Introduction
The intervertebral discs are degenerated due to
ageing and the apoptosis of matrix cells. Imbalances between
apoptosis and regeneration cause the regression of the
intervertebral disc (1). Apoptosis
is as essential as the function of proteoglycan synthesis in
determining the possible degeneration of intervertebral discs. As
early as 1982, nucleus pulposus was observed with typical dead
cells by electron microscopy (2). In
1998, apoptosis was detected by the terminal deoxynucleotidyl
transferase dUTP nick end labelling method in intervertebral discs.
Excessive apoptosis causes the death of nucleus pulposus cells
(3), and apoptosis is associated
with the degeneration of cartilage endplates. In addition, the
apoptotic rate has been demonstrated to increase under long-term
pressure in intervertebral discs (4). Hence, we speculate that apoptosis is
vital in intervertebral disc degeneration.
Apoptosis mediated by Fas (also known as CD95) or
Fas ligand (FasL) is responsible for the loss of disc cells. High
expression of Fas and FasL has been observed in intervertebral disc
protrusions (5) and patients
suffering from degenerative disc disease; however, age is
insignificant with respect to the expression of FasL.
Intervertebral disc cells negate FasL-mediated apoptosis under
autocrine or paracrine Fas. Apoptosis and cell proliferation
maintain relative balance, whereas the apoptotic rate is greater
than the proliferation rate. The negative balance between the two
processes causes cell shrinkage and disappearance, which is
significant in the degeneration of intervertebral discs (6).
Following herniation, the disc cells undergo
apoptosis through autocrine or paracrine FasL (7). Fas and FasL belong to the tumour
necrosis factor family; their combination enables the Fas-FasL
apoptotic pathway. Fas death receptor initiates apoptosis through
the assemblages of Fas-associated death domain (FADD) and
procaspase-8, subsequent proteolytic cleavage of vital structural
substrates, and initiation of the caspase cascade (8). Caspase cascades, which lead to the
activation of caspase-3, are essential in the process of apoptosis
in the injured spinal cord (9). In
this study, Fas and caspase-3 were examined in FasL-induced annulus
fibrosus cells.
Paeonia lactiflora has been used in
traditional Chinese prescriptions to treat certain types of
nociceptive diseases, including muscle and menstrual pain (10–12).
Paeoniflorin (PF) is a bioactive glucoside isolated from the roots
of P. lactiflora Pall. (13).
PF exhibits biological and biomodulating activity, including memory
improvement, as well as antioxidant and anti-inflammatory activity
(14). PF also demonstrates
cytoprotective effects on various cell types. PF inhibits
H2O2-induced apoptosis by suppressing
caspase-3 activity (15). However,
the protective activity of PF in annulus fibrosus cells against
FasL-induced apoptosis is yet to be elucidated. We postulated that
PF mediates its effects through the modulations of the Fas-FasL
signalling pathway and FasL-induced apoptosis.
Materials and methods
Culture and characterisation of
annulus fibrosus cells
Sprague Dawley (SD) rats were anaesthetised with 10%
chloral hydrate (3 µl/g). Disc fibre rings were isolated in a
sterile manner, and superficial disc fibre rings were harvested and
rinsed in 1X phosphate-buffered saline (PBS; Hyclone, Carlsbad, CA,
USA) three times. The disc fibre rings were cut into sections
(volume, 1 mm3). To isolate the cells, the disc tissues
were digested with 0.25% trypsin including 0.02%
ethylenediaminetetraacetic acid (Hyclone) for 20 min, followed by
another treatment with 0.2% collagenase II (Solarbio Science and
Technology Co., Ltd., Beijing, China) for 8 h at 37°C. Following
the enzyme digestion, the suspension was filtered through a 70-µm
mesh. The filtered cells were harvested, and the digestion solution
was refreshed twice. The cells were adjusted to a density of
2×105/ml by a hemocytometre; the cells were then placed
in a humidified incubator with 5% CO2 at 37°C in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 20%
fetal bovine serum (FBS). The cells were passaged at 80%
confluence, which was determined by microscopy.
Third-passage annulus fibrosus cells were plated
onto coverslips, rinsed in 1X PBS, fixed in 4% paraformaldehyde for
30 min, and stained with 1% toluidine blue for 30 min at room
temperature. The sections were briefly rinsed in absolute ethyl
alcohol, dried, transparentised, mounted and observed via
microscopy (original magnification, ×200).
Animals
One-month-old male or female SD rats (n=50; weight,
100–120 g) were purchased from Slaccas Laboratory Animal Co., Ltd.
(Shanghai, China; certification no. SCXX (SH) 2008-0005). The care
and use of animals conformed to the regulations of the Guidelines
for Laboratory Animal Welfare established by the Ministry of
Science and Technology of China (16).
MTT assay
Third-passage annulus fibrosus cells were
trypsinised and subcultured into 96-well plates at 5×103
cells/well. Each group comprised five wells with an untreated group
as a control. When the cells were anchored to the plates, various
concentrations of PF (2080, 208, 20.8, 2.08, 0.208 and 0.0208 µM;
National Institute for Control of Pharmaceutical and Biologic
Products, Fujian, China) were incubated for 24, 48 and 72 h. The
activity of the annulus fibrosus cells was examined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay.
The cells were digested, centrifuged, collected and
rinsed in 1X PBS. Twenty microlitres of 0.5% MTT (Solarbio Science
and Technology Co., Ltd.) solution was added to each well and
incubated at 37°C for 4 h. MTT was discarded and replaced by 150 µl
dimethyl sulfoxide (Solarbio Science and Technology Co., Ltd.), and
the mixture was blended for 10 min. The optical density (OD) was
determined for each group by a microplate reader (Thermo
Scientific, Waltham, MA, USA) at 490 nm; the mean values were
calculated.
Flow cytometric analysis
Third-passage annulus fibrosus cells were
trypsinised and subcultured into 6-well plates at 3×105
cells/well (final volume, 2 ml), and then incubated in DMEM with
20% FBS (Hyclone). After reaching 80% confluence, the cells were
freshly incubated in DMEM with 1% FBS (Hyclone) for 8 h (15) and induced with FasL (R&D Systems,
Minneapolis, MN, USA) at final concentrations of 0, 10, 20 and 40
ng/ml for 24 h. The apoptotic rate was measured by flow cytometry
using Annexin V-FITC/PI (Invitrogen, Carlsbad, CA, USA).
The cells were digested, centrifuged and rinsed in
1X PBS at 4°C. The rinsed cells were resuspended with binding
buffer at a density of 1×106/ml. The cells were stained
with 5 µl Annexin V-FITC and 10 µl PI in the dark at room
temperature for 15 min. A 400 ml volume of the buffer was added to
the resuspending cells; the cells were then sampled by flow
cytometry (BD FACSCalibur, BD Biosciences, San Jose, CA, USA) to
analyse the apoptotic fraction of the annulus fibrosus cells.
Western blot analysis
Third-passage annulus fibrosus cells were
trypsinised and subcultured into 6-well plates at 3×105
cells/well (final volume, 2 ml), and then incubated in DMEM with
20% FBS. After reaching 80% confluence, the cells were replaced by
DMEM with 1% FBS for 8 h and induced with FasL at final
concentrations of 20 ng/ml; the cells were then treated with PF at
final concentrations of 208, 20.8 and 2.08 µM for 24 h.
Total proteins were isolated from the cells; protein
concentrations were tested by the bicinchoninic acid assay method
(Sangon Biotech Co., Ltd., Shanghai, China). Samples of 40 µg total
protein were separated by gel electrophoresis using 12% SDS gel;
the samples were then transferred to polyvinylidene difluoride
(PVDF) membranes. The PVDF membrane was incubated with 1X
Tris-buffered saline with 0.1% Tween-20 (TBST) and 5% dehydrated
skimmed milk to block non-specific protein binding for 2 h; the
proteins were incubated with rabbit anti-Fas (Cell Signaling
Technology, Inc., Beverly, MA, USA).
Subsequently, 1:1,000 each of anti-caspase-3,
anti-GAPDH, and anti-β-actin antibodies (all from Cell Signaling
Technology, Inc.) were incubated overnight at 4°C; anti-rabbit
secondary antibodies (Cell Signaling Technology, Inc.) were
incubated for 1 h at room temperature. The membranes were rinsed in
TBST three times for 5 min, labelled with enhanced
chemiluminescence substrates, and exposed to X-ray. Protein bands
were analysed using a gel imaging analysis system (Bio-Rad,
Hercules, CA, USA) and normalised to β-actin in the sample.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The groups were compared by analysis of variance; P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology and characterisation of
annulus fibrosus cells in vitro
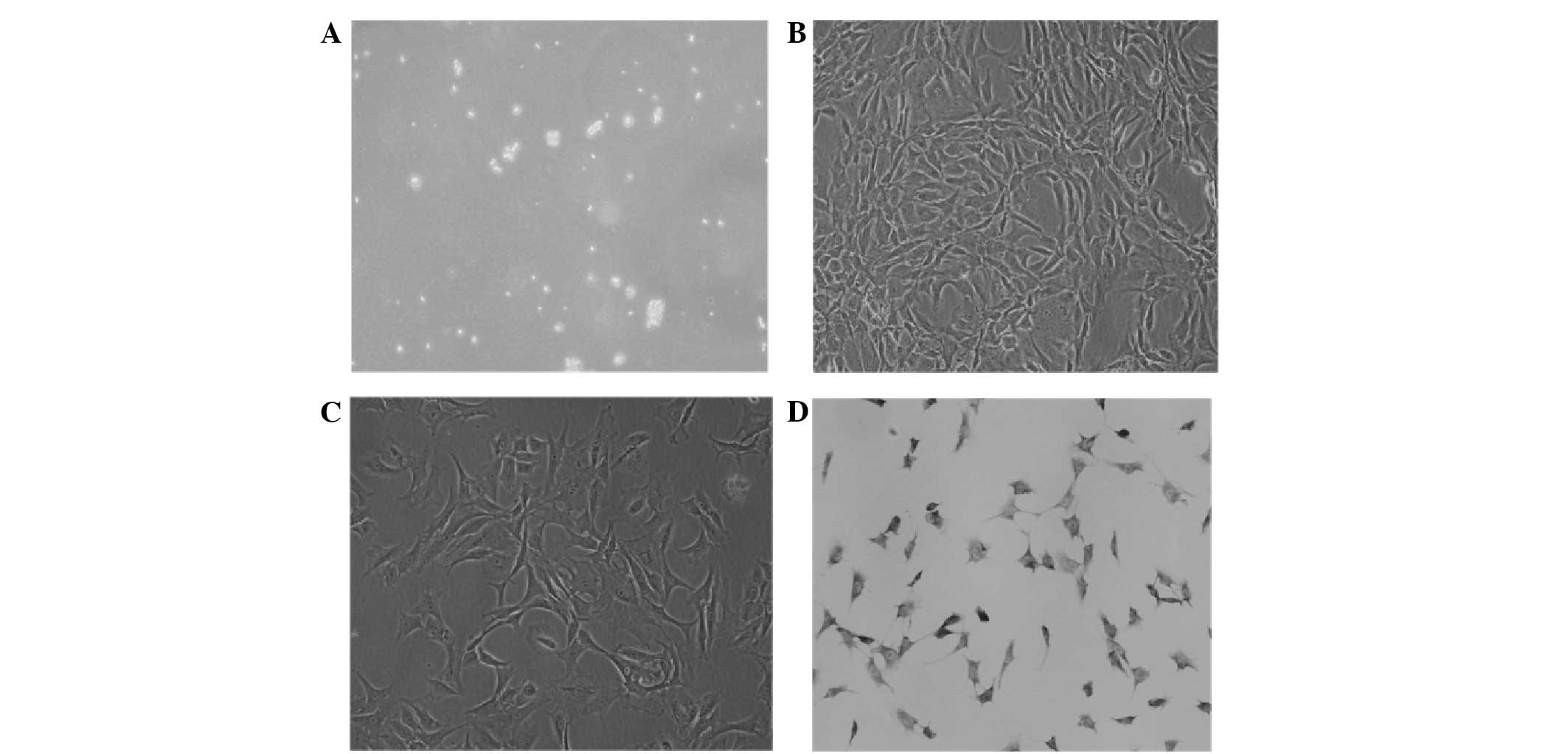
The primary digestion annulus fibrosus cells were
small and round; strong refraction was observed in the medium
(Fig. 1A). On the first day of
culture, the annulus fibrosus cells adhered to the culture flask
and extended pseudopod-like projections; their nuclei were mainly
round or oval. On the fourth day, the cells were mainly polygonal
and in grouped distributions. The cells became confluent and
arranged regularly on the eighth day (Fig. 1B). Second-, third- and fourth-passage
cells grew rapidly; the passaged cells adhered to the culture flask
within 12 h. The period of subculture was 7 days; third-passage
cells were mainly polygonal and all equal in size (Fig. 1C). The annulus fibrosus cells were
detected by toluidine blue staining for characterisation. The
third-passage annulus fibrosus cells were purple and exhibited
metachromatic granules. The nuclei were mainly round or oval and
dark blue (Fig. 1D).
MTT assay
The OD values were significantly higher in the cells
treated with 208, 20.8 and 2.08 µM PF after 24 h than those in
untreated cells (P<0.01). At 48 and 72 h, the OD values of the
cells treated with 208, 20.8 or 2.08 µM PF were also significantly
higher than those of the untreated cells (P<0.01; Fig. 2).
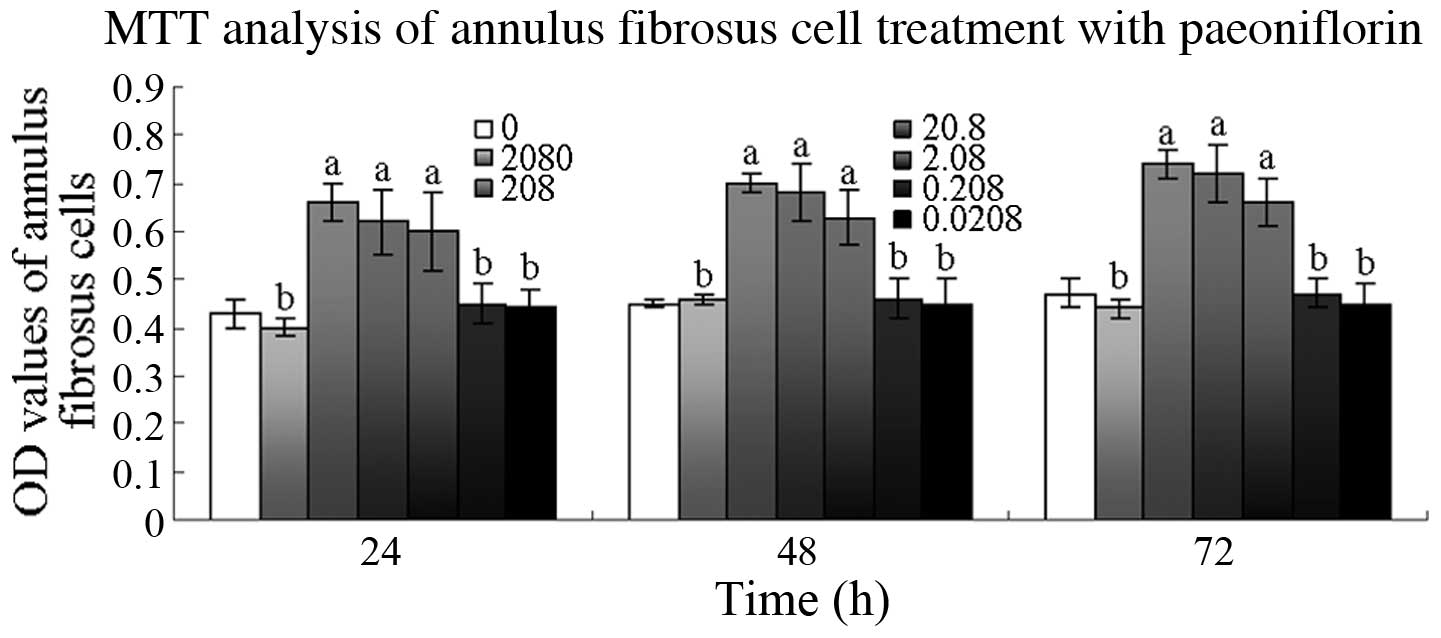 | Figure 2.Effect of paeoniflorin (PF) on annulus
fibrosus cell viability. Cells were treated with 0.0208, 0.208,
2.08, 20.8, 208 and 2080 µM PF for 24, 48 and 72 h, and cell
viability was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. aP<0.01, bP>0.05 vs. 0 µM group.
OD, optical density. |
Inhibitory effects of PF treatment on
FasL-induced apoptosis
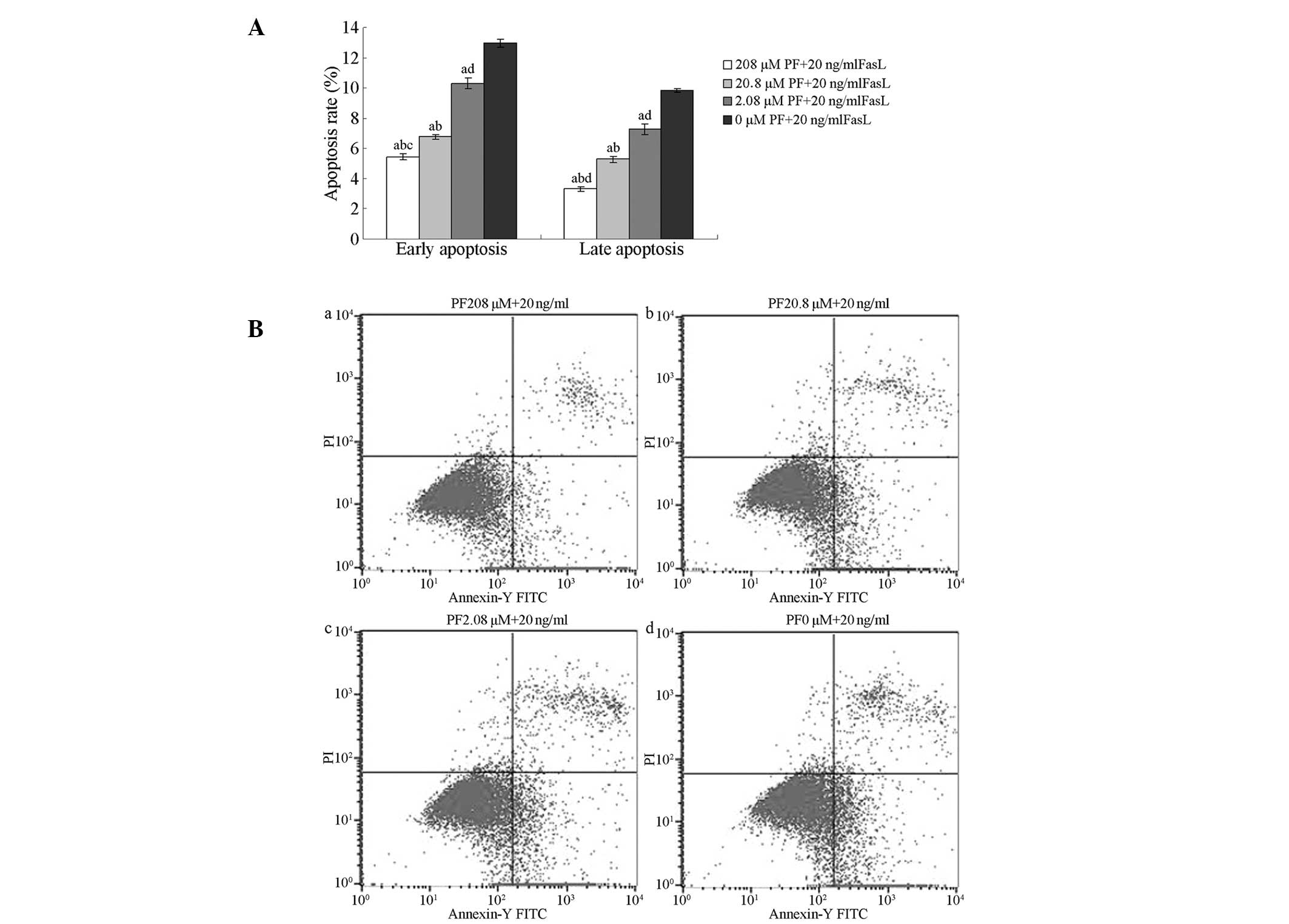
Flow cytometry of annexin V-FITC/PI-stained cells
was used to analyse the inhibitory effects of PF treatment on
FasL-induced apoptosis in annulus fibrosus cells. The percentages
of early and late apoptotic cells following treatment with 208,
20.8 or 2.08 µM PF were significantly lower than those in untreated
cells (P<0.01). The percentages of early and late apoptotic
cells following treatment with 208 µM PF were lower than those of
cells treated with 2.08 µM PF (P>0.05). The percentage of late
apoptotic cells was lower following treatment with 208 µM PF than
when treated with 20.8 µM PF (P>0.05); no significant
differences were observed in the percentages of early apoptotic
cells (P>0.05; Fig. 3).
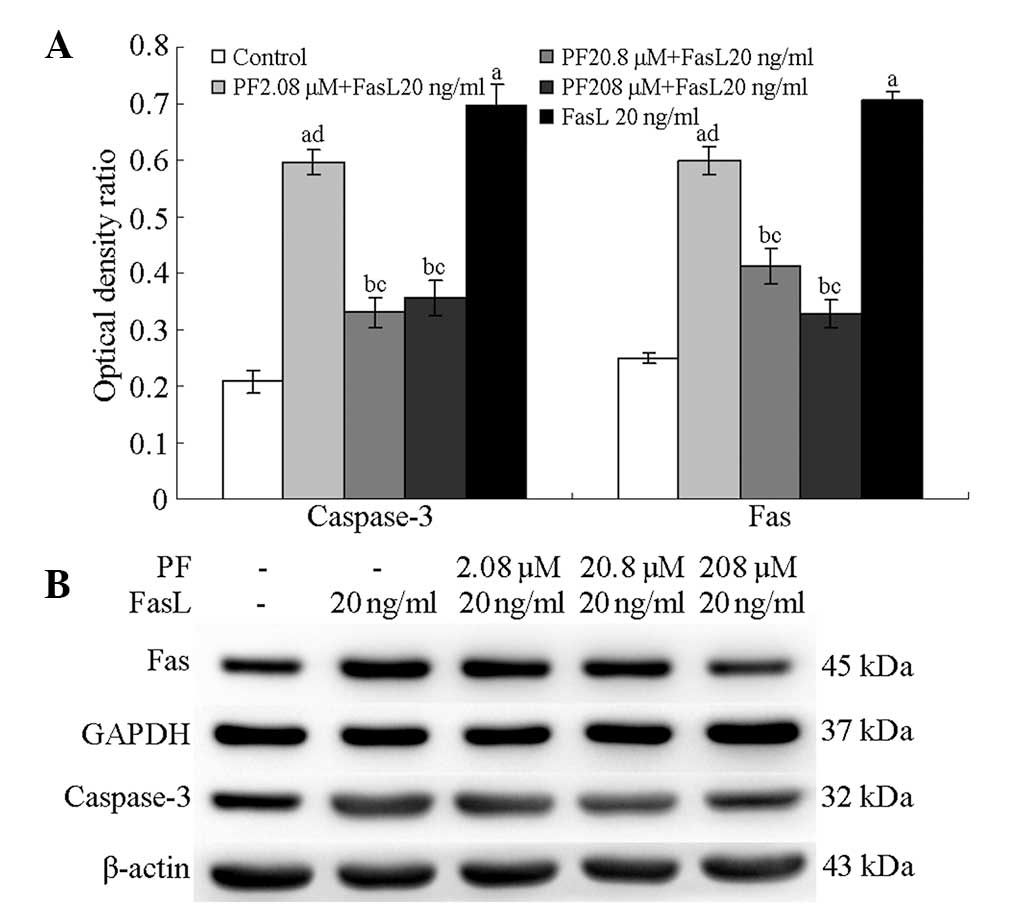
Expression of Fas and caspase-3 in
PF-treated annulus fibrosus cells
Following treatment with 208, 20.8 and 2.08 µM PF,
the annulus fibrosus cells demonstrated significantly lower levels
of caspase-3 protein than untreated cells (P<0.01); the protein
levels in FasL-induced cells were higher than those in normal cells
(P<0.05). Fas levels following 208, 20.8 and 2.08 µM PF
treatment were significantly lower than those in untreated cells
(P<0.05); FasL-induced annulus fibrosus cells exhibited higher
Fas levels compared with normal fibrosus cells (P<0.05; Fig. 4).
Discussion
PF exhibits pharmacological activity, including
antioxidant, anti-inflammatory and neuroprotective effects, on
various types of cells (17–19). The protective activity of PF against
FasL-induced injury was evaluated in annulus fibrosus cells. We
demonstrated that 208, 20.8 or 2.08 µM PF promoted the
proliferation of annulus fibrosus cells and inhibited FasL-induced
apoptosis of annulus fibrosus cells; the results were consistent
with a previous study on the cytoprotective effects of PF (20).
The apoptotic rate of the annulus fibrosus cells was
assessed following treatment with 10, 20 or 40 ng/ml FasL for 24 h.
The cells exhibited varying degrees of apoptosis; the apoptotic
rates significantly increased with the dosage of FasL. The results
revealed that the cells induced with 20 ng/ml FasL resulted in a
moderate apoptotic rate, which was suitable for follow-up
experiments (data not shown). Furthermore, 24 h FasL treatment
effectively activated Fas-FasL signalling and resulted in the
apoptosis of annulus fibrosus cells.
Fas, also known as CD95 and APO-1, is a widely
studied receptor (21). When the Fas
binds with its agonists, the death domain of intracellular gathered
to trimer, then raise Fas-associated death domain (FADD) of
cytoplasm. Then binds through its own death domain to the clustered
receptor death domains. In addition, FADD contains a ‘death
effector domain’ that binds to an analogous domain repeated in
tandem within the zymogen form of caspase-8, which comprises Fas
FADD procaspase 8, referred to as the death-inducing signalling
complex (DISC) (22). DISC activates
the caspase-dependent apoptotic signalling cascade; the activation
results in the cleavage of procaspase-8 and −10 and caspase-3 as
well as the release of active caspase-8 and −10 from the DISC
(23). Caspase-3 initiates apoptosis
by releasing caspase-activated deoxyribonuclease (CAD) from the
inactive complex formed with the CAD inhibitor; CAD triggers the
rapid fragmentation of DNA (24).
Disc cells die during the rapid expression of Fas protein. Factors
upregulated Fas in vivo and in vitro; enhancing the
Fas-FasL system causes apoptosis. The breakage of the balance
between cell proliferation and apoptosis reduces the disc cells.
The change in matrix ingredients and the remodelling damages and
destroys the physiological functions of intervertebral discs. This
change is also referred to as organised intervertebral disc
degeneration (25).
The results revealed that PF reduced the expression
of Fas and caspase-3 protein, which indicates that this compound
reduced the percentage of dead fibre ring cells by the passageway
of Fas-FasL signal transduction, which halted the regression of
intervertebral discs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China: Control system based on ontology sense
discussed bilateral back for ‘theory in the treatment of chronic
lumbago role connotation’ (no. 81303017) and the National 12th
‘Five-Year’ Technology Support Programs (no. 2013BAI10B05).
References
|
1
|
Ahmed SH, El-Shaarawy EA, Ishaq MF and
Moniem MH: Morphological and radiometrical study of the human
intervertebral foramina of the cervical spine. Folia Morphol
(Warsz). 73:7–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kong D, Zheng T, Fang J and Li X:
Apoptosis of endplate chondrocytes in post-laminectomy cervical
kyphotic deformity. An in vivo animal model in sheep. Eur Spine J.
22:1576–1582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang L, Zhang X, Zheng X, et al:
Apoptosis, senescence, and autophagy in rat nucleus pulposus cells:
Implications for diabetic intervertebral disc degeneration. J
Orthop Res. 31:692–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan SC, Ferguson SJ and Gantenbein-Ritter
B: The effects of dynamic loading on the intervertebral disc. Eur
Spine J. 20:1796–1812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma KG, Shao ZW, Yang SH, et al: Autophagy
is activated in compression-induced cell degeneration and is
mediated by reactive oxygen species in nucleus pulposus cells
exposed to compression. Osteoarthritis Cartilage. 21:2030–2038.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guan YJ, Zhang Z, Yu C, Ma L, Hu W and Xu
L: Phospho-SXXE/D motif mediated TNF receptor 1-TRADD death domain
complex formation for T cell activation and migration. J Immunol.
187:1289–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee EW, Seo J, Jeong M, Lee S and Song J:
The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep.
45:496–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Xia P, Shi L and Fan Z: FADD
cleavage by NK cell granzyme M enhances its self-association to
facilitate procaspase-8 recruitment for auto-processing leading to
caspase cascade. Cell Death Differ. 19:605–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Z, Wan ZY, Guo YS, Wang HQ and Luo ZJ:
FasL on human nucleus pulposus cells prevents angiogenesis in the
disc by inducing Fas-mediated apoptosis of vascular endothelial
cells. Int J Clin Exp Pathol. 6:2376–2385. 2013.PubMed/NCBI
|
|
10
|
Wang C, Yuan J, Wu HX, et al: Paeoniflorin
inhibits inflammatory responses in mice with allergic contact
dermatitis by regulating the balance between inflammatory and
anti-inflammatory cytokines. Inflamm Res. 62:1035–1044. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu HQ, Zhang WY, Luo XT, Ye Y and Zhu XZ:
Paeoniflorin attenuates neuroinflammation and dopaminergic
neurodegeneration in the MPTP model of Parkinson's disease by
activation of adenosine A1 receptor. Br J Pharmacol. 148:314–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
Paeoniflorin, a natural neuroprotective agent, modulates multiple
anti-apoptotic and pro-apoptotic pathways in differentiated PC12
cells. Cell Mol Neurobiol. 33:521–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salunga TL, Tabuchi Y, Takasaki I, et al:
Identification of genes responsive to paeoniflorin, a heat shock
protein-inducing compound, in human leukemia U937 cells. Int J
Hyperthermia. 23:529–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu D, Chen J, Zhu H, et al: UPLC-PDA
determination of paeoniflorin in rat plasma following the oral
administration of Radix Paeoniae Alba and its effects on rats with
collagen-induced arthritis. Exp Ther Med. 7:209–217.
2014.PubMed/NCBI
|
|
15
|
Wu YM, Jin R, Yang L, et al:
Phosphatidylinositol 3 kinase/protein kinase B is responsible for
the protection of paeoniflorin upon
H2O2-induced neural progenitor cell injury.
Neuroscience. 14:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
The Ministry of Science and Technology of
the People's Republic of China: Guidance Suggestions for the Care
and Use of Laboratory Animals. 2006.
|
|
17
|
Liu ZH, Sun Z, Wang HQ, et al: FasL
expression on human nucleus pulposus cells contributes to the
immune privilege of intervertebral disc by interacting with
immunocytes. Int J Med Sci. 10:1053–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji Y, Wang T, Wei ZF, Lu GX, Jiang SD, Xia
YF and Dai Y: Paeoniflorin, the main active constituent of Paeonia
lactiflora roots, attenuates bleomycin-induced pulmonary fibrosis
in mice by suppressing the synthesis of type I collagen. J
Ethnopharmacol. 149:825–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang MH, Feng L, Zhu MM, Gu JF, Wu C and
Jia XB: Antioxidative and anti-inflammatory activities of
paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell
damage. Planta Med. 79:1319–1323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wankun X, Wenzhen Y, Min Z, et al:
Protective effect of paeoniflorin against oxidative stress in human
retinal pigment epithelium in vitro. Mol Vis. 17:3512–3522.
2011.PubMed/NCBI
|
|
21
|
Lavrik IN and Krammer PH: Regulation of
CD95/Fas signaling at the DISC. Cell Death Differ. 19:36–41. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashkenazi A and Dixit VM: Death Receptors:
Signaling and modulation. Science. 281(5381): 1305–1308. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JB, Chang H and Kim KW: Expression of
Fas ligand and apoptosis of disc cells in herniated lumbar disc
tissue. Spine. 26:618–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lechardeur D, Drzymala L, Sharma M, et al:
Determinants of the nuclear localization of the heterodimeric DNA
fragmentation factor (ICAD/CAD). J Cell Biol. 150:321–334. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mehrkens A, Karim MZ, Kim S, Hilario R,
Fehlings MG and Erwin WM: Canine notochordal cell-secreted factors
protect murine and human nucleus pulposus cells from apoptosis by
inhibition of activated caspase-9 and caspase-3/7. Evid Based Spine
Care J. 4:154–156. 2013. View Article : Google Scholar : PubMed/NCBI
|