Introduction
Glucose-regulated protein 78 (GRP78) is an important
protein in the endoplasmic reticulum (ER). ER stress is a reaction
that leads to cell death in organisms. During ER stress, GRP78
initiates a cellular self-defense mechanism, the unfolded protein
response signaling cascade, which rebalances the functions of the
ER, allowing the cells to survive under altered living conditions
(1). Excessive and sustained ER
stress triggers pathological processes, such as the nuclear
factor-κB (NF-κB) signaling pathway, inflammatory response and
programmed cell death, in which GRP78 acts as an important factor
closely associated with the occurrence of multiple diseases
(2,3). Therefore, the role of GRP78 in liver
diseases has been attracting increasing attention.
Hepatitis and liver cirrhosis are common diseases
that are severely harmful to human health. During severe liver
disease, the dysfunction or functional decline of the mechanical
and immunological barriers of the intestinal mucosa causes a
reduced defense capability against infections. This can easily
result in infections of tissues and organs throughout the body, as
well as intestinal endotoxemia (IETM) (4). IETM activates a variety of active
substances secreted by Kupffer cells, such as cytokines,
inflammatory mediators and free radicals; this leads to secondary
liver tissue injury (5) and
facilitates the formation of liver fibrosis or even liver
cirrhosis. The main pathological feature of liver cirrhosis is the
abnormal proliferation of collagen fibers, the synthesis of which
is directly associated with the functions of the ER. Experimental
studies have indicated that ER stress plays an important role in
hepatitis, alcoholic liver injury and non-alcoholic fatty liver
diseases (6–8).
In the present study, a rat model of liver cirrhosis
was established through the inductive effects of composite
pathogenic factors in order to investigate the effects that ER
stress have on the pathogenesis of IETM-induced liver fibrosis and
cirrhosis.
Materials and methods
Reagents
CCl4 (analytically pure) was purchased
from Fuyu Chemical Co., Ltd. (Tianjin, China), cholesterol from
Tianjin Chemical Reagent Co., Ltd. (Tianjin, China), and alanine
aminotransferase (ALT) activity and malondialdehyde (MDA) assay
kits were purchased from Nanjing Jiancheng Bioengineering Institute
(Tianjin, China). Endotoxin Chromogenic End-point Tachypleus
Amebocyte Lysate assay kit was obtained from Xiamen Horseshoe Crab
Reagent Manufactory, Co., Ltd. (Xiamen, China), and the tumor
necrosis factor-α (TNF-α) radioimmunoassay kit from Beijing
Purevalley Biotech Co., Ltd., (Beijing, China). The reverse
transcription quantitative polymerase chain reaction (RT-qPCR) kit
was purchased from Takara Biotechnology (Dalian) Co., Ltd. (Dalian,
China) and the homocysteine (HCY) ELISA assay kit from AC
Diagnostics, (San Diego, CA, USA). GRP78 rabbit anti-rat polyclonal
antibody (G9043; Sigma-Aldrich, St. Louis, MO, USA) and
immunohistochemistry streptavidin peroxidase (SP) kit were
purchased from Beijing Biosynthesis Biotechnology Co., Ltd.
(Beijing, China). Procollagen type III radioimmunoassay kit was
purchased from Beijing Huaying Biotechnology Research Institute
(Beijing, China). Commercial brand liquor, cornmeal and lard were
all purchased from public markets.
Sample preparation
Fifty-one clean-grade healthy male Wistar rats,
weighing 200–240 g, were provided by the Experimental Animal Center
of Shanxi Medical University (Taiyuan, China). All animal
experiments were conducted according to the ethical guidelines of
Shanxi Medical University. The rats were randomly divided into four
groups: The liver cirrhosis 4-week (n=11), 6-week (n=11) and 8-week
(n=11) groups, and the normal control group (n=18), which received
a normal diet. Rats in the normal control group were fed with
standard feed and tap water. Rat liver cirrhosis models were
constructed through the induction of composite pathogenic factors
(9): i) The rats were fed with
cornmeal blended with cholesterol (0.5% in weight), and in the
first 2 weeks the feed was additionally blended with 20% lard; ii)
the rats consumed only 5–15% alcohol as a beverage; and iii) the
rats were injected subcutaneously on the back with CCl4
solution. On the first day of the experiment, the rats were
injected with CCl4 at the amount of 0.5 ml/100 g body
weight. They were then injected with 40% CCl4 oil
solution every 3 days at the amount of 0.3 ml/100 g body weight.
Samples were collected at the end of weeks 4, 6 and 8,
respectively. Abdominal aortic blood was drawn in sterile and
endotoxin-free conditions and was centrifuged at 1,200 × g for 15
min at 4°C to separate the plasma, which was stored at −70°C. The
rats were anesthetized with 3% pentobarbital sodium (3 ml/kg body
weight; Shanghai XiTang Biological Technology Co.,Ltd., Shanghai,
China) and then sacrificed at 4-week, 6-week and 8-week after
injection with CCl4, respectively. The rat livers were extracted
and weighed and 10% of the liver tissue was cut and fixed with
neutral formalin for histological examination. The rest of the
liver tissue was immediately stored in liquid nitrogen.
Histopathology
Paraffin-embedded liver tissues were sectioned at 4
µm. Under an optical microscope (CX40; Olympus Corporation, Tokyo,
Japan), liver tissue injury was observed by examination of the
slices stained with hematoxylin and eosin (H&E) and the liver
fibrosis status was visualized using van Gieson (VG) staining.
Determination of biochemical
parameters in plasma and liver
For the plasma sample, ALT activity was determined
by the Reitman and Frankel method (10). The levels of HCY were detected by
ELISA assay, those of endotoxin using the Chromogenic End-point
Tachypleus Amebocyte Lysate kit and those of TNF-α using
radioimmunoassay. For the liver sample, tissue homogenates (10%)
were prepared for the examination of MDA, TNF-α and procollagen
type III peptide (PIIIP) levels, according to the manufacturer's
instructions of the RT-qPCR kit (Takara Biotechnology Co., Ltd.).
Quantification of the proteins was performed using Coomassie
brilliant blue staining.
RT-qPCR
Total RNA was extracted from the liver tissues of
the rats by a one-step method using TRIzol® reagent (Gibco Life
Technologies, Grand Island, NY, USA) and was then subjected to RT
reaction using the RT-qPCR kit. The cDNA proliferation product of
GAPDH was used as an internal control. The rat GAPDH primer
sequences were as follows: Upstream, 5′-GGT CAT CAA CGG GAA
ACCC-3′; downstream, 5′-TCT GAG TGG CAG TGA TGG CA-3′; the amplicon
length was 450 bp. The rat GRP78 primer sequences were as follows:
Upstream, 5′-GGA GGA TGT GGG CAC GGT GGTC-3′; downstream 5′-GTC ATT
CCA AGT GCG TCC GAT GAGG-3′. The amplicon length was 385 bp. The RT
and qPCR reactions were conducted according to the manufacturer's
instructions. PCR amplification conditions were as follows: Initial
denaturation at 95°C for 60 sec, denaturation at 95°C for 15 sec
and annealing at 60°C for 60 sec, repeated for 40 cycles. The bands
of the amplification products were scanned and analyzed using the
Quantity One gel analysis system (Bio-Rad, Hercules, CA, USA). The
absorbance ratio of GRP78 to GAPDH was calculated and served to
indicate the relative content of the expressed GRP78 mRNA.
Immunohistochemistry
Paraffin-embedded slices were dewaxed and rehydrated
through graded alcohols. The slices were then stained using SP
according to the instructions provided with the
immunohistochemistry kit. The primary GRP78 antibody (1:100) was
replaced by phosphate-buffered saline for the negative control.
GRP78 expression in the liver tissue was observed under a
microscope, with the positive staining reaction observed as brown
granules. Five slices were observed for each group of samples, and
10 fields of vision were recorded for each slice. Images were
captured using a digital camera and analyzed using Image-Pro® plus
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) in order
to determine the average optical density of the cells.
Statistical analysis
Data are presented as the mean ± standard deviation.
A one-way analysis of variance and LSD t-test were performed using
SPSS 10.0 software (SPSS Inc., Chicago, IL, USA). Bivariate
correlation method was selected and the Pearson correlation
coefficient and significant correlation were calculated, with a
significant correlation indicated by <0.01. In all other
statistical analyses, P<0.05 was considered to indicate a
statistically significant difference.
Results
Liver cirrhosis induces changes in the
general status and liver histopathology of rats
To investigate the general status of the rats,
visual inspection was carried out. Rats of the normal control group
appeared content and exhibited zero mortality, shiny hair, agile
activities, quick responses, good appetite and normal urine and
feces. Rats of the experimental groups exhibited messy, sparse,
yellow and matted hair and hair loss, as well as listless behavior,
decreased activity and appetite, limited feces, yellow urine, slow
responses to external stimulations, and an infection rate of 18.2%
(6/33), which was calculated based on the precess of abscesses. We
observed the lungs and thoracic cavity of rats with liver cirrhosis
at 4, 6 and 8 weeks (n=33). Anatomical observation indicated that 6
rats had pulmonary abscess, chest abscess or subdiaphragmatic
abscess, and thus the infection rate was calculated to be 6/33.
Histopathological investigation revealed that the
livers of the rats in the normal control group were thin and sharp
at the edge, ruddy in color, soft in texture and smooth on the
surface, while the livers of the rats in the experimental groups
had shrunk in size and were rounded at the edge, pale in color,
hardened in texture and rough and uneven on the surface, which had
numerous nodules.
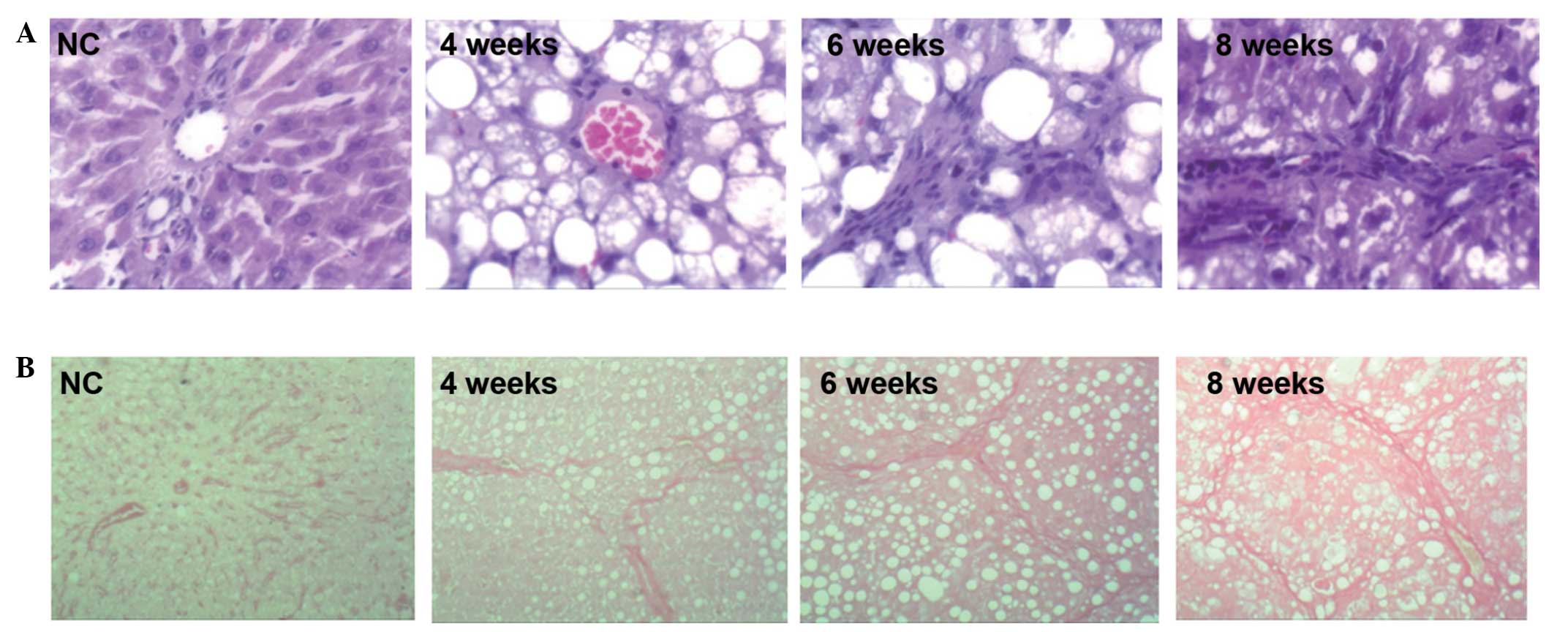
H&E staining revealed the following: In the
control group the hepatic lobules were regular in structure, the
arrangement of the hepatic cords was organized and they were
distributed radially around the central vein, the cytoplasms of the
liver cells were abundant and the nuclei were round and stained
blue. No inflammatory cell infiltration was observed. In the liver
cirrhosis 4 week group, the arrangement of the hepatic cell cords
was disordered and fat vacuoles were observed. In the liver
cirrhosis 6 week and 8 week groups, the structures of the hepatic
lobules were no longer visible, irregular narrowing of the hepatic
sinusoids was observed, the arrangement of the hepatic cells was
disordered, steatosis was significant, bullous fat droplets were
markedly accumulated and abundant fat vacuoles appeared in the
cytoplasm. In addition, ballooning degeneration occurred in the
cells surrounding the liver and inflammatory cell infiltration was
observed, mainly in the portal area (Fig. 1A).
VG staining revealed the following: In the control
group the hepatic lobules were regular in structure; the
arrangement of the hepatic cords was organized, with only a few red
filamentous fiber structures present in the central vein and portal
area. In the liver cirrhosis 4 week group, a greater number of
fibrous structures were observed, fibrous connective tissue
proliferated and formed filamentous cords in the portal area and
hepatic lobules were fragmented by sparse fiber bundles. In the
liver cirrhosis 6 week group, more severe fibroplasia was observed
and the width of the fibers varied. The fibers extended into the
hepatic lobules in stellate shapes, dividing the liver into
pseudolobules with various sizes and irregular shapes, and
additional fibers appeared around the hepatic sinusoids, with those
in the central vein and portal area being the most significant. In
the liver cirrhosis 8 week group, fibrous tissues proliferated
abundantly in the portal area. The majority of the fibrous tissues
were connected to each other, with widened spaces between them.
Furthermore, the normal structures of the liver tissue were altered
(Fig. 1B).
Image analysis of the slices demonstrated that the
content of collagen fibers in the liver tissues of the experimental
groups was higher than that in the control group (P<0.05). These
data suggest that liver cirrhosis caused significant
histopathological changes to the livers of the rats.
Liver cirrhosis induces changes in the
levels of ALT, HCY, endotoxin and TNF-α in rat plasma
During the formation of liver cirrhosis, the ALT
activity levels of all three experimental groups were higher than
that those in the control group (P<0.05). The levels of HCY,
endotoxin and TNF-α in the plasma increased gradually, with those
at the end of week 8 being significantly different from those in
the control group (P<0.05; Table
I). These results suggest that liver cirrhosis increased the
levels of ALT, HCY, endotoxin and TNF-α in the plasma of the
rats.
 | Table I.Levels of ALT, HCY, endotoxin and
TNF-α in the plasma. |
Table I.
Levels of ALT, HCY, endotoxin and
TNF-α in the plasma.
| Groups | n | ALT (u/l) | HCY (µmol/l) | Endotoxin
(Eu/ml) | TNF-α (ng/ml) |
|---|
| NC | 18 |
197.25±26.27 |
26.23±4.10 |
0.04±0.02 |
1.35±0.40 |
| 4 week | 11 |
324.58±22.02a |
13.98±1.00a |
0.08±0.02 |
2.13±0.18a |
| 6 week | 11 |
380.43±30.90a,b |
44.31±7.23a |
0.24±0.12a,b |
2.10±0.23a |
| 8 week | 11 |
364.24±29.89a,b |
49.60±15.56a,b |
0.26±0.18a,b |
2.35±0.08a–c |
Liver cirrhosis induces changes in the
levels of MDA, TNF-α and PIIIP in the rat liver tissue
homogenates
For the quantification of the MDA, TNF-α and PIIIP
levels in liver tissue homogenates, the BCA protein assay kit was
used to determine the protein concentration. As the liver cirrhosis
progressed, the MDA levels were elevated; the MDA levels of all
experimental groups were significantly different from those in the
control group (P<0.05). The concentration of TNF-α was also
markedly increased in the experimental groups, and the TNF-α
concentrations of the liver cirrhosis 6 week and 8 week groups were
significantly different from that of the control group (P<0.05).
The PIIIP levels of all experimental groups were significantly
higher than those of the control group (P<0.05; Table II). These data indicate that liver
cirrhosis elevated the levels of MDA, TNF-α and PIIIP in the liver
tissue.
 | Table II.Levels of MDA, TNF-α and PIIIP in the
liver tissues. |
Table II.
Levels of MDA, TNF-α and PIIIP in the
liver tissues.
| Groups | n | MDA (nmol/ml) | TNF-α (ng/ml) | PIIIP (µg/l) |
|---|
| NC | 18 |
1.86±0.20 |
0.92±0.23 |
33.65±34.53 |
| 4 week | 11 |
4.20±0.53a |
1.21±0.05 |
65.55±12.50a |
| 6 week | 11 |
5.61±1.80a,b |
1.74±0.77a |
78.74±28.60a |
| 8 week | 11 |
11.06±1.99a–c |
2.01±0.32a,b |
75.54±29.11a |
Liver cirrhosis induces increases in
the GRP78 mRNA and protein expression levels in rat liver
tissues
In order to investigate the GRP78 mRNA expression in
the liver tissue of the rats, RT-qPCR was performed (Fig. 2A). Positive bands of GRP78 mRNA were
observed for all experimental groups and the control group. GRP78
mRNA transcription in the liver cirrhosis groups was gradually
enhanced by the end of weeks 4, 6 and 8, respectively, and the
increased levels were significantly different from that of the
control group (P<0.05).
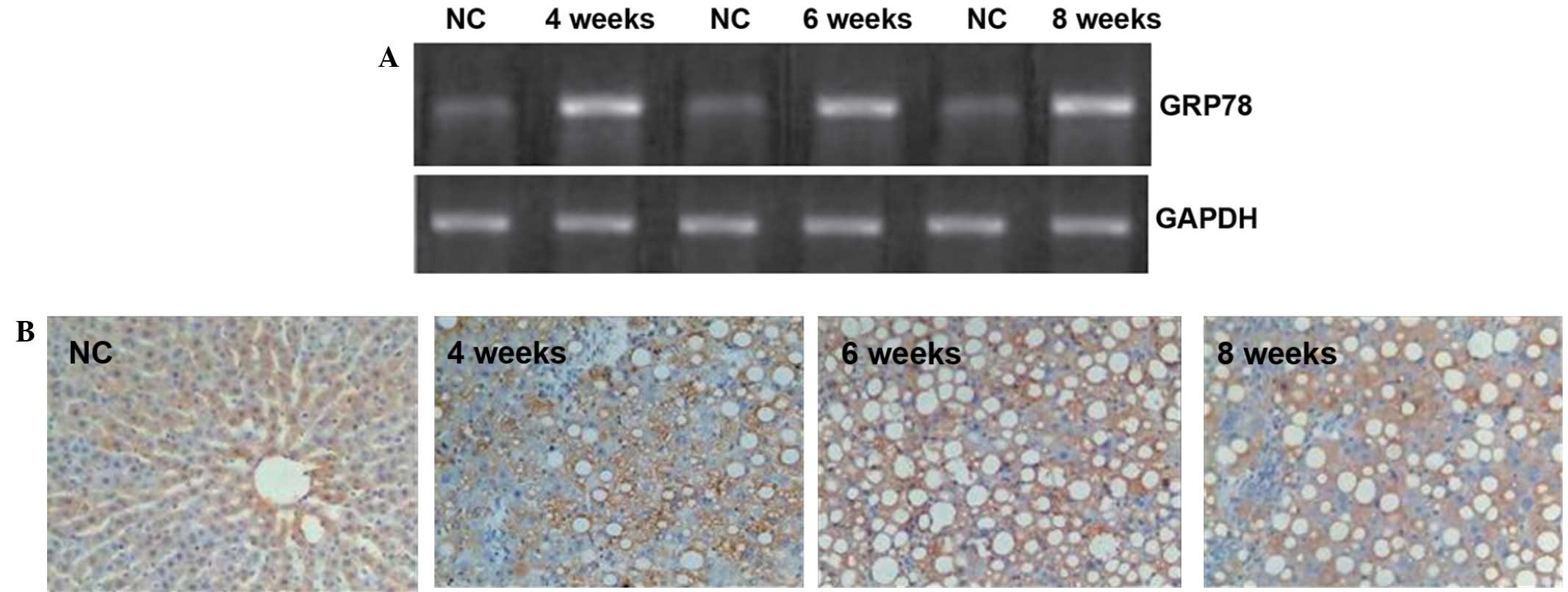 | Figure 2.GRP78 expression in rat livers. (A)
Gel assay for the detection of GRP78 mRNA in the rat liver tissues.
Optical density values obtained using the Quantity One gel analysis
system were as follows: NC, 1.16±0.03; 4 weeks, 1.32±0.22; 6 weeks,
1.36±0.08, 8 weeks, 1.47±0.12 (all P<0.05 compared with the NC
group). (B) Immnunohistochemistry of GRP78 protein expression in
the rat liver tissues (magnification, ×400). The number of GRP78
positive cells in the liver tissues of the rats as determined by
semi-quantitative analysis were as follows: NC, 2.2±0.2; 4 weeks,
8.2±1.5; 6 weeks, 9.4±1.0; 8 weeks, 10.2±1.6 (all P<0.05
compared with the NC group). GRP78, glucose-regulated protein 78;
NC, normal control. |
To visualize the GRP78 protein
expression, immunohistochemistry was employed
In the control group, only a few GRP78-positive
cells with brown granules were visible in the cytoplasm of cells in
the liver tissues. By contrast, the number of GRP78-positive
granules observed in the experimental groups was significantly
increased compared with the number observed in the control group
(Fig. 2B). Furthermore, fat vacuoles
appeared in the cells, and positive staining was observed in the
cytoplasm and cell membranes (Fig.
2B). These results suggest that the GRP78 protein expression in
the liver tissue was increased by liver cirrhosis in the rats.
Correlation between increases in GRP78
expression and MDA levels during the formation of liver cirrhosis
in rats
Statistical analysis was conducted to test the
correlation between various factors during the formation of liver
cirrhosis in the rats. Endotoxin levels in the plasma were found to
correlate with MDA levels in the liver homogenates, HCY levels in
the plasma and the GRP78 positive cell number. The GRP78 protein
expression level was found to be positively correlated with the MDA
and HCY levels in the plasma (Table
III); therefore, the present data suggest that MDA levels in
rats were affected by the elevation of the GRP78 protein expression
during the formation of liver cirrhosis
 | Table III.Correlation analysis. |
Table III.
Correlation analysis.
| Factor 1 | Factor 2 | Correlation
coefficient | P-value |
|---|
| Endotoxin in
plasma | MDA in liver
homogenate | 0.861 | <0.01 |
| Endotoxin in
plasma | HCY in the
plasma | 0.895 | <0.01 |
| Endotoxin in
plasma | GRP78 positive cell
number | 0.833 | <0.01 |
| GRP78 protein | MDA in the
plasma | 0.800 | <0.01 |
| GRP78 protein | HCY in the
plasma | 0.617 | <0.01 |
Discussion
In the present study, the rat liver underwent
pathological processes, including inflammatory cell infiltration,
hepatic steatosis, fibrosis and even cirrhosis, following the
impairment induced by composite pathogenic factors. The elevated
levels of ALT indicated that the hepatocytes were severely injured,
and the increased levels of plasma HCY implied that liver
metabolism was dysfunctional. Yellow skin and mucosa and dark urine
suggested that the secretory function of the liver was decreased.
The elevated MDA levels in liver tissue homogenates demonstrated
the occurrence of oxidative stress. Increased endotoxin levels in
the plasma, combined with similar elevations of TNF-α in the plasma
and liver tissue homogenates, demonstrated the formation of IETM.
Liver injury induced by idiopathic pathogenic factors is known to
lead to gastrointestinal disorders, intestinal bacterial
overgrowth, the functional decline of mechanical and immunological
barriers of the intestinal mucosa and injury of phagocytic function
Kupffer cells, which subsequently act as important causes of IETM
(11) and bacteremia.
It has previously been demonstrated that, following
secondary liver injury, IETM formed during various liver diseases
is the primary cause of the transformation of acute hepatitis to
chronic and then severe hepatitis. Endotoxins not only are directly
toxic to hepatocytes, but also cause further damage to the liver by
sequentially binding with lipolysaccharide binding protein CD14 and
the transmembrane signaling receptor toll-like receptor 4, to
activate endotoxin-related signaling pathways, such as the NF-κB
pathway, and to facilitate the transcription, synthesis and release
of various cytokines and chemical factors (12). Notably, TNF-α not only mediates
multiple biological effects of endotoxin by itself, but also
induces other inflammatory mediators to jointly take effect in
inducing transforming growth factor-β, and promoting the activation
and proliferation of hepatic stellate cells and their
transformation to fibroblasts, thus increasing fibronectin and
proteoglycan synthesis and facilitating liver fibrosis and
cirrhosis (13). Liver fibrosis is
the intermediate phase in the transformation from hepatitis and
liver damage to liver cirrhosis and its pathology involves the
excessive precipitation of extracellular matrix including collagen,
glycoprotein and proteoglycan, among which collagen type I and III
are the main components. It may, therefore, be hypothesized that
IETM plays a key role in liver cirrhosis. For a long time, NF-κB
was deemed to be a molecular target for antagonizing the biological
effect of endotoxin (14); however,
blocking the effect of endotoxin by targeting a single signaling
molecule is unlikely to be successful due to the existence of a
network among different signaling pathways; therefore, it is
important for the mechanisms of cirrhosis caused by IETM to be
elucidated, in order to provide effective strategies to prevent and
treat liver cirrhosis.
GRP78 is a molecule that appears in the earliest
stage of the development of the endoplasmic reticulum (ER) and
possesses important functions. Studies have discovered that GRP78
is a key factor in the ER that is closely associated with multiple
diseases. GRP78 has been shown to be directly involved in the onset
of hepatitis, alcoholic liver injury and non-alcoholic fatty liver
disease (7–9). In the present study, it was found that
in the formation of rat liver cirrhosis, GRP78 protein expression
in the liver tissues increased with the progression of the disease,
and the percentage increase correlated with the plasma levels of
endotoxin, MDA and HCY. Studies have indicated that endotoxins
cause ER stress directly or indirectly through the induction of
oxidative stress, which could also be promoted by ER stress. These
two types of stress interact with each other in the progression of
the disease (15–17).
In the present study, IETM-induced oxidative stress
and mitochondrial dysfunction may have led to an imbalance of
Ca2+, while the endotoxin induced a great number of
inflammatory mediators that are likely to cause the accumulation of
unfolded proteins in the ER, or cause ER stress by insulin
resistance or by increasing free fatty acids and hence, increased
the expression of GRP78 (18). In
previous studies of rats with acute and alcoholic liver injuries,
increases in GRP78 protein expression coincided with hepatocyte
apoptosis and pathological injuries (19,20).
Furthermore, the reduction of GRP78 protein expression induced by
chlorogenic acid has been reported to significantly inhibit liver
fibrosis (21). Prostaglindin E1 has
also exhibited inhibitory effects on the precipitation of liver
collagen, while effectively reducing GRP78 levels (22). In addition, hyper-homocysteinemia
(HHCY), which is caused by methionine cycle disorders, is also an
important cause of ER stress and the high expression of GRP78
(8). Ji et al (8) showed that HCY increased the production
of intracellular O2 by promoting the release of
inflammatory mediators, such as NF-κB, interleukin-6 and
interleukin-1B, reduced nitric oxide levels and induced ER stress,
thereby facilitating the occurrence of liver cirrhosis.
On the basis of the aforementioned findings, the
present study suggests that intestinal endotoxin causes ER stress,
directly or indirectly through oxidative stress, and sustained ER
stress generates positive feedback to aggravate the injury and
cause a continuous increase of GRP78 expression levels, which may
trigger a sterol regulatory cascade reaction (18). This would lead to the abnormal
accumulation of hepatic lipids, causing steatosis, inflammation,
necrosis and apoptosis of the liver cells, being the key factor to
promote liver fibrosis formation. In addition, HHCY, which occurred
simultaneously, is also likely to play a crucial role in the
formation of liver fibrosis.
A previous study has reported that the
overexpression of GRP78 in the livers of obese rats, achieved using
adenoviral vectors, decreased the cleavage of sterol regulatory
element binding protein-1c, inhibited the expression of lipogenesis
enzymes and significantly alleviated hepatic steatosis (23). This particular study further
demonstrated the close association between GRP78 expression and
liver fibrosis; however, it remains unclear whether GRP78 is a
switch for fat metabolism promoting hepatic steatosis in the
condition of pathological high expression, and inhibiting hepatic
steatosis when its expression level exceeds the regulating levels
in the organisms following human intervention.
In conclusion, the data of the present study
indicate that IETM and HHCY aggravate liver injuries, possibly by
triggering ER stress in liver tissues, and facilitate the formation
of hepatic steatosis, fibrosis and even cirrhosis. Therefore,
during the treatment of liver diseases, in addition to reducing the
production of endotoxin and lowering plasma HCY levels, it is also
important to regulate GRP78 protein expression levels, in order to
rebalance ER functions and delay or stop the progression of liver
fibrosis and cirrhosis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81070339), the
International Science and Technology Cooperation Project in Shanxi
Province (grant no. 2010081068), the Director Funding of MOE Key
Laboratory on Cellular Physiology Established by Province and
Ministry in Shanxi Medical University (grant no. 2010-09) and the
Returned Overseas Expert Foundation in Shanxi Province (grant no.
211-091). In addition, funding was obtained from the US National
Institutes of Health (grant nos. R01AA018612 and R01AA014428).
References
|
1
|
Gonzalez-Gronow M, Selim MA, Papalas J and
Pizzo SV: GRP78: A multifunctional receptor on the cell surface.
Antioxid Redox Signal. 11:2299–2306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Görlach A, Klappa P and Kietzmann T: The
endoplasmic reticulum: Folding, calcium homeostasis, signaling, and
redox control. Antioxid Redox Signal. 8:1391–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji C: Dissection of endoplasmic reticulum
stress signaling in alcoholic and non-alcoholic liver injury. J
Gastroenterol Hepatol. 23(Suppl 1): S16–S24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X
and Zhao HZ: Experimental study on the role of endotoxin in the
development of hepatopulmonary syndrome. World J Gastroenterol.
11:567–572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito K, Kiyosawa N, Kumagai K, Manabe S,
Matsunuma N and Yamoto T: Molecular mechanism investigation of
cycloheximide-induced hepatocyte apoptosis in rat livers by
morphological and microarray analysis. Toxicology. 219:175–186.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esfandiari F, Villanueva JA, Wong DH,
French SW and Halsted CH: Chronic ethanol feeding and folate
deficiency activate hepatic endoplasmic reticulum stress pathway in
micropigs. Am J Physiol Gastrointest Liver Physiol. 289:G54–G63.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gentile CL and Pagliassotti MJ: The role
of fatty acids in the development and progression of Nonalcoholic
fatty liver disease. J Nutr Biochem. 19:567–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji C and Kaplowitz N: Betaine decreases
hyperhomocysteinemia, endoplasmic reticulum stress and liver injury
in alcohol-fed mice. Gastroenterology. 124:1488–1499. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HY, Han DW, Zhao ZF, Liu MS, Wu YJ,
Chen XM and Ji C: Multiple pathogenic factor-induced complications
of cirrhosis in rats: A new model of hepatopulmonary syndrome with
intestinal endotoxemia. World J Gastroenterol. 13:3500–3507. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reitman S and Frankel S: A colorimetric
method for the determination of serum glutamic oxaloacetic and
glutamic pyruvic transaminase. Am J Clin Pathol. 28:56–63.
1957.PubMed/NCBI
|
|
11
|
Zhang HY, de Han W, Su AR, Zhang LT, Zhao
ZF, Ji JQ, Li BH and Ji C: Intestinal endotoxemia exerts a central
role in development of hepatopulmonary syndrome in a cirrhotic rat
model induced by multiple pathogenic factors. World J
Gastroenterol. 13:6385–6395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu CP, Liu J, Liu JC, Han DW, Zhang Y and
Zhao YC: Dynamic changes and mechanism of intestinal endotoxemia in
partially hepatectomized rats. World J Gastroenterol. 13:3592–3597.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Kim YS, Hwang JW, Han YK, Lee JS,
Kim SK, Jeon YJ, Moon SH, Jeon BT, Bahk YY and Park PJ: Sulfated
chitosan oligosaccharides suppress LPS-induced NO production via
JNK and NF-κB inactivation. Molecules. 19:18232–18247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Qin J, Liu X, Han Y, Yang Z, Chang
X and Ji X: Nitric oxide-mediated neuronal apoptosis in rats with
recurrent febrile seizures through endoplasmic reticulum stress
pathway. Neurosci Lett. 443:134–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang K: Integration of ER stress,
oxidative stress and the inflammatory response in health and
disease. Int J Clin Exp Med. 3:33–40. 2010.PubMed/NCBI
|
|
17
|
Ilieva EV, Naudí A, Kichev A, Ferrer I,
Pamplona R and Portero-Otín M: Depletion of oxidative and
endoplasmic reticulum stress regulators in Pick disease. Free Radic
Biol Med. 48:1302–1310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiramatsu N, Kasai A, Hayakawa K, Yao J
and Kitamura M: Real-time detection and continuous monitoring of ER
stress in vitro and in vivo by ES-TRAP: Evidence for systemic,
transient ER stress during endotoxemia. Nucleic Acids Res.
34:e932006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen T, Wu ZM, Liu Y, Tan YF, Ren F and Wu
H: Upregulation of heme oxygenase-1 with hemin prevents
D-galactosamine and lipopolysaccharide-induced acute hepatic injury
in rats. Toxicology. 237:184–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji C and Kaplowitz N:
Hyperhomocysteinemia, endoplasmic reticulum stress and alcoholic
liver injury. World J Gastroenterol. 10:1699–1708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi H, Dong L, Bai Y, Zhao J, Zhang Y and
Zhang L: Chlorogenic acid against carbon tetrachloride-induced
liver fibrosis in rats. Eur J Pharmacol. 623:119–124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L and Zhu HG: Inhibition of
prostaglandin E1 on acumulation of collagen type I and III in liver
of rabit with schistosoma japonicum. Zhong Guo Pu Tong Wai Ke Za
Zhi She. 16:577–580. 2007.(In Chinese).
|
|
23
|
Kammoun HL, Hainault I, Ferré P and
Foufelle F: Nutritional related liver disease: Targeting the
endoplasmic reticulum stress. Curr Opin Clin Nutr Metab Care.
12:575–582. 2009. View Article : Google Scholar : PubMed/NCBI
|