluorescence
spectrometry, in the present study this effect was detected at a
higher (millimolar) concentrations of 7-HC in cells exposed for 3
h. Furthermore, in the present study, experiments were conducted to
determine how rapidly the exposure of A549 cells to 7-HC induced
the activation of C-3. To the best of our knowledge, single-cell
microinjection has not previously been employed by other
researchers in this type of study. The results indicate that 7-HC
rapidly induced C-3 activation. The concentration that induced this
rapid C-3 activation effect (1.85 mM) decreased cell viability by
only 10% after 3 h of exposure.
The majority of previous studies have employed a
maximum simple coumarin concentration of 100 µM and reported the
reduction of viability at 24 or 48 h (10–17).
However, it was not clear whether this rapid C-3 activation effect
was induced by the binding of 7-HC with particular intracellular
ligands. Zlabinger et al (27) demonstrated that in human monocytes,
coumarin binding sites appeared to be present in relatively high
numbers (7.5×108/cell); however, their affinity was low
(Ka~2×102 M−1). Furthermore,
inhibition experiments performed with 7-HC revealed that an ~4-fold
molar concentration of 7-HC was necessary to induce a 50%
displacement of coumarin from its binding site (27). It may be hypothesized that the C-3
activation effect, observed at higher concentrations compared with
those previously reported, may be due to A549 cells possessing
these binding sites.
Cytotoxic and cytostatic activity, in addition to
the mechanisms by which these effects are produced, have been
reported for a number of naturally occurring coumarins, such as
esculetin (11,13) and osthole (16,18), in
addition to and synthetic coumarins such as quercetin (17). However, the majority of these
coumarins have not been subjected to testing beyond the
pre-clinical phase, in contrast with the coumarins whose effects
and pharmacokinetic properties in humans are widely known. The
prompt conversion of coumarin into 7-HC, which has displayed the
most relevant activity, as well as its rare toxicity among patients
(7) is well documented. In 1994,
Marshall et al (28) reported
the testing of 7-HC in a phase I trial in which patients who
received daily dosages of between 1,000 and 7,000 mg presented few
collateral effects. It has also been reported that 7-HC is not
transported by multidrug resistance-associated proteins (4–7,29). As 7-HC has been previously employed
in a number of phase II clinical studies, and has already been
designated an Orphan drug by the US FDA, there is a possibility of
successfully conducting further studies using 7-HC combined with
conventional therapy as a lung cancer treatment.
Acknowledgements
This study constitutes a partial fulfillment of the
Graduate Program in Biological Sciences of the National Autonomous
University of México. The authors thank Hiram Molina Espinosa for
critically reading the final version of the manuscript and making
valuable suggestions. Grants were obtained from the National
Science and Technology Council (no. 98729) and the Research and
Technological Innovation Support Program from the National
University of Mexico (no. IN216812).
References
|
1
|
U.S. National Cancer Institute:
Surveillance, Epidemiology, and End Results Program. SEER Stat Fact
Sheets: Lung and Bronchus Cancer. http://seer.cancer.gov/statfacts/html/lungb.htmlAccessed.
April 14–2015
|
|
2
|
U.S. National Cancer Institute: Targeted
Cancer Therapies. http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheetAccessed.
April 12–2015
|
|
3
|
U.S. Food and Drug Administration (FDA):
Search Orphan Drug Designations and Approvals. http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfmAccessed.
April 14–2015
|
|
4
|
Jain PK: Coumarin: Chemical and
pharmacological profile. J App Pharm Sci. 2:236–240. 2012.
|
|
5
|
Casley-Smith JR and Casley-Smith JR: The
pathophysiology of lymphedema and the action of benzo-pyrones in
reducing it. Lymphology. 21:190–194. 1988.PubMed/NCBI
|
|
6
|
Thornes RD, Lynch G and Seehan MV:
Cimetidine and coumarin therapy of melanoma. Lancet. 2:3281982.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cox D, O'Kennedy R and Thornes RD: The
rarity of liver toxicity in patients treated with coumarin
(1,2-benzopyrone). Hum Toxicol. 8:501–506. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thornes D, Daly L, Lynch G, Browne H,
Tanner A, Keane F, O'Loughlin S, Corrigan T, Daly P and Edwards G:
Irish Melanoma Group: Prevention of early recurrence of high risk
malignant melanoma by coumarin. Eur J Surg Oncol. 15:431–435.
1989.PubMed/NCBI
|
|
9
|
Dexeus FH, Logothetis CJ, Sella A, Fitz K,
Amato R, Reuben JM and Dozier N: Phase II study of coumarin and
cimetidine in patients with metastatic renal cell carcinoma. J Clin
Oncol. 8:325–329. 1990.PubMed/NCBI
|
|
10
|
Mohler JL, Gomella LG, Crawford D, Glode
LM, Zippe CD, Fair WR and Marshal ME: Phase II evaluation of
coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma.
Prostate. 20:123–131. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chu CY, Tsai YY, Wang CJ, Lin WL and Tseng
TH: Induction of apoptosis by esculetin in human leukemia cells.
Eur J Pharmacol. 416:25–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lopez-Gonzalez JS, Prado-Garcia H,
Aguilar-Cazares D, Molina-Guarneros JA, Morales-Fuentes J and
Mandoki JJ: Apoptosis and cell cycle disturbances induced by
coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines.
Lung Cancer. 43:275–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JY, Della-Fera MA and Baile CA:
Esculetin induces mitochondria-mediated apoptosis in 3T3-L1
adipocytes. Apoptosis. 11:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang JY, Huang YF, Lu HF, Ho HC, Yang
JS, Li TM, Chang NW and Chung JG: Coumarin induces cell cycle
arrest and apoptosis in human cervical cancer HeLa cells through a
mitochondria- and caspase-3 dependent mechanism and NF-kappaB
down-regulation. Vivo. 21:1003–1009. 2007.
|
|
15
|
Alvarez-Delgado C, Reyes-Chilpa R, Estrada
Muñiz E, Mendoza-Rodríguez A, Quintero-Ruiz A, Solano J and Cerbón
MA: Coumarin A/AA induces apoptosis-like cell death in HeLa cells
mediated by the release of apoptosis-inducing factor. J Biochem Mol
Toxicol. 23:263–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Jiang G, Yao F, He Y, Liang G,
Zhang Y, Hu B, Wu Y, Li Y and Liu H: Growth inhibition and
apoptosis induced by osthole, a natural coumarin, in hepatocellular
carcinoma. PLoS One. 7:e378652012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng SY, Li Y, Jiang D, Zhao J and Ge JF:
Anticancer effect and apoptosis induction by quercetin in the human
lung cancer line A-549. Mol Med Rep. 5:822–826. 2012.PubMed/NCBI
|
|
18
|
Shokoohinia Y, Hosseinzadeh L, Alipour M,
Mostafaie A and Mohammadi-Motlagh HR: Comparative evaluation of
cytotoxic and apoptogenic effects of several coumarins on human
cancer cell lines: Osthole induces apoptosis in p53-deficient H1299
cells. Adv Pharmacol Sci. 2014:8475742014.PubMed/NCBI
|
|
19
|
Finn G, Creaven B and Egan D: Modulation
of mitogen-activated protein kinases by 6-nitro-7-hydroxycoumarin
mediated apoptosis in renal carcinoma cells. Eur J Pharmacol.
481:159–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Musa MA, Badisa VL, Latinwo LM, Patterson
TA and Owens MA: Coumarin-based benzopyranone derivatives induced
apoptosis in human lung (A549) cancer cells. Anticancer Res.
32:4271–4276. 2012.PubMed/NCBI
|
|
21
|
Goel A, Prasad AK, Parmar VS, Ghosh B and
Saini N: Apoptogenic effect of 7,8-diacetoxy-4-methylcoumarin and
7,8-diacetoxy-4-methylthiocoumarin in human lung adenocarcinoma
cell line: Role of NF-kappa B, Akt, ROS and MAP kinase pathway.
Chem Biol Interact. 179:363–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goel A, Chhabra R, Ahmad S, Prasad AK,
Parmar VS, Ghosh B and Saini N: DAMTC regulates cytoskeletal
reorganization and cell motility in human lung adenocarcinoma cell
line: An integrated proteomics and transcriptomics approach. Cell
Death Dis. 3:e4022012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schilling WH, Crampton RF and Longland RC:
Metabolism of coumarin in man. Nature. 221:664–665. 1969.
View Article : Google Scholar
|
|
24
|
Mossman T: Rapid colorimetric assay for
cellular growth and survival: Applications to proliferation and
cytotoxicity assay. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y: Single-cell microinjection
technologies. Single-cell Analysis: Methods and Protocols.
Lindström S and Andersson-Svahn H: (Heidelberg). Humana Press.
169–176. 2012. View Article : Google Scholar
|
|
26
|
Elinos-Báez CM, León F and Santos E:
Effects of coumarin and 7-OH-coumarin on bcl-2 and Bax expression
in two human lung cancer cell lines in vitro. Cell Biol Int.
29:703–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zlabinger GJ, Nöhammer C, Böhmig GA and
Menzel JE: Mode of action of coumarin in immune cells. J Cancer Res
Clin Oncol. 120(Suppl): S17–S18. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marshall ME, Mohler JL, Edmonds K,
Williams B, Butler K, Ryles M, Weiss L, Urban D, Bueschen A and
Markiewicz M: An updated review of the clinical development of
coumarin (1,2-benzopyrone) and 7-hydroxycoumarin. J Cancer Res Clin
Oncol. 120(Suppl): S39–S42. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wittgen HG, van den Heuvel JJ, van den
Broek PH, Siissalo S, Groothuis GM, de Graaf IA, Koenderink JB and
Russel FG: Transport of the coumarin metabolite 7-hydroxycoumarin
glucuronide is mediated via multidrug resistance-associated
proteins 3 and 4. Drug Metab Dispos. 40:1076–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|