Introduction
Cerebral atherosclerosis is an important risk factor
for ischemic stroke, and is the cause of stroke in 8–15% of
patients (1). Factors that lead to
stroke and that are associated with cerebral artery stenosis
include intraluminal embolus, perforating artery occlusion and low
perfusion (2). Currently, common
techniques used for cerebrovascular evaluation include computed
tomography angiography, magnetic resonance angiography (MRA) and
digital subtraction angiography (DSA); however, the majority of
these only examine the vascular lumen. With the development of
interventional vascular therapy, pre-operative evaluation for
interventional treatment of cerebrovascular diseases should not be
limited to the location and stenosis of afflicted vessels. Rather,
it is also important to determine the distribution, shape and
properties of plaques on stenotic artery walls in order to properly
evaluate surgical procedures and patient prognoses. High-resolution
magnetic resonance imaging (HR-MRI) is a novel technique developed
to examine blood vessels, specifically in the evaluation of
cerebral vessel wall plaques (3).
Previous HR-MRI studies demonstrated that vascular lumen morphology
was significantly different between patients with symptomatic and
asymptomatic cerebral infarction (4). However, vessel wall morphology and
plaque enhancement patterns during different periods of cerebral
infarction have not yet been reported. In the present study, 3.0-T
HR-MRI was performed to investigate the morphological
characteristics of afflicted vessels during different periods of
cerebral infarction in patients with acute and non-acute cerebral
infarction.
Materials and methods
Clinical data
Informed consent was obtained from all patients
prior to an MRI scan. The study was approved by the institutional
review board and ethics commission of the Affiliated Hospital of
Xuzhou Medical College (Xuzhou, China), and 32 patients were
prospectively included. Clinical data were collected from patients
with unilateral middle cerebral artery (MCA) stenosis confirmed by
brain MRA, which was performed in the Affiliated Hospital of Xuzhou
Medical College between November 2013 and April 2014. Patients with
any of the following conditions were excluded: An age of <50
years; non-atherosclerotic cerebrovascular disease, including
vascular malformations, arterial dissection and arteritis; <50%
MCA stenosis; and HR-MRI of insufficient quality to meet
measurement requirements.
HR-MRI examination
Time-of-flight (TOF) MRA was performed using a
Discovery MR750 3.0-T superconducting MRI scanner (GE Healthcare,
Piscataway, NJ, USA) equipped with a 16-channel phased array
coil.
Scan parameters were as follows: Repetition time
(TR)/echo time (TE), 27/6.9 msec; field of view (FOV), 24×16 cm;
layer thickness, 1.6 mm; flip angle, 20°; number of excitations
(NEX), 1; and matrix size, 320×256. For diffusion-weighted imaging
(DWI) these parameters were as follows: TR/TE, 4,500/90 msec; FOV,
24×24 cm; layer thickness, 6.0 mm; and NEX, 3.
For HR-MRI, proton density-weighted imaging (PDWI)
scan parameters were as follows: TR/TE, 2,900/20 msec; FOV, 12×12
cm; layer thickness, 2.5 mm; NEX, 4; echo train length (ETL), 10;
matrix size, 256×256; and voxel size, 0.22×0.22×2.5 mm. For T1WI
and enhanced T1WI, the parameters were as follows: TR/TE, 600/12
msec; FOV, 12×12 cm; layer thickness, 2.5 mm; NEX, 4; ETL, 3;
matrix size, 256×256; and voxel size, 0.22×0.22×2.5 mm.
Contrast-enhanced T1-weighted images were captured 2
min after intravenous injection of gadopentetate dimeglumine
(Magnevist; Bayer Schering Pharma AG, Leverkusen, Germany) at 0.1
mmol/kg of body weight. A total of 6 slices were imaged in each
HR-MRI sequence, and the scan time was 2–3 min for each.
Image analysis
All images were uploaded to an AW Volume Share 5
(4.6) workstation (GE Healthcare) for analysis. Image quality was
rated on a scale of 1–5 (in which a score of 5 indicated excellent
image quality). Parameter measurement and quantitative analysis
were conducted on images with a score of ≥3 (5). Patients were classified into the acute
and non-acute cerebral infarction (including no infarction and old
infarction) groups based on the presence or absence of high DWI
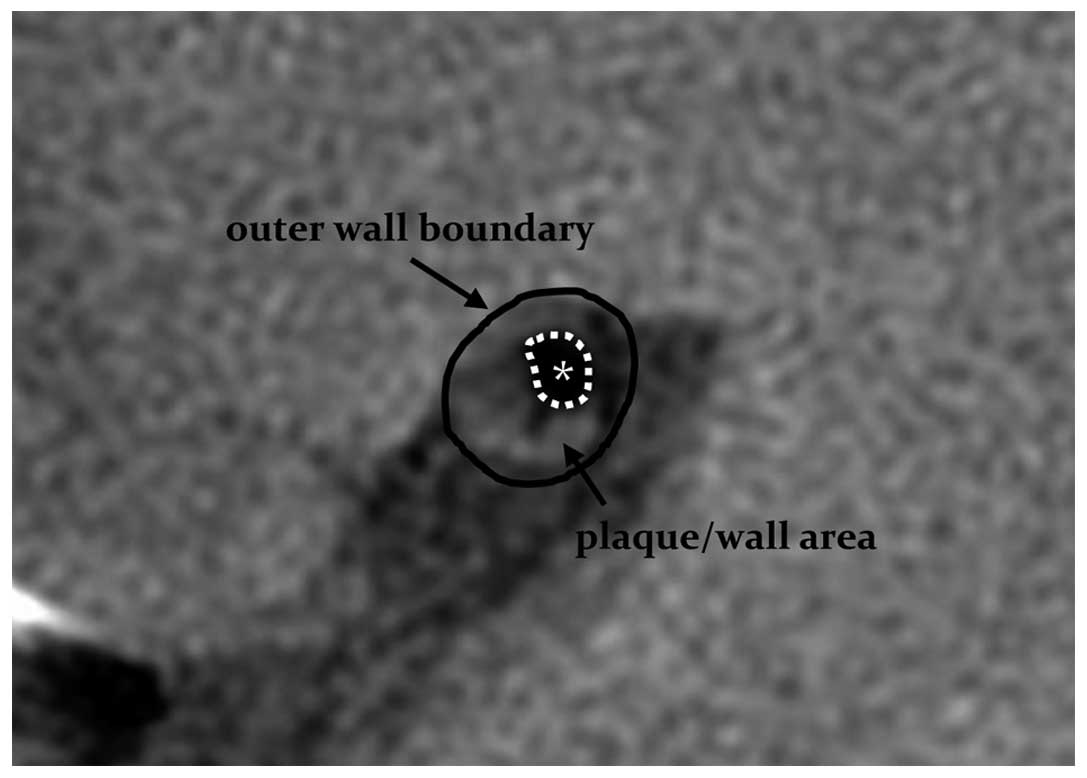
signal intensity. The outer wall boundary and the vessel lumen of
the narrowest vessel segments were manually drawn using the PDWI
sequences (Fig. 1), and lumen and
vessel areas were automatically calculated by the workstation
software. Wall area = vessel area - lumen area. A normal layer at
the proximal end of the lesion was selected as the reference layer;
if there was no suitable layer at the proximal end, a normal layer
at the distal end was selected. Stenosis rate = (1 - lumen area of
the narrowest layer / lumen area of the reference layer) × 100;
remodeling rate = vessel area of the narrowest layer / vessel area
of the reference layer. A remodeling rate of ≤0.95 was defined as
negative remodeling (NR), and a rate of ≥1.05 was considered
positive remodeling (PR) (6). An
eccentric plaque was defined as localized plaque surrounding <75%
of the vessel wall. Image quality and target vessels were analyzed
by two experienced imaging physicians, with 16 and 14 years of
experience, in a blinded manner, and a consensus was achieved
through discussion when disagreements occurred.
Statistical analysis
All data were analyzed using SPSS 13.0 (SPSS Inc.,
Chicago, IL, USA). The basic clinical data, stenosis rate, wall
area, lumen area, plaque area, plaque enhancement patterns and wall
remodeling rate were compared between the acute and non-acute
infarction groups. Measurement data were expressed as the mean ±
standard deviation and compared between the two groups using a
two-sample Student's t-test. Enumerated data were expressed as
frequency and percentage and compared between the two groups using
χ2 or Fisher's exact tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
A total of 32 patients met the inclusion criteria,
including 15 acute and 17 non-acute cerebral infarction patients.
The time from onset to MRI ranged from 6 h to 3 days for acute
patients. The patients' general information and atherosclerosis
risk factors are listed in Table I.
The quality of all images met the aforementioned quantitative
analysis requirements. There was no significant difference in image
quality between the DWI-positive and -negative groups (3.5 vs. 3.6;
P>0.05).
 | Table I.Demographic data. |
Table I.
Demographic data.
| Demographic
group | DWI(+) (n=15) | DWI(−) (n=17) | P-value |
|---|
| Age,
yearsa | 65.2±14.8 | 66.4±11.9 | 0.614 |
| Male, n (%) | 8
(53.3) | 7
(41.2) | 0.723 |
| Hypertension, n
(%) | 13 (86.7) | 12 (70.6) | 0.402 |
| Diabetes mellitus, n
(%) | 5
(33.3) | 4
(23.5) | 0.699 |
| Smoking, n (%) | 6
(40.0) | 5
(29.4) | 0.712 |
| Hypercholesterolemia,
n (%) | 10 (66.7) | 9
(52.9) | 0.491 |
| Cardiovascular
history, n (%) | 5
(33.3) | 7
(41.2) | 0.726 |
Overall, eccentric plaques were observed in 29
patients, including 13 acute and 16 non-acute infarction patients.
The vascular stenosis rates were 70.12±16.42 and 68.22±12.87% in
the acute and non-acute patients, respectively (P=0.281). The acute
group was significantly different from the non-acute group with
regard to wall area, lumen remodeling rate and plaque enhancement
(P=0.002, P=0.002 and P=0.004, respectively). The wall area of
stenotic segments was significantly greater in the acute group
compared with the non-acute group (11.84±2.81 vs. 8.75±2.02
mm2; P=0.002). No significant difference was observed in
lumen area (3.24±1.38 vs. 3.05±1.42 mm2; P=0.873) and
plaque area (6.44±2.61 vs. 5.61±2.17 mm2; P=0.257)
between the acute and non-acute groups. Plaque enhancement was
observed in 12 acute and 4 non-acute infarction patients (P=0.004;
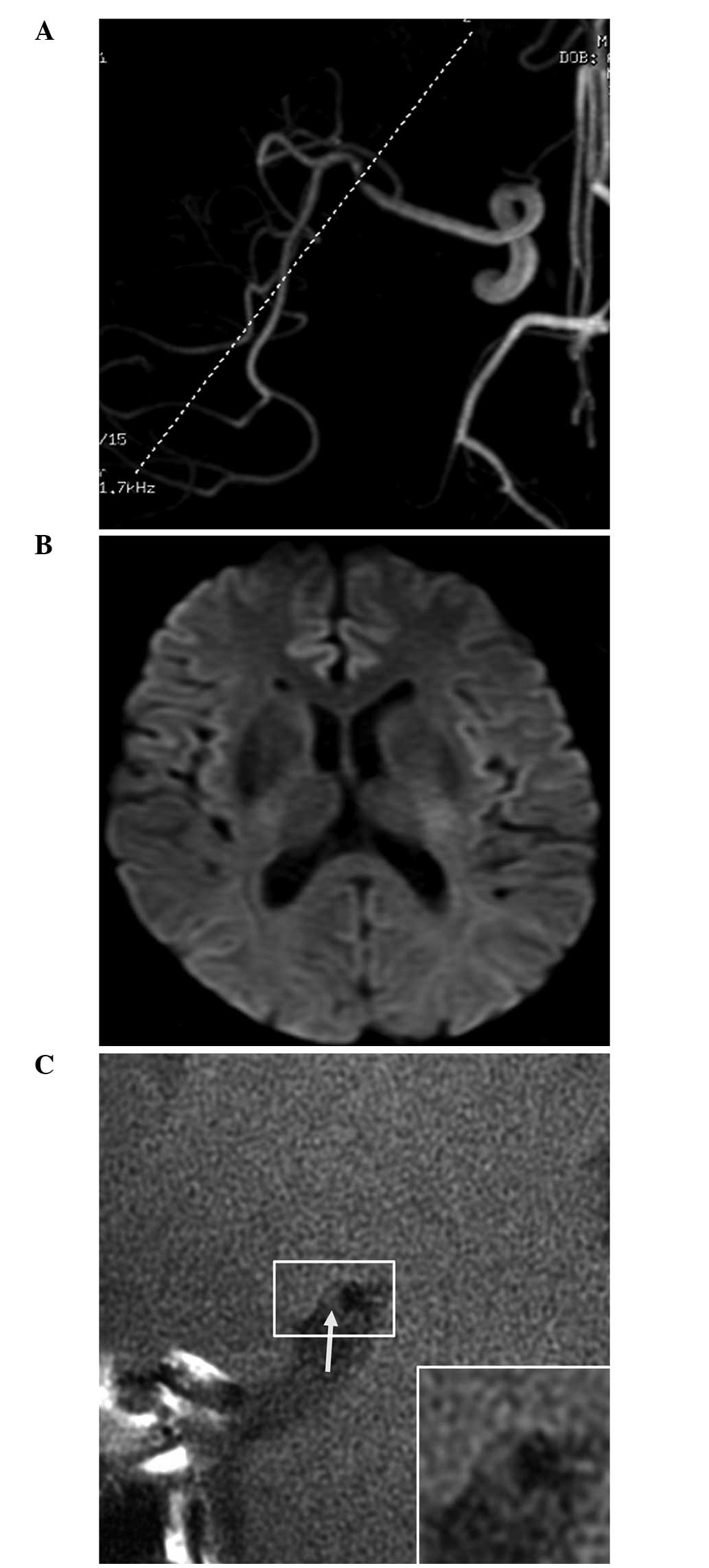
Table II; Figs. 2 and 3).
 | Table II.Comparison of stenotic arteries
between acute and non-acute cerebral infarction. |
Table II.
Comparison of stenotic arteries
between acute and non-acute cerebral infarction.
| Features | DWI(+) (n=15) | DWI(−) (n=17) | P-value |
|---|
| Stenosis degree,
% |
70.12±16.42 | 68.22±12.87 | 0.281 |
| Wall area,
mm2 | 11.84±2.81 | 8.75±2.02 | 0.002 |
| Lumen area,
mm2 |
3.24±1.38 | 3.05±1.42 | 0.873 |
| Plaque area,
mm2 |
6.44±2.61 | 5.61±2.17 | 0.257 |
| Eccentric plaque, n
(%) | 13 (86.7) | 16 (94.1) | 0.589 |
| Plaque enhancement, n
(%) | 12 (80.0) | 4
(23.5) | 0.004 |
| Remodeling
ratea |
1.08±0.19 | 0.87±0.14 | 0.002 |
Discussion
Cerebral atherosclerosis is a common disease, and
plaque formation and rupture are important factors in the
pathogenesis of ischemic stroke and transient ischemic attack
(TIA). Previous carotid artery studies have indicated that MRI
could effectively evaluate carotid artery plaque characteristics
(7). Due to the small lumen of
cerebral arteries and the limitations of MRI, only a few studies
have evaluated cerebral arterial walls and plaques (3,8), but the
development of HR-MRI has made it possible to assess intracranial
arterial wall morphology using MRI.
Klein et al (9) demonstrated that HR-MRI could produce
images of cerebral arterial walls and lumina; in specific cases in
which conventional TOF MRA provides normal findings or images of a
rough wall, HR-MRI can detect wall plaques that are not visible
using MRA. This is hypothesized to be associated with vascular
lumen remodeling. Early studies investigating the coronary arteries
identified two patterns of lumen remodeling: PR and NR. The former
is characterized by outward expansion of blood vessels, which aids
in maintaining vascular lumen size, but makes the plaques
vulnerable to rupture, thus causing TIA and acute stroke. The
latter is characterized by inward blood vessel contraction, which
can aggravate stenosis, but allows the plaques to be relatively
stable (9,10). Thus, a large wall plaque may not
cause stenosis due to vascular lumen remodeling. For this reason,
traditional TOF MRA and DSA have certain limitations and can be
misleading when evaluating stenosis or risk factors. Li et
al (11) performed TOF MRA and
HR-MRI examinations in 48 patients with suspected MCA stenosis. The
study reported that HR-MRI detected abnormal vessel walls that were
diagnosed as normal by TOF MRA in 5 patients, and 2 patients were
diagnosed with vascular stenosis using TOF MRA, but no abnormal
lumen was reported by HR-MRI. These findings were considered to be
associated with the different remodeling patterns in the stenotic
lumen.
In the present study, the acute cerebral infarction
group had a wall area significantly greater than that of the
non-acute group, and predominantly exhibited PR. No significant
differences were identified in lumen area or plaque area, and
therefore, it was suggested that the presence or absence of
increased DWI signal intensity was due to vascular remodeling. In
previous studies, the remodeling pattern of stenotic arteries has
been associated with interventional surgery complications (12); patients with NR may be at greater
risk of injury during interventional therapy, and pre-operative
HR-MRI aids in the assessment of the risk of surgery (13).
HR-MRI is currently the best way to evaluate
cerebral artery plaque stability. Due to the limited resolution of
MRI equipment, cerebral arterial plaque composition analysis
remains in the exploratory stage (14). Conversely, the analysis of carotid
atherosclerotic plaque composition using HR-MRI is relatively
mature, and intracranial atherosclerotic plaques have properties
similar to those of carotid atherosclerotic plaques (15).
Unstable plaques are characterized by a large
intraplaque lipid core or intraplaque hemorrhage, and a thin or
discontinuous fibrous cap, whereas stable plaques are mainly
composed of fiber, have a small or no lipid core, and a thick and
continuous fibrous cap (16,17). Plaque enhancement, in addition to
signal characteristics, is associated with plaque stability.
Enhanced imaging reveals enhanced wall plaques of afflicted
arteries in acute stroke patients and plaque enhancement is
associated with the infarction distribution (18,19). In
the present study, wall plaque enhancement was observed in 12 and 4
patients in the DWI-positive and -negative groups, respectively.
The plaque enhancement rate was significantly higher in the
DWI-positive group, and wall plaque enhancement was considered to
be associated with internal neovascularization or inflammatory
reactions (20).
The present study has certain limitations. First,
although HR-MRI is more effective than conventional TOF MRA in
evaluating cerebral arterial wall plaques, the majority of the
results are based on associated results of carotid or coronary
plaques from earlier studies and it is difficult to obtain
pathological results of cerebral artery plaques. For this reason,
the evaluation of cerebral artery plaque morphology and properties
is subjective to a certain extent. Second, due to the small total
sample size, the cerebral infarction patients were divided into
acute and non-acute groups based on DWI only, and were not further
divided according to infarction time. Moreover, the severity of
cerebral infarction in the acute patients was not clearly defined,
meaning that there were a few uncertain factors in the stenotic
artery analysis. Third, plaque enhancement was mainly determined
based on the subjective judgment of the physicians; no quantitative
analysis was performed. In future studies, quantitative analyses of
plaque signal intensities prior to and following enhancement may
aid in determining the association between plaque properties and
cerebral infarction.
In conclusion, the present study showed that HR-MRI
can provide high-quality images of vessel wall structure that
cannot be achieved with conventional MRI, and thus can serve as a
clear complement to TOF MRA to perform a more detailed analysis of
stenotic artery walls in patients with cerebral infarction,
including resolving plaque properties. HR-MRI can thus assist in
clinical treatment and in determining patient prognosis.
Acknowledgements
This study was supported by funding from Xuzhou
Medical College (grant no. 2014KJ17).
References
|
1
|
Wityk RJ, Lehman D, Klag M, Coresh J, Ahn
H and Litt B: Race and sex differences in the distribution of
cerebral atherosclerosis. Stroke. 27:1974–1980. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong KS, Gao S, Chan YL, Hansberg T, Lam
WW, Droste DW, Kay R and Ringelstein EB: Mechanisms of acute
cerebral infarctions in patients with middle cerebral artery
stenosis: A diffusion-weighted imaging and microemboli monitoring
study. Ann Neurol. 52:74–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryu CW, Jahng GH, Kim EJ, Choi WS and Yang
DM: High resolution wall and lumen MRI of the middle cerebral
arteries at 3 tesla. Cerebrovasc Dis. 27:433–442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M,
Peng B, Feng F, Jin ZY and Cui LY: In vivo high-resolution MR
imaging of symptomatic and asymptomatic middle cerebral artery
atherosclerotic stenosis. Atherosclerosis. 212:507–511. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan C, Mitsumori LM, Ferguson MS,
Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS and
Hatsukami TS: In vivo accuracy of multispectral magnetic resonance
imaging for identifying lipid-rich necrotic cores and intraplaque
hemorrhage in advanced human carotid plaques. Circulation.
104:2051–2056. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF
and Zhao TQ: Arterial remodeling of advanced basilar
atherosclerosis: a 3-tesla MRI study. Neurology. 75:253–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan C, Beach KW, Smith LH Jr and
Hatsukami TS: Measurement of atherosclerotic carotid plaque size in
vivo using high resolution magnetic resonance imaging. Circulation.
98:2666–2671. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niizuma K, Shimizu H, Takada S and
Tominaga T: Middle cerebral artery plaque imaging using 3-Tesla
high-resolution MRI. J Clin Neurosci. 15:1137–1141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein IF, Lavallée PC, Mazighi M,
Schouman-Claeys E, Labreuche J and Amarenco P: Basilar artery
atherosclerotic plaques in paramedian and lacunar pontine
infarctions: A high-resolution MRI study. Stroke. 41:1405–1409.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schoenhagen P, Ziada KM, Kapadia SR, Crowe
TD, Nissen SE and Tuzcu EM: Extent and direction of arterial
remodeling in stable versus unstable coronary syndromes: An
intravascular ultrasound study. Circulation. 101:598–603. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li ML, Xu WH, Song L, Feng F, You H, Ni J,
Gao S, Cui LY and Jin ZY: Atherosclerosis of middle cerebral
artery: Evaluation with high-resolution MR imaging at 3T.
Atherosclerosis. 204:447–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamagishi M, Terashima M, Awano K, Kijima
M, Nakatani S, Daikoku S, Ito K, Yasumura Y and Miyatake K:
Morphology of vulnerable coronary plaque: Insights from follow-up
of patients examined by intravascular ultrasound before an acute
coronary syndrome. J Am Coll Cardiol. 35:106–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang WJ, Yu W, Ma N, Du B, Lou X and
Rasmussen PA: High resolution MRI guided endovascular intervention
of basilar artery disease. J Neurointerv Surg. 3:375–378. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swartz RH, Bhuta SS, Farb RI, Agid R,
Willinsky RA, Terbrugge KG, Butany J, Wasserman BA, Johnstone DM,
Silver FL and Mikulis DJ: Intracranial arterial wall imaging using
high-resolution 3-tesla contrast-enhanced MRI. Neurology.
72:627–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fayad ZA and Fuster V: Characterization of
atherosclerotic plaques by magnetic resonance imaging. Ann NY Acad
Sci. 902:173–186. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saam T, Cai J, Ma L, Cai YQ, Ferguson MS,
Polissar NL, Hatsukami TS and Yuan C: Comparison of symptomatic and
asymptomatic atherosclerotic carotid plaque features with in vivo
MR imaging. Radiology. 240:464–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saam T, Yuan C, Chu B, Takaya N, Underhill
H, Cai J, Tran N, Polissar NL, Neradilek B, Jarvik GP, et al:
Predictors of carotid atherosclerotic plaque progression as
measured by noninvasive magnetic resonance imaging.
Atherosclerosis. 194:e34–e42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JM, Jung KH, Sohn CH, Moon J, Han MH
and Roh JK: Middle cerebral artery plaque and prediction of the
infarction pattern. Arch Neurol. 69:1470–1475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demarco JK, Ota H, Underhill HR, Zhu DC,
Reeves MJ, Potchen MJ, Majid A, Collar A, Talsma JA, Potru S, et
al: MR carotid plaque imaging and contrast-enhanced MR angiography
identifies lesions associated with recent ipsilateral
thromboembolic symptoms: An in vivo study at 3T. AJNR Am J
Neuroradiol. 31:1395–1402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen XY, Wong KS, Lam WW, Zhao HL and Ng
HK: Middle cerebral artery atherosclerosis: Histological comparison
between plaques associated with and not associated with infarct in
a postmortem study. Cerebrovasc Dis. 25:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|