Introduction
Pulmonary embolism (PE) and deep vein thrombosis
(DVT), referred to collectively as venous thromboembolism (VTE),
are life-threatening conditions that may arise in patients
following acute trauma and major surgery, particularly after total
hip and knee replacement (1,2). Patients are usually asymptomatic until
the occurrence of a fatal PE (3).
Prevention of VTE requires a reliable tool for the stratification of
the risk for developing VTE, screening strategies and effective
prophylaxis to significantly reduce mortality in intensive care
unit (ICU) patients. Basic indications of VTE, including
endothelial abnormality, stasis of blood flow and
hypercoagulability, are typically observed in the critically ill
(4). Pharmacological prophylaxis
with low-molecular-weight heparin (LMWH) has been demonstrated to
reduce VTE rates (5,6). In addition, LMWH may be associated with
a reduction in major bleeding compared with unfractionated heparin
(7). Furthermore, the trade-off
between VTE prevention and excess hemorrhage has prevented the
development of a consensus in the guidelines of major professional
societies (8). The reliable
identification of patients that could potentially benefit from
high-potency prophylaxis may help to resolve this controversy and
facilitate the selection of prophylaxis by ICU teams. Previous
studies on the thromboprophylactic effect of intermittent pneumatic
compression (IPC) have indicated that its use may protect against
major DVT events (9–11). However, compliance has previously
been a limitation, and this treatment is not applicable to patients
with a pulmonary edema or heart failure. To the best of our
knowledge, no study has directly compared the thromboprophylactic
effect of IPC with that of LMWH. Therefore, the aim of the present
study was to compare the efficacy and safety of IPC combined with
LMWH, IPC and LMWH as VTE prophylactic treatments in 500 patients
undergoing major treatment in an ICU.
Materials and methods
Study design and patient
enrolment
This retrospective study was performed in the
general and surgical ICU of the Affiliated Hospital of Jiangsu
University (Zhenjiang, China) between January 2010 and March 2014.
After the trial was approved by the Institutional Ethics Committee,
500 patients were enrolled following admission to the ICU. Informed
consent was obtained either from the patient or the patients
family. The causes of ICU admission were various and included:
Traumatic brain injury and cerebral infarction; multiple injuries
such as multiple rib fractures, hemothorax with pneumothorax,
pulmonary contusion, ruptured spleen and intestinal rupture;
orthopedic hip replacement, pelvic fractures and femoral shaft
fractures; cancer such as lung, esophageal, stomach, colon and
pancreatic cancer, in addition to post-operative treatment
following the removal of other tumors; severe acute pancreatitis,
cirrhosis decompensation, severe pneumonia and acute respiratory
distress syndrome; shock, multiple organ dysfunction syndrome and
cardiopulmonary arrest resuscitation, and active peptic ulcer,
acute infective endocarditis, hemorrhagic stroke and severe renal
impairment (Table I). The patients
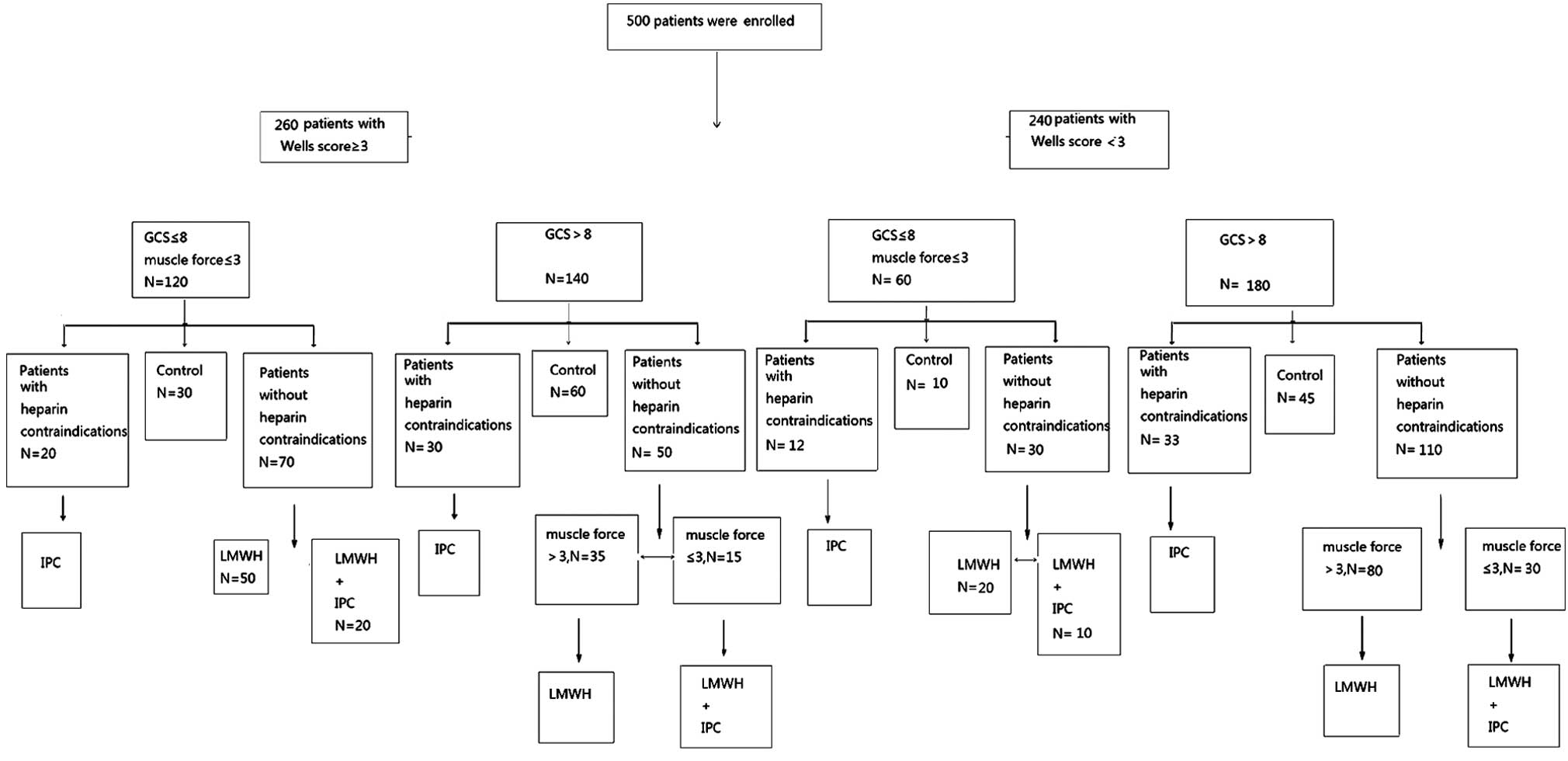
were divided into four groups according to the prophylaxis of DVT,
as follows: IPC group, LMWH group, LMWH + IPC group and control
group (in which the patients were not treated with IPC or LMWH)
(Fig. 1). Severity of patient
illness was evaluated using the Glasgow Coma Scale, Wells scoring
system and an assessment of muscle force.
 | Table I.Comparison of basic characteristics
among the four patient groups. |
Table I.
Comparison of basic characteristics
among the four patient groups.
| Characteristic | IPC | LMWH | LMWH + ICP | Control |
|---|
| Total cases | 95 | 185 | 75 | 145 |
| Gender
(male/female) | 65/30 | 125/60 | 55/20 | 110/35 |
| Age (mean years ±
SE) | 55.0±4.0 | 58.9±3.4 | 59.6±3.5 | 60.3±3.3 |
| Craniocerebral
trauma | 9 | 18 | 7 | 11 |
| Brain infarction | 5 | 36 | 10 | 12 |
| Multiple injury | 6 | 14 | 3 | 12 |
| Tumor
postoperative | 3 | 22 | 10 | 11 |
| Severe acute
pancreatitis | 5 | 28 | 9 | 12 |
| Decompensation of
cirrhosis | 4 | 12 | 10 | 8 |
| Severe pneumonia | 1 | 18 | 6 | 8 |
| Acute respiratory
distress syndrome | 3 | 14 | 5 | 6 |
| Hemorrhagic
shock | 12 | 1 | 2 | 15 |
| Multiple organ
dysfunction syndrome | 3 | 9 | 5 | 12 |
| Cardiopulmonary
resuscitation | 4 | 12 | 7 | 5 |
| Active peptic
ulcer | 9 | 0 | 0 | 4 |
| Acute infective
endocarditis | 8 | 0 | 0 | 6 |
| Hemorrhagic
stroke | 14 | 0 | 0 | 14 |
| Severe renal
impairment | 9 | 1 | 1 | 9 |
Treatment
Patients that had been admitted to the ICU for
<30 days were excluded from the study.
Risk factors of all patients were assessed and
scored (12): Low risk, 1 point;
medium risk, 2 points; high risk, 3 or 4 points; and ultra-high
risk, ≥5 points. Higher scores indicated a higher risk of
developing DVT. Patients who had heparin contraindication were
grouped into a physiotherapy group and received the IPC
intervention (Shengsi Haichuan Medical Equipment, Co., Ltd.,
Shanghai, China) for 2 h each time, twice a day; patients in the
LMWH and LMWH + IPC groups received LMWH by subcutaneous injection
if their platelet count and prothrombin time were within the normal
ranges (5,000 units by subcutaneous injection, every 12 h). The
dose was changed on day 3 (5,000 units by subcutaneous injection,
once daily) to maintain the prothrombin time at 1.5–2.0-fold the
normal value.
The four chambers of the IPC device (Wonjin
manufacturer, Shengsi Haichuan Medical Equipment Co. Ltd.,
Shanghai, China) were placed on the calf, with two placed on the
thigh (avoiding the knee). Then, pneumatic pressure was adjusted
from the ankle to the calf and thigh, so that the pressure on the
ankle was the highest, and the pressure on the thighs was the
lowest, in order to promote effective venous return from the limb.
Pressure was increased rapidly to evacuate the deep vein of the
lower extremity within 11 sec, and subsequently reduced for 60 sec.
Finally, complete refilling of the deep vein was allowed.
The entire leg from the ankle to the thigh was
wrapped in inflatable sleeves for IPC, allowing for an appropriate
space of ≤1 cm between the IPC sleeve and the limb. The limb skin
temperature, color and dorsalis pedis artery pulse were monitored
closely by a nurse using an electronic thermometer and by manual
measurement.
Evaluation criteria
Simplified Wells' scoring systems were used to
evaluate DVT and PE (13). The most
common symptoms of DVT include: Pain deep in the calf or thigh,
unilateral swelling, increased temperature of the leg, tenderness
and redness. Positive signs for DVT additionally included
tenderness of the deep vein, swelling of the lower limb or calf
circumference >3 cm more than the normal size (10 cm below
tibial tuberosity), limb or pitting edema of the lower extremities
and visible superficial veins (excluding varicose veins) (14–16).
Heparin contraindications include: i) Allergic reaction to heparin
and LMWH; ii) severe coagulopathy; iii) history of reduced platelet
count induced by treatment with LMWH or heparin; iv) active peptic
ulcer or bleeding tendency of organ damage; and v) acute infective
endocarditis, with the exception of infection following heart valve
replacement surgery.
Color Doppler ultrasonography
Over the last two decades, venous compression
ultrasonography has become the imaging test of choice for
diagnosing DVT in the lower extremities of patients.
Ultrasonography for detection on venous thromboembolism has been
used widely (17–19).
Ultrasound examination (LOGIQ P3 ultrasound system;
GE Healthcare, Shanghai, China) of the symptomatic leg(s) was
performed by compression of the proximal veins. Compression
maneuvers of the symptomatic leg were conducted at 1-cm intervals
along the length of the femoral vein (from the inguinal ligament)
and popliteal veins to the level of the calf vein trifurcation
(20).
In addition, the ipsilateral external iliac vein was
imaged in all patients. Intraluminal echogenic masses consistent
with a thrombus were noted on B-mode imaging, and spontaneous
venous flow was assessed using Doppler interrogation. Absent or
reduced flow was further evaluated via ultrasound apparatus
(20). DVT was diagnosed based on
noncompressibility at any two contiguous segments of the femoral or
popliteal vein. Furthermore, DVT was diagnosed in the iliac veins
by the absence of flow within the iliac vein and/or the presence of
a visible thrombus by Doppler imaging. DVT was excluded based on
full compressibility of the femoral and popliteal veins and normal
Doppler imaging of the iliac veins.
Imaging was performed by trained technicians, and
the images were reviewed by local radiologists. In all cases,
abnormal results were confirmed by the local radiologist.
Ultrasound measurements for the deep vein of a lower
extremity were performed on days 1, 3, 7 and 14 after admission.
Ultrasound diagnostic criteria for lower extremity DVT (21) included: Widening of deep vein lumen
of the affected region after thrombosis, observation of substantial
hypoechoic lumen, filled or occupied portions of affected deep
vein, force required to compress the affected region, inability to
flatten the venous lumen and irregularly shaped thrombus. The
affected region (thrombus) showed no color flow signals on the
screen of the B-mode ultrasonic diagnostic equipment (GE
Healthcare) when the venous lumen was completely blocked. When the
lumen was partially blocked, reduced blood flow with an outline
containing points or in a scattered pattern could be observed near
the middle or edge of the thrombus. In certain cases blood flow
appeared as spots when the distal limb was compressed.
Enhanced helical computed tomography (CT)
examination (SOMATOM Definition AS 64-slice spiral CT scanner;
Siemens AG, Munich, Germany) was used in patients with symptoms
highly suggestive of DVT but with negative findings on
ultrasonography.
Statistical analysis
Ages are presented as the mean ± standard error, and
gender, rates of DVT and PE are presented as percentages.
Statistical analysis was conducted using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). Rates among the four groups
were compared using χ2 tests, whereas means among the
four groups were compared using t-tests. Ages among the four groups
were compared using analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
Incidence of DVT
A total of 500 patients were divided into four
groups: IPC, LMWH, LMWH + ICP or control (without LMWH or IPC). The
incidence rates of DVT were 9.5% in the IPC group, 9.2% in the LMWH
group, 0% in the LMWH + ICP group and 23.4% in the control group.
Statistically significant significances were detected between the
three prophylaxis groups and the control group, and were also noted
between the LMWH + ICP group and the IPC and LMWH groups. No
statistically significant difference was observed in DVT incidence
between the IPC group and the LMWH group (Table II).
 | Table II.Incidence of DVT, PE and
complications of treatment in the four groups. |
Table II.
Incidence of DVT, PE and
complications of treatment in the four groups.
| Group | No. of
patients | DVT cases, n
(%) | PE cases, n
(%) | Bleeding cases, n
(%) |
|---|
| IPC | 95 |
9
(9.5)a,b |
3
(3.2)a | 0 |
| LMWH | 185 | 17
(9.2)a,b | 2
(1.1)a | 10 (5.4) |
| LMWH + IPC | 75 | 0 (0)a | 0 (0)a | 4
(5.3) |
| Control | 145 | 34 (23.4) | 20 (13.6) | 0 |
Incidence of PE
The incidence rates of PE were 3.2% in the IPC
group, 1.1% in the LMWH group, 0% in the LMWH + ICP group and 13.6%
in the control group. There were observed to be statistically
significant differences between the three prophylaxis groups and
the control group, but no statistically significant differences
were noted in PE incidence among the prophylaxis groups (Table II).
Incidence of hemorrhage
The incidence rates of hemorrhage complications were
0% in the IPC group, 5.4% in the LMWH group, 5.3% in the LMWH + IPC
group and 0% in the control group. Patients that presented with
subcutaneous hemorrhage complications in the LMWH and LMWH + IPC
groups stopped bleeding following drug withdrawal, and no patients
suffered hemorrhage complications in the IPC and control
groups.
Wells' scoring system
Among the patients that had a Wells score of ≥3, the
incidence of DVT was 14% in the IPC group, 17.6% in the LMWH group,
0% in the LMWH + IPC group and 35.4% in the control group. Results
showed statistically significant differences between the three
prophylaxis groups and the control group and between the LMWH + IPC
group and the IPC and LMWH groups. No statistically significant
differences were identified between the IPC and LMWH groups
(Table III). Among the patients
with a Wells score of <3 (data not shown), the incidences of DVT
were 4.4% in the IPC group, 2% in the LMWH group, 0% in the LMWH +
IPC group and 3.6% in the control group. These results showed no
statistically significant differences among the four groups.
 | Table III.Incidence of DVT in patients from the
four groups with a Wells score ≥3. |
Table III.
Incidence of DVT in patients from the
four groups with a Wells score ≥3.
| Group | No. of
patients | DVT cases, n
(%) |
|---|
| IPC | 50 | 7
(14)a,b |
| LMWH | 85 | 15
(17.6)a,b |
| LMWH + IPC | 35 | 0 (0)a |
| Control | 90 | 32 (35.4) |
Discussion
Prophylaxis of VTE in critically ill patients that
are at a high risk for thrombosis and bleeding simultaneously poses
a major challenge (22), as in a
previous study it was found that 22% of trauma patients who had
ongoing bleeding or injuries were at high risk for serious bleeding
complications (23). The observation
that the risk of VTE increases as the number of risk factors
increases should be taken into account when assessing risk levels
and considering thromboprophylaxis (24). The American College of Chest
Physicians and the American Society of Colon and Rectal Surgeons
have recommended that every hospital should formulate a risk
stratification approach to categorize trauma patients in terms of
thromboembolic risk (25). With
improvements in detection methods, DVT and PE detection rates have
increased; however, the methods for preventing DVT require further
improvement. Due to its simplicity of use, IPC has been used in our
department for several years, despite reports that further research
is required to observe the validity of IPC device for preventing
VTE (26).
As patients may be admitted to a general ICU for a
wide variety of reasons, a single method for preventing DVT may
have limitations. In clinical practice, patients that have suffered
trauma or fracture of the leg are not good candidates for the use
of IPC, whereas in other cases, such as patients suffering
hypovolemic shock, trauma or other coagulation disorders, there is
a risk of bleeding with heparin use. However, LMWH is associated
with a low risk of bleeding (27).
Numerous reports have indicated that LMWH is able to reduce the
incidence of DVT in patients with cancer and patients undergoing
major surgery (28–30), in a dose-dependent manner (31). Dennis et al (32) reported that IPC was effective for DVT
prophylaxis. Sekine and Koh (33)
recommended IPC for DVT prophylaxis in patients with low or medium
risk and low dose heparin for high-risk patients.
IPC is an effective mechanical method of DVT
prophylaxis, exerting an antithrombotic effect that appears to be a
result of reduced plasminogen activator inhibitor-1 levels and
increased tissue plasminogen activator activity. These actions
stimulate fibrinolytic activity and increase venous blood flow
velocity, thereby reducing stasis and altering hypercoagulability
(34). However, the efficacy of this
treatment is determined in part by patient compliance with the
treatment protocol. Furthermore, the use of ICP is limited in cases
with certain diseases such as pulmonary edema and non-congestive
heart failure.
LMWH is among the most significant recent
developments in prophylaxis against DVT (24). The anticoagulatory effects of LMWH
have been well characterized and function by reducing the activity
of coagulation factor XIIa and thrombin (35). LMWH interacts with platelets and has
been reported to inhibit platelet aggregation, thus prolonging the
duration of bleeding in patients. Thus, the clinical application of
LMWH remains limited by its primary side-effect, bleeding (35). Although it is likely that the
hemorrhaging observed in the present study was associated with the
anticoagulatory effects of LMWH, other factors may also be
important. The evaluation of efficacy was based on the frequency of
VTE (36). It was observed that
there was a statistical significance between the three prophylaxis
groups and the control group, which indicated the efficacy of those
treatments. ICP may exert its antithrombotic effect by stimulating
fibrinolytic activity and increasing venous blood flow velocity,
while LMWH is well characterized and functions by accelerating the
inhibitory effect of antithrombin on factors XIIa, XIa, Xa and IXa
and thrombin (24), whether the IPC
and LMWH treatments produced a significant difference in efficacy
was of no importance. However, by combining ICP and LMWH
treatments, an improved response may be achieved as each treatment
functions via an independent mechanism.
The results of the present study require
interpretation within the context of its limitations. Firstly, this
study did not involve true randomization, and was an observational
study since the allocation of treatment was determined by the
provider not by a random sequence. However, this protocol was
necessary as certain patients exhibited heparin contraindications
or pulmonary edema and were unable to receive certain treatments.
Secondly, it remains unclear whether an improved benefit/risk ratio
of prophylaxis of thromboembolic events versus risk of bleeding
complications may be established for LMWH (24). LMWH-induced thrombocytopenia has been
reported in clinical therapy (37).
In addition, it was observed in the present study that the LMWH +
ICP treatment had a significant improvement in prophylactic effect,
with decreased side effects, which provided a better prospective
for the prevention of thromboprophylaxis in critically ill
patients.
In conclusion, the results of the present study
suggest that LMWH combined with IPC exhibited an excellent
prophylactic effect against DVT and PE. The effect of IPC was
comparable to that of LMWH anticoagulation therapy. As for the
patients in the ICU who were at high risk for DVT but had
contraindications for heparin, this therapy may effectively reduce
the incidence of DVT if administered selectively on the basis of
the patient's condition. For patients at a high risk of DVT, with
no contraindications for heparin, it is proposed that IPC in
combination with anticoagulant therapy may effectively reduce the
incidence of DVT and is potentially applicable in a clinical
context.
Acknowledgements
This study was supported by Zhenjiang City
Technology Support Program-Social Development Project (no.
SH2013037) and Clinical Science and Technology Development Fund of
Jiangsu University (no. JLY20120164).
References
|
1
|
Geerts WH, Jay RM, Code KI, Chen E, Szalai
JP, Saibil EA and Hamilton PA: A comparison of low-dose heparin
with low-molecular-weight heparin as prophylaxis against venous
thromboembolism after major trauma. N Engl J Med. 335:701–707.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kapoor A, Chew P, Silliman RA, Hylek EM,
Katz JN, Cabral H and Berlowitz D: Venous thromboembolism after
joint replacement in older male veterans with comorbidity. J Am
Geriatr Soc. 61:590–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shorr AF and Williams MD: Venous
thromboembolism in critically ill patients. Observations from a
randomized trial in sepsis. Thromb Haemost. 101:139–144.
2009.PubMed/NCBI
|
|
4
|
Welsby I and Ortel TL: Is it time for
individualized thromboprophylaxis regimens in the ICU. Crit Care
Med. 43:500–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knudson MM: Venous thromboembolism
prophylaxis: One size does not fit all: Comment on ‘Comparative
effectiveness of unfractionated and low-molecular-weight heparin
for prevention of venous thromboembolism following bariatric
surgery’. Arch Surg. 147:998–999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahmoudi M and Sobieraj DM: The
cost-effectiveness of oral direct factor Xa inhibitors compared
with low-molecular-weight heparin for the prevention of venous
thromboembolism prophylaxis in total hip or knee replacement
surgery. Pharmacotherapy. 33:1333–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costantino G, Ceriani E, Rusconi AM, Podda
GM, Montano N, Duca P, Cattaneo M and Casazza G: Bleeding risk
during treatment of acute thrombotic events with subcutaneous LMWH
compared to intravenous unfractionated heparin; a systematic
review. PLoS One. 7:e445532012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schenkeveld L, Pedersen SS, van Nierop JW,
Lenzen MJ, de Jaegere PP, Serruys PW and van Domburg RT:
Health-related quality of life and long-term mortality in patients
treated with percutaneous coronary intervention. Am Heart J.
159:471–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergqvist D and Lindblad B: The
thromboprophylactic effect of graded elastic compression stockings
in combination with dextran 70. Arch Surg. 119:1329–1331. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones NA, Webb PJ, Rees RI and Kakkar VV:
A physiological study of elastic compression stockings in venous
disorders of the leg. Br J Surg. 67:569–572. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarmiento A and Goswami AD: Thromboembolic
prophylaxis with use of aspirin, exercise and graded elastic
stockings or intermittent compression devices in patients managed
with total hip arthroplasty. J Bone Joint Surg Am. 81:339–346.
1999.PubMed/NCBI
|
|
12
|
Clagett GP, Anderson FA Jr, Geerts W, Heit
JA, Knudson M, Lieberman JR, Merli GJ and Wheeler HB: Prevention of
venous thromboembolism. Chest. 114(5 Suppl): 531S–560S. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wells PS, Anderson DR, Rodger M, Ginsberg
JS, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain
M, et al: Derivation of a simple clinical model to categorize
patients probability of pulmonary embolism: Increasing the models
utility with the SimpliRED D-dimer. Thromb Haemost. 83:416–420.
2000.PubMed/NCBI
|
|
14
|
Kearon C, Kahn SR, Agnelli G, Goldhaber S,
Raskob GE and Comerota AJ: American College of Chest Physicians:
Antithrombotic therapy for venous thromboembolic disease: American
College of Chest Physicians evidence-based clinical practice
guidelines (8th Edition). Chest. 133(6 Suppl): 454S–545S. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Dongen CJ, Prandoni P, Frulla M,
Marchiori A, Prins MH and Hutten BA: Relation between quality of
anticoagulant treatment and the development of the postthrombotic
syndrome. J Thromb Haemost. 3:939–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells PS, Anderson DR, Bormanis J, Guy F,
Mitchell M, Gray L, Clement C, Robinson KS and Lewandowski B: Value
of assessment of pretest probability of deep-vein thrombosis in
clinical management. Lancet. 350:1795–1798. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heijboer H, Büller HR, Lensing AW, Turpie
AG, Colly LP and ten Cate JW: A comparison of real-time compression
ultrasonography with impedance plethysmography for the diagnosis of
deep-vein thrombosis in symptomatic outpatients. N Engl J Med.
329:1365–1369. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaeger KA and Staub D: Ultrasonography for
venous thromboembolism. Ultraschall Med. 32:237–240. 2011.(In
English and German). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawaguchi T, Kumabe T, Kanamori M,
Nakamura T, Saito R, Yamashita Y, Sonoda Y, Watanabe M and Tominaga
T: Early detection of venous thromboembolism in patients with
neuroepithelial tumor: Efficacy of screening with serum D-dimer
measurements and Doppler ultrasonography. J Neurooncol.
101:495–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan WS, Spencer FA, Lee AY, Chunilal S,
Douketis JD, Rodger M and Ginsberg JS: Safety of withholding
anticoagulation in pregnant women with suspected deep vein
thrombosis following negative serial compression ultrasound and
iliac vein imaging. CMAJ. 185:E194–E200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Leary DH, Kane RA and Chase BM: A
prospective study of the efficacy of B-scan sonography in the
detection of deep venous thrombosis in the lower extremities. J
Clin Ultrasound. 16:1–8. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiasson TC, Manns BJ and Stelfox HT: An
economic evaluation of venous thromboembolism prophylaxis
strategies in critically ill trauma patients at risk of bleeding.
PLoS Med. 6:e10000982009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geerts WH: Prevention of venous
thromboembolism in high-risk patients. Hematology AM Soc Hematol
Educ Program. 2006:462–466. 2006. View Article : Google Scholar
|
|
24
|
De A, Roy P, Garg VK and Pandey NK:
Low-molecular-weight heparin and unfractionated heparin in
prophylaxis against deep vein thrombosis in critically ill patients
undergoing major surgery. Blood Coagul Fibrinolysis. 21:57–61.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rogers FB, Cipolle MD, Velmahos G, Rozycki
G and Luchette FA: Practice management guidelines for the
prevention of venous thromboembolism in trauma patients: The EAST
practice management guidelines work group. J Trauma. 53:142–164.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao JM, He ML, Xiao ZM, Li TS, Wu H and
Jiang H: Different types of intermittent pneumatic compression
devices for preventing venous thromboembolism in patients after
total hip replacement. Cochrane Database Syst Rev.
12:CD0095432012.
|
|
27
|
Streiff MB and Lau BD: Thromboprophylaxis
in nonsurgical patients. Hematology Am Soc Hematol Educ Program.
2012:631–637. 2012.PubMed/NCBI
|
|
28
|
Larocca A, Cavallo F, Bringhen S, Di
Raimondo F, Falanga A, Evangelista A, Cavalli M, Stanevsky A,
Corradini P, Pezzatti S, et al: Aspirin or enoxaparin
thromboprophylaxis for patients with newly diagnosed multiple
myeloma treated with lenalidomide. Blood. 119:933–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jameson SS, Rymaszewska M, Hui AC, James
P, Serrano-Pedraza I and Muller SD: Wound complications following
rivaroxaban administration: A multicenter comparison with
low-molecular-weight heparins for thromboprophylaxis in lower limb
arthroplasty. J Bone Joint Surg Am. 94:1554–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hull RD, Liang J and Townshend G:
Long-term low-molecular-weight heparin and the post-thrombotic
syndrome: A systematic review. Am J Med. 124:756–765. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riha GM, Van PY, Differding JA and
Schreiber MA: Oregon Health & Science University Trauma
Research Group: Incidence of deep vein thrombosis is increased with
30 mg twice daily dosing of enoxaparin compared with 40 mg daily.
Am J Surg. 203:598–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dennis M, Sandercock P, Reid J, Graham C
and Forbes J: CLOTS Trials Collaboration: Does intermittent
pneumatic compression reduce the risk of post stroke deep vein
thrombosis? The CLOTS 3 trial: Study protocol for a randomized
controlled trial. Trials. 13:262012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sekine Y and Koh E: Thoracic surgery for
patients with deep vein thrombosis. Kyobu Geka. 65:697–700.
2012.(In Japanese). PubMed/NCBI
|
|
34
|
Comerota AJ, Chouhan V, Harada RN, Sun L,
Hosking J, Veermansunemi R, Comerota AJ Jr, Schlappy D and Rao AK:
The fibrinolytic effects of intermittent pneumatic compression:
Mechanism of enhanced fibrinolysis. Ann Surg. 226:306–313. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carter C, Kelton J, Hirsh J, Cerskus A,
Santos A and Gent M: The relationship between the hemorrhagic and
antithrombotic properties of low molecular weight heparin in
rabbits. Blood. 59:1239–1245. 1982.PubMed/NCBI
|
|
36
|
Greer IA and Nelson-Piercy C:
Low-molecular-weight heparins for thromboprophylaxis and treatment
of venous thromboembolism in pregnancy: A systematic review of
safety and efficacy. Blood. 106:401–407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rota E, Bazzan M and Fantino G:
Fondaparinux-related thrombocytopenia in a previous
low-molecular-weight heparin (LMWH)-induced heparin-induced
thrombocytopenia (HIT). Thromb Haemost. 99:779–781. 2008.PubMed/NCBI
|