Introduction
Neuropathic pain can arise as a consequence of a
lesion or disease affecting the somatosensory system (1). Symptoms may include hypersensitivity to
noxious (hyperalgesia) and non-noxious (allodynia) stimuli, as well
as spontaneous pain (2). An
estimated 7–8% of the general population suffers from mild to
moderate forms of neuropathic pain, and 5% may be severely affected
by it (3). Such pain is a major
health problem that substantially reduces quality of life for the
afflicted individuals and poses a significant economic burden to
the health system and society (2).
Understanding the pathophysiological process of neuropathic pain
and the underlying molecular mechanisms will facilitate the
development of novel therapies for neuropathic pain. Among the
several widely used experimental animal models for neuropathic
pain, chronic constriction injury (CCI) of the sciatic nerve is one
of the most popular (4).
Angiotensin II (Ang II) is a principle component of
the renin-angiotensin system (RAS), which has a pivotal role in the
regulation of blood pressure and fluid homeostasis in mammals
(5). Numerous studies have
demonstrated that Ang II, which interacts with the autonomic
nervous system, is involved in the central and peripheral
regulation of sensory information (6,7). In
several rodent pain models, the intracerebroventricular injection
of Ang II has been shown to exert antinociceptive effects (6,8). These
findings indicate that the Ang II/Ang II type 1 (AT1) receptor axis
has a key function in nociception.
In addition to the angiotensin-converting enzyme
(ACE)/Ang II/AT1 receptor axis, the RAS involves a
counter-regulatory axis, in which ACE2, Ang-(1–7) and the
Mas receptor play a role (9).
Ang-(1–7) performs a wide range of actions, several
of which counteract the actions of Ang II, and is recognized as a
key component of the RAS (10). The
direct generation of Ang-(1–7) can occur with high efficiency through
the action of ACE2 on Ang II (10–12), as
well as through the action of neutral endopeptidase and prolyl
endopeptidase on Ang I (13,14). It is well established that the G
protein-coupled receptor Mas has a functional binding site for
Ang-(1–7) (15,16), and
previous studies have suggested that the actions of Ang-(1–7) may be
mediated by an interaction with Mas in the central nervous system
(17,18). The findings that Mas is localized in
the rat dorsal root ganglion (DRG) and that Ang-(1–7) produces
a peripheral antinociceptive effect in rats through Mas have been
previously described (19). Thus, as
an essential component of the RAS, the Ang-(1–7)/Mas axis
may play an important role in antinociception.
The aim of the present study was to explore for the
first time, to the best of our knowledge, the expression pattern of
Mas in DRG neurons following chronic nerve injury and to examine
the effects of Mas inhibition and activation on neuropathic pain in
a CCI rat model.
Materials and methods
Animals and reagents
Male inbred Sprague Dawley rats (weight, 250–300 g)
were purchased from Central South University (Changsha, China) and
were housed at the Xi'an Jiaotong University School of Medicine
BioResources Center (Xi'an, China). Animals were placed in a quiet,
temperature- (22±2°C) and humidity- (60±6%) controlled room with a
12-h light/dark cycle (light beginning at 8:00 a.m.), and all tests
were performed during the light phase of the cycle.
125I-Sodium iodide (carrier free, 100 mCl/ml) was
purchased from Amersham Biosciences (Piscataway, NJ, USA).
Ang-(1–7) was purchased from Sigma Chemical Co.
(St. Louis, MO, USA). D-Pro7-Ang-(1–7) was
purchased from the American Peptide Company (Sunnyvale, CA, USA).
Mouse monoclonal anti-MAS1 (G-1) antibody (cat. no. sc-390453) was
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
TRIzol® reagent and the SYBR® Green Master Mix were purchased from
Invitrogen (Life Technologies, Carlsbad, CA, USA) and Applied
Biosystems (Foster City, CA, USA), respectively.
Establishment of the CCI rat model and
treatments
The rat CCI model was established as previously
described (20). Briefly, each
animal was anesthetized via an intraperitoneal injection of sodium
pentobarbital (60 mg/kg). The common sciatic nerve of the animal
was exposed and freed from adherent tissue at the mid-thigh level
by using blunt dissection to separate the biceps femoris muscles.
Four loose ligatures were placed 1 mm apart, using chromic gut
suture (4-0 absorbable suture; Jorgensen Laboratories, Inc.,
Loveland, CO, USA). The animals were randomly assigned to one of
six groups (n=20 per group): Sham + Saline (animals subject to sham
surgery plus intrathecal injection of 20 µl saline every 3 days
from 3 days before surgery), Sham + Mas receptor inhibitor (Mas-I)
[animals subject to sham surgery plus intrathecal injection of 20
µl 10 nM Mas-I D-Pro7-Ang-(1–7) every 3
days from 3 days before surgery], Sham + Ang-(1–7) [animals
subject to sham surgery plus intrathecal injection of 20 µl 120 pM
Mas agonist Ang-(1–7) every 3 days from 3 days before surgery],
CCI + Saline (animals subject to CCI surgery plus intrathecal
injection of 20 µl saline every 3 days from 3 days before surgery),
CCI + Mas-I [animals subject to CCI surgery plus intrathecal
injection of 20 µl 10 µM D-Pro7-Ang-(1–7) every 3
days from 3 days before surgery], CCI + Ang-(1–7) [animals
subject to CCI surgery plus intrathecal injection of 20 µl 120 pM
Ang-(1–7) every 3 days from 3 days before surgery].
All animals were sacrificed within 13 days after the surgery. This
study was conducted in accordance with the Xi'an Jiaotong
University School of Medicine guidelines on the use of live animals
for research, and the experimental protocol was approved by the
Laboratory Animal Users Committee at Xi'an Jiaotong University
School of Medicine.
Behavior tests
Thermal hyperalgesia was assessed in accordance with
a previously described method (21)
using a plantar analgesia instrument (Stoelting Co., Wood Dale, IL,
USA) every day from 3 days before to 13 days after surgery. The
intensity of the radiant infrared heat source stimulus was set to
IR50 and the cut-off time was set at 15 sec. Prior to each testing
session, the rats were placed on a glass platform and left to
habituate to the surroundings for ≥15 min. The thermal stimulus was
applied to the plantar surface of the paw. The thermal threshold
was defined as the latency (sec) to the first sign of pain
behavior. Signs of pain behavior included paw withdrawal,
flinching, biting and/or licking of the stimulated paw. To assess
mechanical allodynia, von Frey monofilaments (Stoelting Co.) with a
range of stiffness levels (2.0–15.0 g) were used every day from 3
days before to 13 days after surgery. Prior to each testing
session, the rats were placed on a metallic platform and left to
habituate to the surroundings for ≥15 min. The stimulus strength
was sequentially increased and/or decreased to determine the paw
withdrawal threshold response.
DRG neuron cell isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
On days 1, 3, 7 and 13 after the CCI surgery, 5
randomly selected rats were sacrificed at each time-point, and DRG
neurons were isolated from the enlarged section of the lumbar
spinal cord in accordance with a previously described method
(22). Cells were used for
experiments 24–48 h after isolation. RNA was prepared using TRIzol
reagent and then purified using the Turbo DNA-free™ system (Ambion,
Austin, TX, USA). The cDNAs were synthesized using SuperScript™ II
reverse transcriptase (Invitrogen, Life Technologies). qPCR was
performed using the LightCycler® thermal cycler system (Roche
Diagnostics, Indianapolis, IN, USA) and a SYBR Green I kit,
following the manufacturer's instructions. The PCR cycling
conditions were as follows: 20 sec at 95°C, followed by 40 cycles
of 3 sec at 95°C and 30 sec at 60°C. The results were normalized
against those of the reference gene GAPDH in the same sample. The
primers used (Baolong Oligos, Beijing, China) were as follows: Rat
Mas forward, 5′-GAC CAG CCC ACA GTT ACC AGTT-3′ and reverse, 5′-CCA
GGG TTC CCC TTC TGA CT-3′; rat GAPDH forward, 5′-TGG TCT ACA TGT
TCC AGT ATG ACT-3′ and reverse, 5′-CCA TTT GAT GTT AGC GGG ATC
TC-3′. Each experiment was performed in duplicate and repeated
three times.
Western blot analysis
DRG neurons were incubated at 95°C for 10 min
following lysis in 250 µl 2X sodium dodecyl sulfate (SDS) loading
buffer (62.5 mM TrisHCl, pH 6.8, 2% SDS, 25% glycerol, 0.01%
bromphenol blue and 5% 2-mercaptoethanol). Equal quantities of
lysates were loaded onto 10% SDS-polyacrylamide gels, and proteins
were then blotted onto a microporous polyvinylidene difluoride
membrane (Millipore, Billerica, MA, USA). The membranes were
incubated for 1 h with anti-MAS1 antibody (Santa Cruz
Biotechnology, Inc.) at a 1:1,000 dilution and then washed and
incubated with a horseradish peroxidase-conjugated secondary
antibody for 1 h (1:5,000; Santa Cruz Biotechnology, Inc.).
Peroxidase was revealed with an enhanced chemiluminescence kit from
GE Healthcare (Shanghai, China).
[125I]Ang-(1–7) binding
assay
Isolated rat DRG neurons were rinsed twice with
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Waltham, MA, USA) and equilibrated on ice with incubation buffer
(DMEM containing 0.2% bovine serum albumin and a protease inhibitor
cocktail, pH 7.4) for 30 min. The plates were then incubated at 4°C
for 60 min with incubation buffer containing 0.5 nmol/l
125I-Ang-(1–7), labeled as described in a previous study
(23). Incubation was terminated by
rinsing the cells three times with ice-cold phosphate-buffered
saline. Cell solubilization was achieved through incubation with
0.1 mol/l NaOH for 60 min, and the radioactivity was then measured.
Nonspecific binding was determined in the presence of 10 µmol/l
unlabeled Ang-(1–7) and found to be ≤15%. Specific binding
was calculated by subtracting the nonspecific binding from the
total binding. The disintegration per minute data were normalized
against cell number (per 10,000 cells). Each experiment was
performed in duplicate and repeated three times.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows 10.0 (SPSS Inc., Chicago, IL, USA). Data values are
expressed as the mean ± standard deviation. Comparisons of means
among multiple groups were conducted using one-way analysis of
variance followed by post hoc pairwise comparisons using Tukey's
tests. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of Mas and Ang-(1–7)
binding
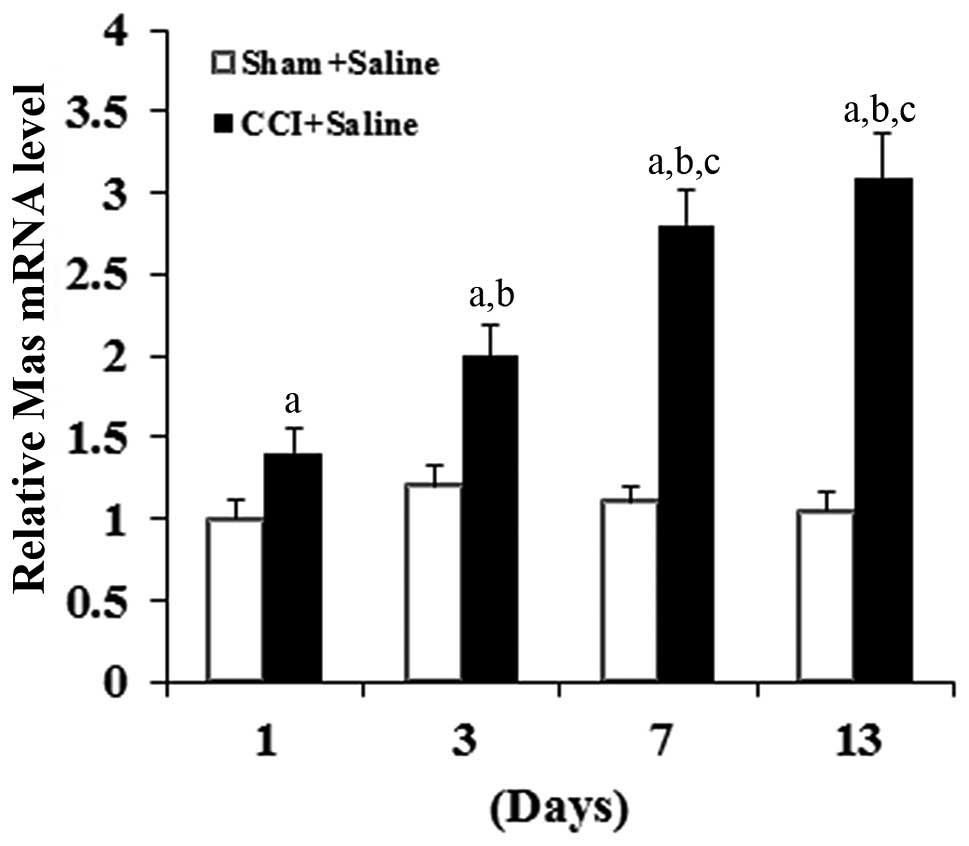
As shown in Fig. 1,
compared with the sham group, CCI time-dependently increased the
Mas mRNA level in the DRG neurons. Western blot analyses confirmed
that CCI time-dependently increased the Mas protein level in the
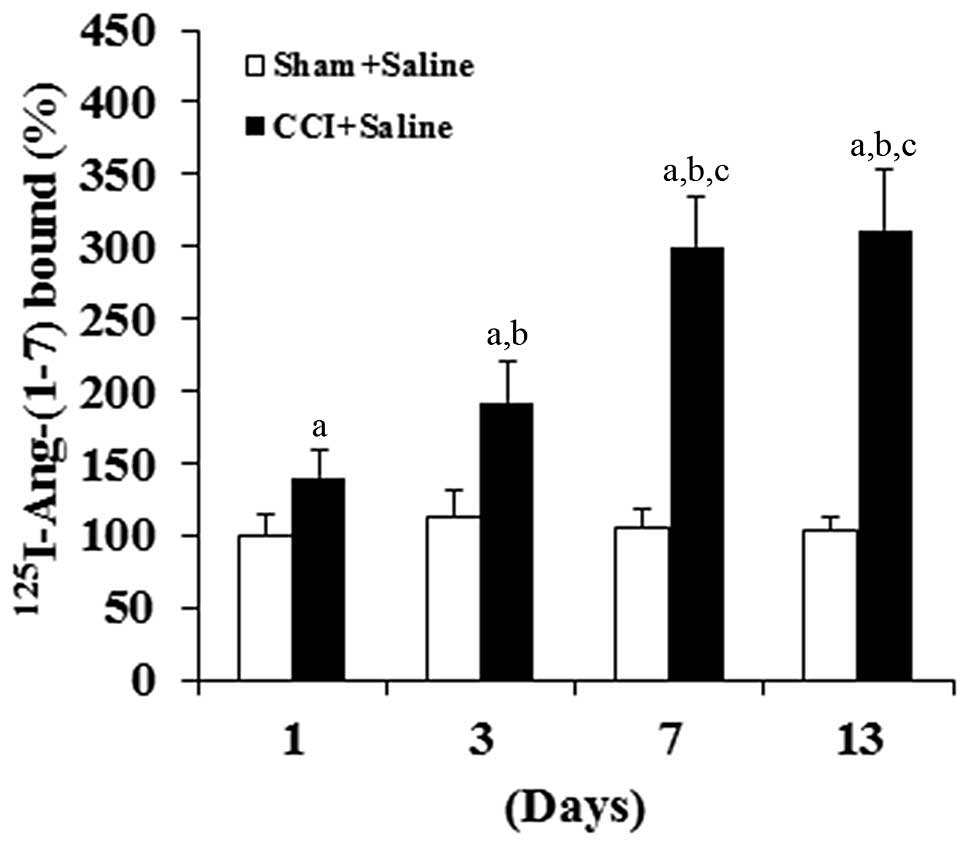
DRG neurons compared with the sham group (Fig. 2). Consistent with these results,
isolated DRG neurons showed a time-dependent increase in
Ang-(1–7) binding on the cell membrane following
the CCI surgery, but not the sham surgery (Fig. 3). In combination, the results suggest
that CCI can significantly increase the density of Mas-ligand
binding on the cell membrane of DRG neurons by inducing the
expression of Mas at the mRNA level.
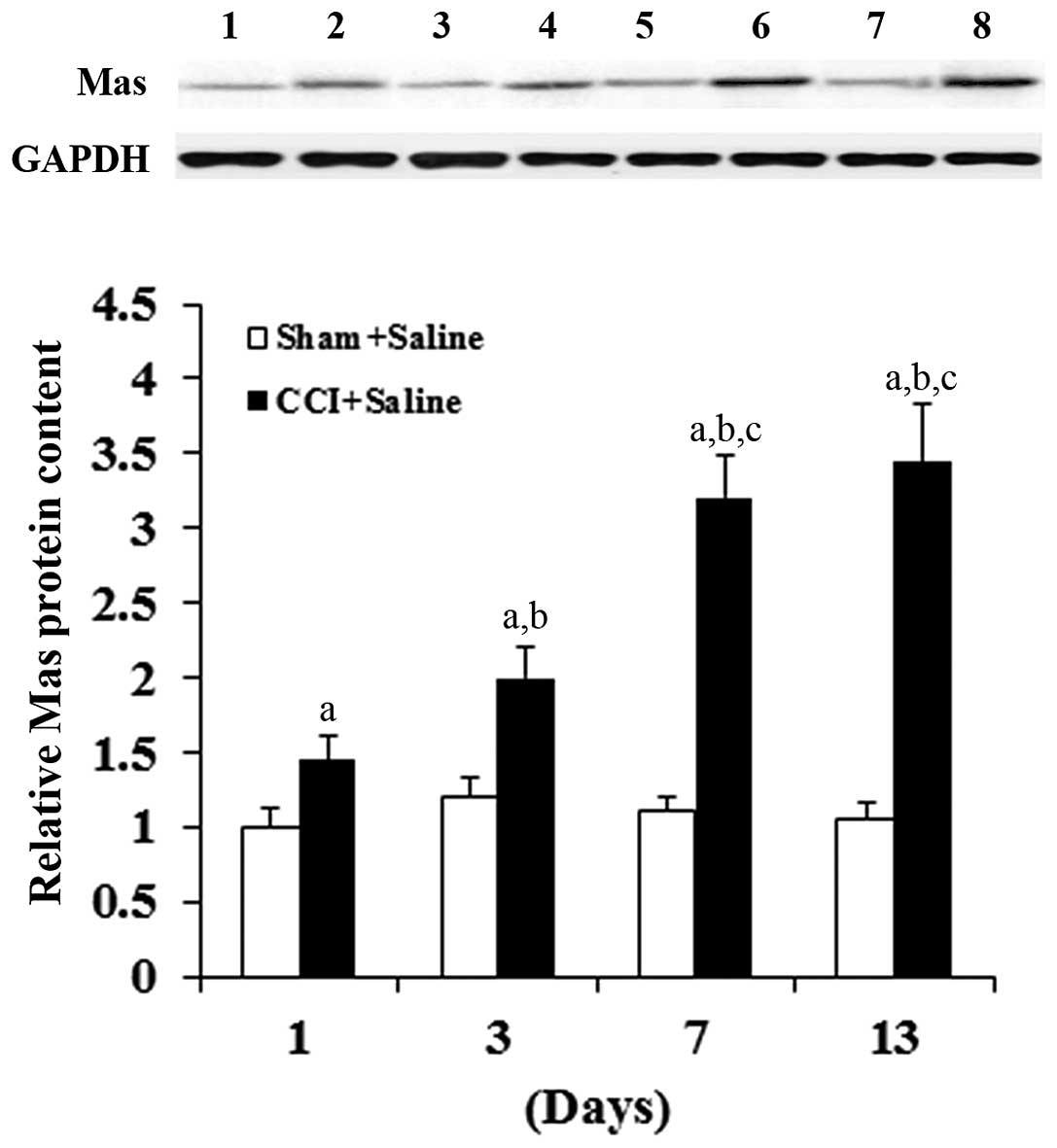 | Figure 2.Mas receptor protein level in DRG
neurons of rats with or without CCI. The expression of Mas receptor
protein in the rat DRG neurons was determined using western blot
analyses on days 1, 3, 7 and 13 after sham or CCI surgery. Lanes 1,
3, 5, 7 represent the Sham + Saline group on days 1, 3, 7 and 13,
respectively; lanes 2, 4, 6 and 8 represent the CCI + Saline group
on days 1, 3, 7 and 13, respectively. GAPDH blotting was used as a
loading control. Protein blots were measured densitometrically. The
density of the Mas receptor blot was normalized against that of
GAPDH to obtain a relative blot density, which was expressed as a
fold change relative to that of the Sham + Saline group on day 1
(designated as 1). Three independent experiments were performed for
each western blot analysis. Data values are expressed as the mean +
standard deviation; n=5 in each group at each time-point.
aP<0.05 vs. Sham + Saline; bP<0.05 vs.
CCI + Saline on day 1; cP<0.05 vs. CCI + Saline on
day 3. DRG, dorsal root ganglion; CCI, chronic constriction
injury. |
Hyperalgesia and allodynia tests
To explore the functional significance of
CCI-induced Mas expression in DRG neurons, thermal hyperalgesia and
mechanical allodynia tests were next performed in rats treated with
the selective Mas agonist Ang-(1–7) (200 pM)
or selective Mas inhibitor D-Pro7-Ang-(1–7) (10 nM).
As shown in Fig. 4, no significant
group differences were found in the paw withdrawal latency or
threshold among the animals receiving sham surgery plus saline,
Ang-(1–7) or D-Pro7-Ang-(1–7).
Compared with the sham control groups, CCI significantly decreased
the paw withdrawal latency and threshold, and this effect was
markedly improved and aggravated by intrathecal injection of
Ang-(1–7) and D-Pro7-Ang-(1–7),
respectively. Intrathecal injection of 20 µl Ang-(1–7) at 200
pM or D-Pro7-Ang-(1–7) at 10 nM did not cause any noticeable
side effects in the rats.
 | Figure 4.Thermal hyperalgesia and mechanical
allodynia in rats with or without CCI. Thermal hyperalgesia and
mechanical allodynia were measured in rats 3 days before surgery
(day −3) and on days 1, 3, 7 and 13 after sham or CCI surgery. In
the Sham + Saline group, rats received sham surgery plus an
intrathecal injection of 20 µl saline every 3 days from day −3; in
the Sham + Mas-I group, rats received sham surgery plus intrathecal
injection of 20 µl 10 nM Mas-I D-Pro7-Ang-(1–7) every 3
days from day −3; in the Sham + Ang-(1–7) group,
rats received sham surgery plus intrathecal injection of 20 µl 200
pM Mas agonist Ang-(1–7) every 3 days from day −3; in the CCI +
Saline group, rats received CCI surgery plus intrathecal injection
of 20 µl saline every 3 days from day −3; in the CCI + Mas-I group,
rats received CCI surgery plus intrathecal injection of 20 µl 10 µM
D-Pro7-Ang-(1–7) every 3 days from day −3; in the CCI +
Ang-(1–7) group, rats received CCI surgery plus
intrathecal injection of 20 µl 200 pM Ang-(1–7) every 3
days from day −3. Data values are expressed as the mean + standard
deviation; n=5 in each group at each time-point.
aP<0.05 vs. Sham + Saline, Sham + Mas-I and Sham +
Ang-(1–7); bP<0.05 vs. CCI + Mas-I;
cP<0.05 vs. CCI + Saline. CCI, chronic constriction
injury; Mas-I, Mas receptor inhibitor; Ang-(1–7),
angiotensin-(1–7). |
Discussion
Neuropathic pain, characterized by hyperalgesia,
allodynia, and spontaneous pain, is one of the most painful
symptoms that can be experienced in the clinic (24) and often occurs as a result of injury
to the peripheral nerves, DRG, spinal cord or brain (24). The RAS is considered to have an
important role in nociception (5–8), and
Ang-(1–7) is a biologically active member of the
RAS (25). The physiological role of
Ang-(1–7) has been firmly established by two
discoveries: i) Identification of the ability of ACE2, an enzyme
that generates Ang-(1–7) from Ang I or Ang II (25); ii) characterization of the G
protein-coupled receptor Mas as a receptor that is associated with
several actions of Ang-(1–7) (26).
Costa et al (19)
demonstrated that Mas is expressed in rat DRG neurons and showed,
through the subcutaneous injection of prostaglandin E2
and Ang-(1–7) into the rat's hind paw, that
Ang-(1–7) produced a peripheral antinociceptive
effect in rats via Mas (19). In the
present study, a rat CCI model was employed to provide the first
evidence, to the best of our knowledge, that the Mas expression in
DRG neurons is time-dependently induced by chronic nerve injury.
Furthermore, it was found that intrathecal activation and
inhibition of Mas could improve and aggravate CCI-induced
neuropathic pain, respectively.
The present results showed that Mas expression at
both the mRNA and the protein level was time-dependently increased
in DRG neurons following CCI, but not the sham surgery, suggesting
that chronic nerve injury can induce Mas expression in DRG neurons
at the mRNA level. The increased Mas expression led to an increased
density of Mas-ligand binding on the cell membrane of DRG neurons,
suggesting the functional significance of this phenomenon. This was
confirmed by behavioral tests, which showed that the intrathecal
injection of the Mas-I/antagonist D-Pro7-Ang-(1–7) markedly
aggravated thermal hyperalgesia and mechanical allodynia in CCI
rats, while the intrathecal injection of the Mas agonist
Ang-(1–7) significantly improved thermal
hyperalgesia and mechanical allodynia in the CCI rats. Taking into
account the relatively low concentrations of D-Pro7-Ang-(1–7) (10 nM)
and Ang-(1–7) (200 pM) used and the marked effects
observed, the Ang-(1–7)/Mas axis could be an effective
therapeutic target for neuropathic pain, warranting further study.
This is particularly important since neuropathic pain, particularly
the nerve-injured neuropathy, is opioid resistant (24).
Notably, the inhibition or activation of Mas in rats
with sham surgery did not cause any significant differences in
thermal hyperalgesia and mechanical allodynia, which may have been
due to the fact that the Ang-(1–7)/Mas axis
in the DRG neurons was only activated following pathophysiological
changes ensuing from chronic nerve injury. This theory is supported
by the present finding that the Mas expression was time-dependently
increased only following the CCI, but not the sham surgery, which
also suggests that the increased Mas expression is a compensatory
mechanism to reduce chronic nerve injury-induced neuropathic pain.
Further studies are required to elucidate the underlying molecular
mechanisms. There are several experimental animal models for
neuropathic pain (24); since only
the CCI model was employed in this study, an examination of the
effects of Mas inhibition and activation on neuropathic pain in
other neuropathic pain models would be of particular interest in
future studies.
In conclusion, the present study has demonstrated
that Mas expression in DRG neurons is time-dependently induced by
chronic nerve injury; intrathecal activation and inhibition of Mas
can improve and aggravate CCI-induced neuropathic pain,
respectively. This study provides novel insights into the
pathophysiological process of neuropathic pain and suggests that
the Ang-(1–7)/Mas axis could be an effective
therapeutic target for neuropathic pain.
Acknowledgements
This study was supported by the Science and
Technology Bureau of Hunan Province (grant no. 2014-HJ-2561).
References
|
1
|
Jensen TS, Baron R, Haanpüü M, Kalso E,
Loeser JD, Rice AS and Treede R: A new definition of neuropathic
pain. Pain. 152:2204–2205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grace PM, Hurley D, Barratt DT, Tsykin A,
Watkins LR, Rolan PE and Hutchinson MR: Harnessing pain
heterogeneity and RNA transcriptome to identify blood-based pain
biomarkers: A novel correlational study design and bioinformatics
approach in a graded chronic constriction injury model. J
Neurochem. 122:976–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin HC, Huang YH, Chao TH, Lin WY, Sun WZ
and Yen CT: Gabapentin reverses central hypersensitivity and
suppresses medial prefrontal cortical glucose metabolism in rats
with neuropathic pain. Mol Pain. 10:632014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obata K, Yamanaka H, Fukuoka T, Yi D,
Tokunaga A, Hashimoto N, Yoshikawa H and Noguchi K: Contribution of
injured and uninjured dorsal root ganglion neurons to pain behavior
and the changes in gene expression following chronic constriction
injury of the sciatic nerve in rats. Pain. 101:65–77. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Gasparo M, Catt KJ, Inagami T, Wright
JW and Unger T: International union of pharmacology. XXIII. The
angiotensin II receptors. Pharmacol Rev. 52:415–472.
2000.PubMed/NCBI
|
|
6
|
Pelegrini-da-Silva A, Martins AR and Prado
WA: A new role for the renin-angiotensin system in the rat
periaqueductal gray matter: Angiotensin receptor-mediated
modulation of nociception. Neuroscience. 132:453–463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakagawa T, Okuyama S, Kawashima N, Hozumi
S, Nakagawasai O, Tadano T, Kisara K, Ichiki T and Inagami T: Pain
threshold, learning and formation of brain edema in mice lacking
the angiotensin II type 2 receptor. Life Sci. 67:2577–2585. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavel J, Tang H, Brimijoin S, Moughamian
A, Nishioku T, Benicky J and Saavedra JM: Expression and transport
of Angiotensin II AT1 receptors in spinal cord, dorsal root ganglia
and sciatic nerve of the rat. Brain Res. 1246:111–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferreira AJ, Murça TM, Fraga-Silva RA,
Castro CH, Raizada MK and Santos RA: New cardiovascular and
pulmonary therapeutic strategies based on the
Angiotensin-converting enzyme 2/angiotensin-(1–7)/mas receptor
axis. Int J Hypertens. 2012:1478252012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raizada MK and Ferreira AJ: ACE2: A new
target for cardiovascular disease therapeutics. J Cardiovasc
Pharmacol. 50:112–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vickers C, Hales P, Kaushik V, Dick L,
Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, et al:
Hydrolysis of biological peptides by human angiotensin-converting
enzyme-related carboxypeptidase. J Biol Chem. 277:14838–14843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Der Sarkissian S, Huentelman MJ, Stewart
J, Katovich MJ and Raizada MK: ACE2: A novel therapeutic target for
cardiovascular diseases. Prog Biophys Mol Biol. 91:163–198. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rice GI, Thomas DA, Grant PJ, Turner AJ
and Hooper NM: Evaluation of angiotensin-converting enzyme (ACE),
its homologue ACE2 and neprilysin in angiotensin peptide
metabolism. Biochem J. 383:45–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stanziola L, Greene LJ and Santos RA:
Effect of chronic angiotensin converting enzyme inhibition on
angiotensin I and bradykinin metabolism in rats. Am J Hypertens.
12:1021–1029. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferreira AJ and Santos RA: Cardiovascular
actions of angiotensin-(1–7). Braz J Med Biol Res. 38:499–507.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomes ER, Santos RA and Guatimosim S:
Angiotensin-(1–7)-mediated signaling in cardiomyocytes. Int J
Hypertens. 2012:4931292012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santos RA, Campagnole-Santos MJ and
Andrade SP: Angiotensin-(1–7): An update. Regul Pept. 91:45–62.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao L, Xun J, Jiang X and Tan R: Propofol
up-regulates Mas receptor expression in dorsal root ganglion
neurons. Pharmazie. 68:677–680. 2013.PubMed/NCBI
|
|
19
|
Costa AC, Becker LK, Moraes ER, Romero TR,
Guzzo L, Santos RA and Duarte ID: Angiotensin-(1–7) induces
peripheral antinociception through mas receptor activation in an
opioid-independent pathway. Pharmacology. 89:137–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennett G and Xie Y: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zanella JM, Burright EN, Hildebrand K,
Hobot C, Cox M, Christoferson L and Mckay WF: Effect of etanercept,
a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the
rat chronic constriction injury model. Spine (Phila Pa 1976).
33:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melli G and Höke A: Dorsal root ganglia
sensory neuronal cultures: A tool for drug discovery for peripheral
neuropathies. Expert Opin Drug Discov. 4:1035–1045. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gironacci MM, Adamo HP, Corradi G, Santos
RA, Ortiz P and Carretero OA: Angiotensin (1–7) induces MAS
receptor internalization. Hypertension. 58:176–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizoguchi H, Watanabe C, Yonezawa A and
Sakurada S: New therapy for neuropathic pain. Int Rev Neurobiol.
85:249–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silva DM, Vianna HR, Cortes SF,
Campagnole-Santos MJ, Santos RA and Lemos VS: Evidence for a new
angiotensin-(1–7) receptor subtype in the aorta of Sprague-Dawley
rats. Peptides. 28:702–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Medeiros MA, França MS, Boileau G, Juliano
L and Carvalho KM: Specific fluorogenic substrates for neprilysin
(neutral endopeptidase, EC 3.4.24.11) which are highly resistant to
serine- and metalloproteases. Braz J Med Biol Res. 30:1157–1162.
1997. View Article : Google Scholar : PubMed/NCBI
|