Introduction
Liver cirrhosis is the final stage of a chronic
fibrotic process in the liver and is the primary cause of portal
hypertension. Hyperdynamic circulation, which is secondary to the
presence of systemic vasodilation, is an important factor in the
aggravation and persistence of portal hypertension (1). Numerous mechanisms are involved in the
development of systemic vasodilation, including increased synthesis
of nitric oxide (NO) and carbon monoxide (CO) and the activation of
KATP channels in the systemic and splanchnic arterial
circulation (2). Previous studies
have demonstrated that the hepatic heme oxygenase-1 (HO-1)/CO
system (3) and NO/nitric oxide
synthetase activity are overexpressed in rats with cirrhosis and
contribute to portal hypertension (4)
In a previous study, hydrogen sulfide
(H2S) was identified as the third endogenous signaling
gasotransmitter, in addition to NO and CO, and was found to serve
crucial functions in normal physiological conditions and in the
process/progress of numerous diseases (5). H2S is produced endogenously
from cysteine by pyridoxal-5-phosphate-dependent enzymes, including
cystathionine-β-synthase and cystathionine-γ-lyase (CSE) (6), and is involved in vasorelaxation by
activating the KATP channel, a different mechanism from
that of NO and CO (7,8).
NO is endogenously generated by vascular endothelial
cells, while H2S is derived from vascular smooth muscle
cells and CO is endogenously produced by vascular endothelial and
smooth muscle cells; therefore, it is plausible that these gaseous
transmitters may interact in the regulation of biological functions
(9–12). It has previously been shown that NO
is able to upregulate the endogenous production of H2S
by increasing CSE gene expression (8). Additionally, H2S is able to
enhance vasodilation by NO at very low concentrations (8).
The effect of CO on the production of H2S
remains unclear. CSE is the primary enzyme involved in catalyzing
the endogenous production of H2S in mammalian hepatic
tissue (13), and >50% of the gas
in the liver appears to be derived from CSE (13). The aim of the present study was to
investigate the effect of endogenous CO on the H2S/CSE
pathway in cirrhotic livers of rats by manipulating HO-1 enzyme
activity via the intraperitoneal injection of zinc protoporphyrin
IX (ZnPP), a specific HO-1 enzyme inhibitor, or cobalt
protoporphyrin (CoPP), a specific HO-1 enzyme inducer.
Materials and methods
Animal care
The experimental protocols were approved by the
Animal Care and Use Committee of Dalian Medical University (Dalian,
China), in accordance with the guidelines established by the
Canadian Council on Animal Care. A total of 45 healthy male Sprague
Dawley rats (weight, 200–220 g; age, 6 weeks) were obtained from
the Animal Center of Dalian Medical University (Dalian, China).
Reagents
TRIzol® reagent was obtained from Nanjing KeyGen
Biotech, Co., Ltd. (KGA1203; Nanjing, China); a PrimeScript® RT
Master Mix Perfect Real Time kit (DRR036A) and SYBR® Premix Ex Taq™
(DRR420A) were purchased from Takara Biotechnology Co., Ltd.
(DRR036A; Dalian, China); polyclonal rabbit anti-mouse HO-1 and
rabbit anti-mouse CSE antibodies were obtained from Wuhan Boster
Biological Technology Co., Ltd. (Wuhan, China); anti-rabbit IgG was
obtained from Fuzhou Maixin Biotechnology Development Co., Ltd.
(MaxVision™2; Fuzhou, China); a Takara RNA polymerase chain
reaction (PCR) kit (alfalfa mosaic virus) Ver. 3.0 was purchased
from Takara Biotechnology Co., Ltd.; and CoPP and ZnPP were
obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animal model and grouping
Rats were randomly divided into four groups: Sham
(n=8), cirrhosis (n=8), CoPP (n=12) and ZnPP (n=12). The rats were
housed in a specific pathogen-free center, at a temperature of
24–26°C and at a relative humidity of 60–65%. Rats were housed for
3 days prior to experimental protocols being initiated, were well
fed and received water ad libitum. Bile duct ligation (BDL)
was used to induce cirrhosis in rats in the cirrhosis, CoPP and
ZnPP groups, according to the method described in a previous study
(14). Laparotomy was performed
under anesthesia with ether. The bile duct was isolated and double
ligated with 3-0 silk. The abdominal wall and the skin were closed
with 4-0 silk sutures, and antibiotic benzathine benzylpenicillin
powder was sprinkled over the closed incision. The rats were
continuously fed and housed for an additional 4-week period after
surgery, and samples were collected. Rats in the sham group
underwent laparotomy, with the bile duct isolated but not ligated.
ZnPP and CoPP (Sigma-Aldrich) were dissolved in 0.2 mol/l NaOH,
adjusted to pH 7.4 and diluted in 0.85% NaCl. The final
concentration was 1 mg/ml, as previously described (15), and the resulting ZnPP and CoPP
solutions were used to inhibit or induce HO-1 expression,
respectively. Rats in the ZnPP and CoPP groups received an
intraperitoneal injection of ZnPP or CoPP (5 mg/kg/day),
respectively, for a week prior to sample collection. In addition, 5
of the initial 45 rats died prior to sample collection.
Sample collection
At 4 weeks after surgery, the rats were anesthetized
with ether and the portal vein was isolated. A catheter, connected
to pressure transducers (BL-420F data acquisition and analysis
system; Chengdu Technology & Market Co., Ltd., Chengdu, China),
was placed in the portal vein to measure portal vein pressure
(PVP). Arterial blood (1 ml) was then collected using a heparinized
syringe through the arterial catheter to measure carboxyhemoglobin
(COHb) levels using a RapidLab 1245 blood gas analyzer (Siemens
Healthcare, Malvern, PA, USA) as an index for the CO level in
arterial blood. Subsequently, 4 ml portal venous blood was
collected from the rats to measure serum levels of alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and total
bilirubin (TBIL) using a Hitachi 7600-110 automatic biochemical
analyzer (Hitachi, Ltd., Tokyo, Japan). One lobe of the liver was
excised and tissue samples were fixed in 10% neutral formalin
solution and embedded in paraffin; the remaining tissue was
preserved at −80°C for subsequent quantitative PCR (qPCR)
analysis.
Measurement of serum H2S
Plasma samples (75 µl) were mixed with 100 µl
distilled water and 300 µl 10% trichloroacetic acid. Then, 150 µl
1% zinc acetate was added to Eppendorf tubes, along with 20 µM
N,N-Dimethyl-p-phenylenediamine sulfate in 7.2 M HCl
and FeCl3 (30 µM; 133 µl) in 1.2 M HCl. After 15 min of
incubation, the absorbance of the solution was measured at a
wavelength of 670 nm using a UV-2550 spectrophotometer (Shimadzu
Corp., Kyoto, Japan). All samples were assayed in duplicate, and
the H2S concentration was calculated against a
calibration curve of NaHS (0.122–250 µM).
Hepatic hydroxyproline (HYP)
content
The HYP content in the liver was evaluated as an
indirect index of tissue collagen content, according to a
previously described method with modification (16), and was expressed in micrograms per
gram of wet weight (µg/g).
Pathological analysis
Hematoxylin and eosin (H&E) staining was
performed according to standard procedures. The changes in liver
cells, portal areas and central veins were assessed. In addition,
Van Gieson's staining was conducted to visualize collagen type I.
Sections were stained with Weigerts Resorcin Fuchsin at room
temperature for 25 min, washed with water, differentiated in acid
alcohol, then washed in water for a further 10 min. The sections
were subsequently stained with Van Gieson for 5 min, washed in
water, dehydrated with ethanol, cleared using xylene and mounted
for observation.
Immunohistochemical analysis
Liver tissues were fixed in a 10% neutral formalin
solution, embedded in paraffin wax and cut into sections of 1 × 0.8
× 0.0004 cm. Certain sections were stained with H&E, while
others underwent deparaffinization, rehydration and inactivation,
prior to being incubated with rabbit-anti-mouse CSE and HO-1
polyclonal antibodies (1:50) at room temperature for 60 min.
Following incubation with the primary antibody, the sections were
incubated with a secondary antibody (MaxVision 2) at room
temperature for 15 min. The sections were protected by coverslips
following staining. The primary antibody was replaced by
phosphate-buffered saline to serve as a negative control. Samples
were incubated with 3% H2O2 for 10 min at
room temperature to eliminate endogenous peroxidase activity and to
block nonspecific background staining. Subsequently, the sections
were washed with distilled water, 0.01 M citrate buffer (pH=6.0)
was added and the sections were heated by microwave oven for 10
min. Following cooling, the sections were washed with 0.1 M PBS
wash buffer 3 times. The immunoreactive signal was visualized by
color deposition, using diaminobenzidine as a substrate. Yellow
material in the cytoplasm was considered to indicate positive
cells. Images were analyzed using Image-Pro Plus software, version
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) to calculate the
area and mean density of positive expression. Mean density was
calculated as follows: Integrated optical density/area of interest.
Results from five visual fields were averaged for each sample.
qPCR analysis
Quantification of the expression level of target
genes was performed using qPCR. Total RNA was extracted from rat
livers using TRIzol reagent (Nanjing KeyGen Biotech. Co., Ltd.).
After extraction of RNA, reverse transcription was performed using
random primers provided with the Takara PCR kit, following the
manufacturer's instructions. The PCR amplification conditions were
as follows: Pre-denaturation at 95°C for 30 sec, followed by 40
cycles of amplification by denaturing at 95°C for 5 sec, annealing
at 60°C for 1 min and extending at 72°C for 30 sec. PCR cycling was
performed using a Mx3005P qPCR system (Agilent Technologies, Inc.,
Santa Clara, CA, USA). The relative quantity of mRNA for each gene
was normalized against the quantity of the housekeeping gene
β-actin. Each sample was run and analyzed in triplicate. The primer
sequences for CSE were as follows: 5′-GAG CCG GAG CAA TGG AGT TC-3′
(forward) and 5′-GGA TTT CCA GAG CGG CTG TA-3′ (reverse). The
primer sequences for β-actin were: 5′-GGA GAT TAC TGC CCT GGC TCC
TA-3′ (forward) and 5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′
(reverse). The primers were designed and synthesized by Takara
Biotechnology Co., Ltd.
Statistical analysis
Data analysis was performed using SPSS software,
version 10.0 (SPSS, Inc., Chicago, IL, USA). Analysis of variance
or Wilcoxon statistical methods were used to determine statistical
differences. All data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Biochemical examination
At 4 weeks after surgery, ascites and jaundice were
observed in the groups subjected to BDL, indicating that the BDL
model was successfully established.
The serum levels of AST, ALT and TBIL in the
cirrhosis group were significantly higher than those in the sham
group (P<0.05). Furthermore, the levels were higher in the CoPP
group and reduced in the ZnPP group compared with those in the
cirrhosis group (P<0.05) (Table
I). The serum levels of H2S in the cirrhosis group
were significantly lower than those in the sham group (P<0.05).
Furthermore, serum H2S was higher in the ZnPP group and
lower in the CoPP group compared with that in the cirrhosis group
(P<0.05). The levels of COHb in the arterial blood were
significantly higher in the cirrhosis group than those in the sham
group (P<0.05). Compared with the cirrhosis group, the COHb
levels were significantly decreased in the ZnPP group (P<0.05)
and significantly increased in the CoPP group (P<0.05). The PVP
was significantly higher in the cirrhosis group than that in the
sham group (P<0.05). Compared with the cirrhosis group, the PVP
was significantly higher in the CoPP group and reduced in the ZnPP
group (P<0.05) (Table II).
 | Table I.Comparison of serum ALT, AST and TBIL
among the groups. |
Table I.
Comparison of serum ALT, AST and TBIL
among the groups.
| Group | ALT (U/l) | AST (U/l) | TBIL (mg/dl) |
|---|
| Sham | 37.25±5.32 | 172.61±7.32 | 0.81±0.22 |
| Cirrhosis |
96.43±8.02a |
287.58±10.49a |
11.85±1.87a |
| CoPP |
120.33±9.83b |
410.51±12.53b |
14.65±2.26b |
| ZnPP |
45.42±5.59b |
205.08±8.03b |
5.59±2.24b |
 | Table II.PVP and concentrations of endogenous
H2S and COHb among the groups. |
Table II.
PVP and concentrations of endogenous
H2S and COHb among the groups.
| Group | H2S
(µmol/l) | COHb (%) | PVP
(cmH2O) |
|---|
| Sham | 369.54±51.28 | 0.23±0.05 | 9.05±0.53 |
| Cirrhosis |
142.85±38.58a |
0.50±0.20a |
14.87±2.02a |
| CoPP |
109.23±27.32b |
0.83±0.39b |
17.58±1.23b |
| ZnPP |
215.38±33.56b |
0.23±0.06b |
13.21±1.14b |
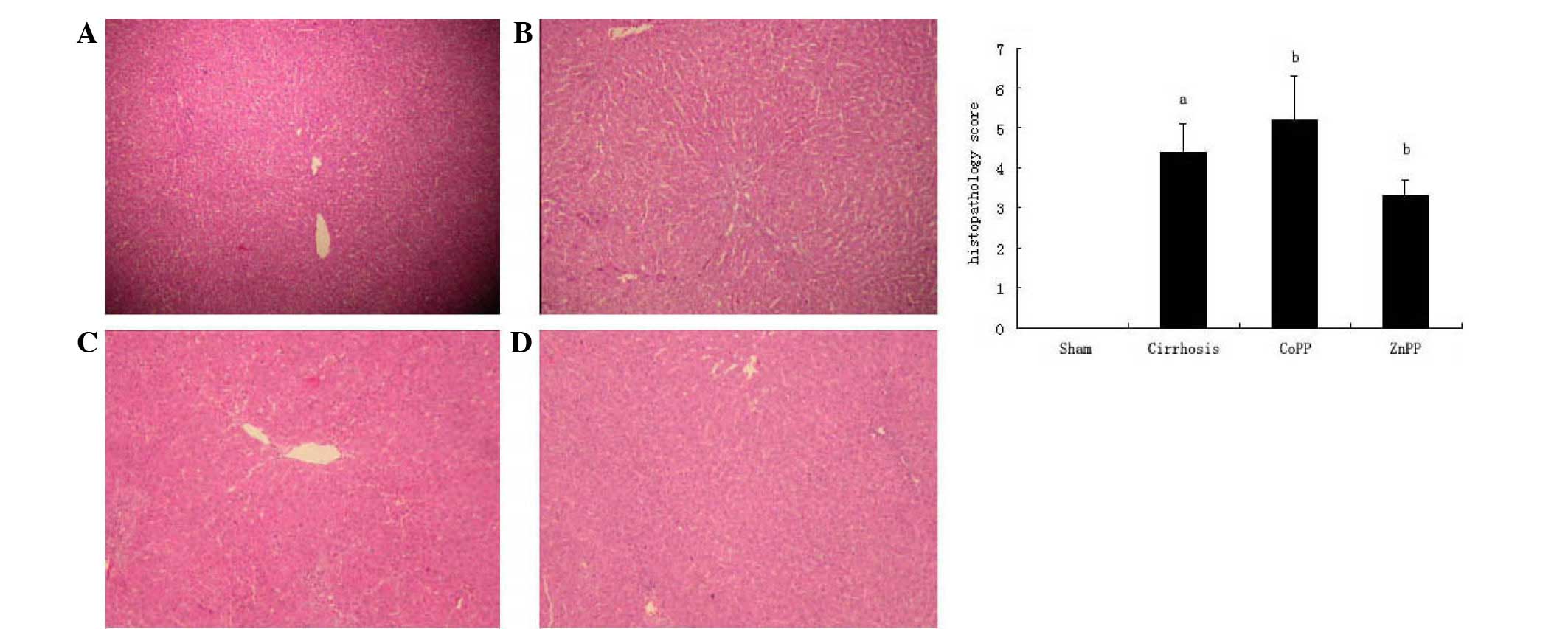
Histopathological analysis of the
liver
The degree of hepatic fibrosis was evaluated by
H&E staining. The sham group exhibited normal hepatic
architecture, whereas the cirrhosis group exhibited the
histological characteristics of bile duct proliferation and
extensive fibrosis. Compared with the cirrhosis group, fibrous
hyperplasia and fibrotic extensions with fibroblast proliferation
were less prevalent in the ZnPP group and more prominent in the
CoPP group (Fig. 1).
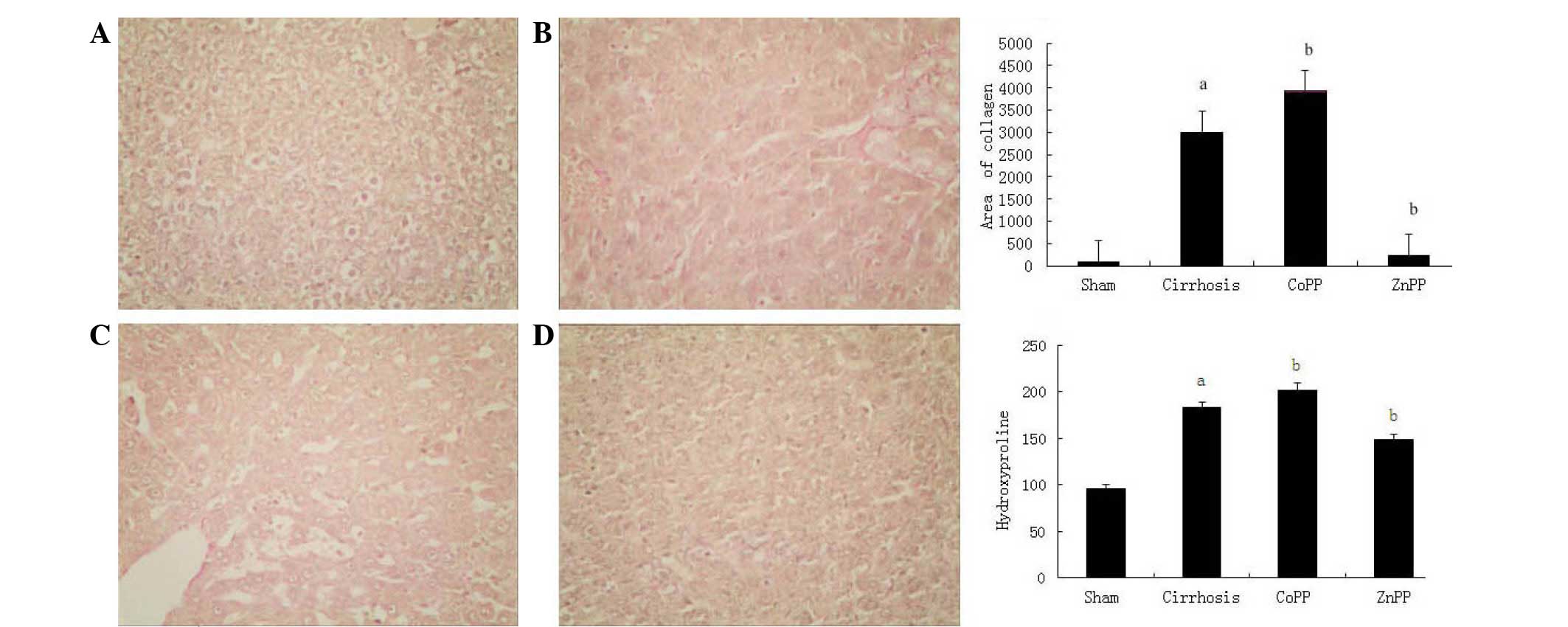
Collagen type I was observed using Van Gieson's
staining (Fig. 2). In the cirrhosis
group, collagen type I in the portal area and bile duct wall was
markedly thicker than that in the sham group (P<0.01). Compared
with the cirrhosis group, the extent of fibrosis was increased in
the CoPP group and decreased in the ZnPP group. The change in HYP
content in the liver tissue was in accordance with the change in
type I collagen. Compared with the sham group, the HYP content was
higher in the cirrhosis group. Compared with the cirrhosis group,
the HYP content was higher in the CoPP group and lower in the ZnPP
group (P<0.05).
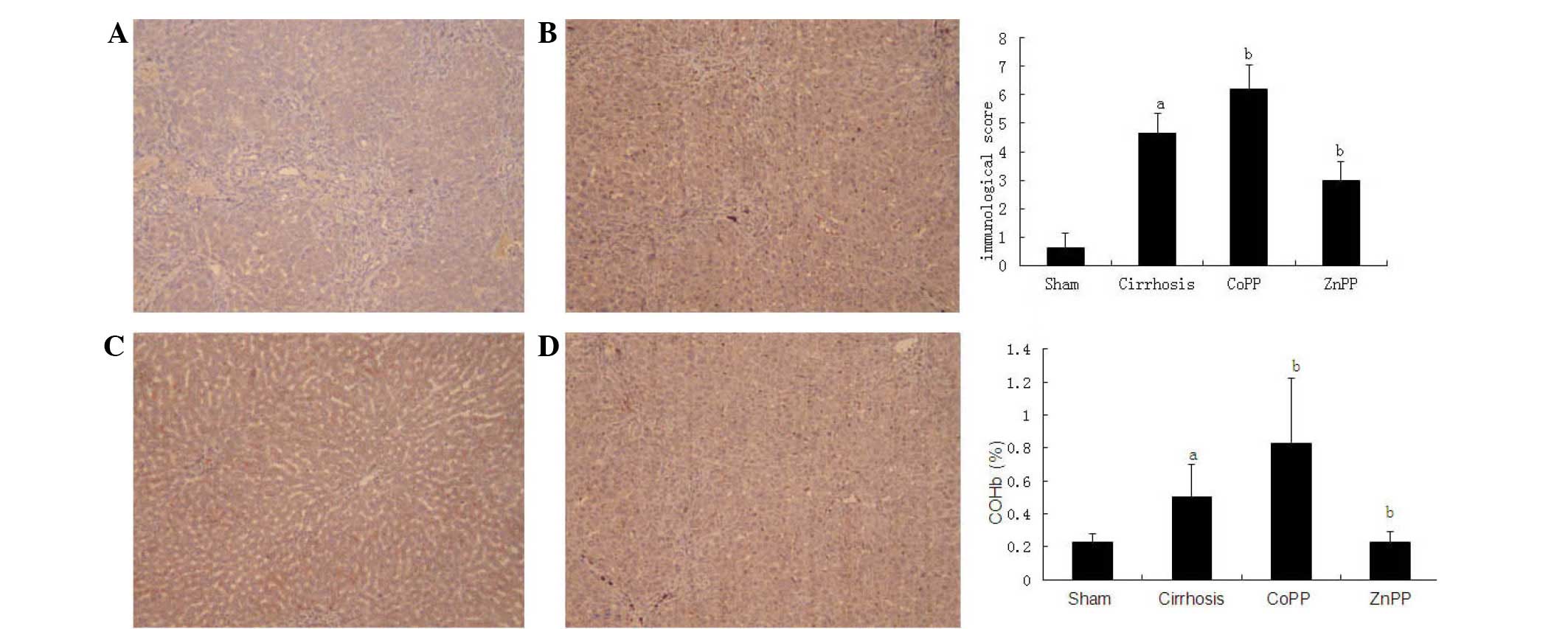
Immunohistochemical detection of CSE
and HO-1 protein expression levels
To localize the CSE and HO-1 protein expression
levels in the livers, immunohistochemical analysis was conducted
using specimens from the four groups. As shown in Fig. 3, the expression of the HO-1 protein
was primarily located in Kupffer's cells and hepatocytes, which is
consistent with the observations of previous studies (17,18). In
addition, the intensity and percentage of cells expressing HO-1
protein in the liver were detected. Mild staining was observed in
hepatic tissue samples from the sham group, with a score of
0.63±0.51. The HO-1 immunoreactivity was strongly positive in the
cirrhosis group with a score of 4.63±0.72, which was significantly
higher than that in the sham group (P<0.01). Compared with the
cirrhosis group, the HO-1 score was increased in the CoPP group
(6.21±0.85) and decreased in the ZnPP group (2.98±0.68) (both
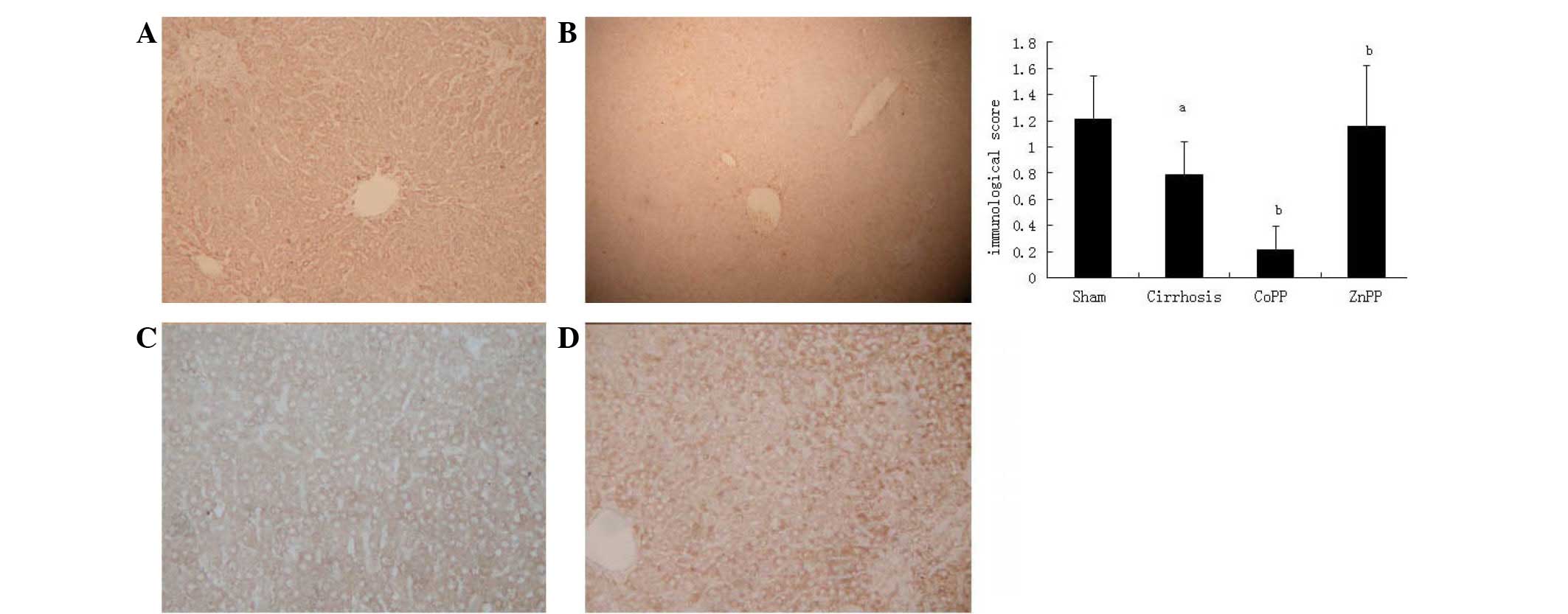
P<0.05). The expression of the CSE protein was predominantly
located in hepatocytes and hepatic stellate cells (HSCs) (Fig. 4), which is consistent with previous
studies (5,19). The intensity and percentage of cells
expressing hepatic CSE protein were additionally evaluated. There
was moderate positive staining in the sham group, with an overall
score of 1.21±0.33. The CSE score in the cirrhosis group was
0.79±0.25, which was significantly reduced compared with that in
the sham group (P<0.01). Compared with the cirrhosis group, the
CSE score was further decreased in the CoPP group (0.21±0.18),
whereas it was increased in the ZnPP group (1.16±0.46) (both
P<0.05).
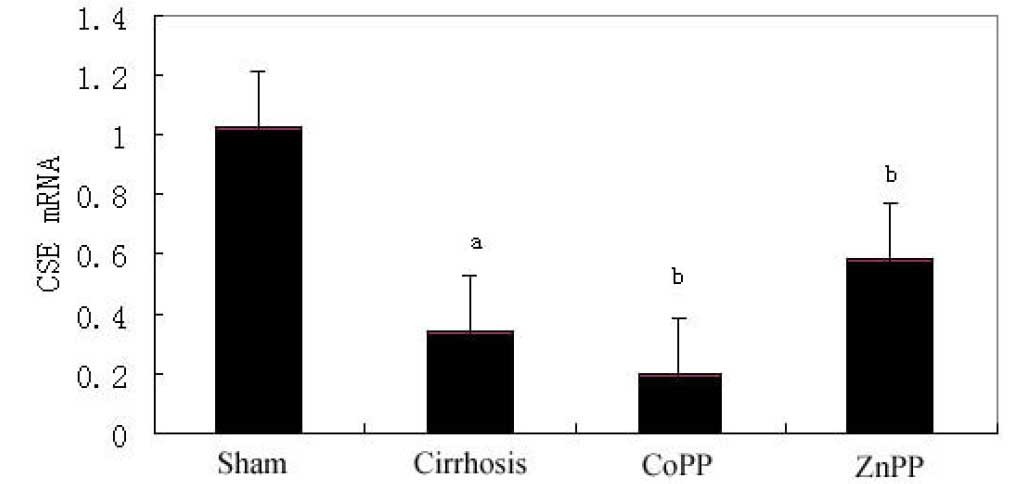
Hepatic CSE mRNA expression
levels
As determined by qPCR, the hepatic expression levels
of CSE mRNA in the cirrhosis group were significantly lower than
those in the sham group (P<0.01). Furthermore, compared with the
cirrhosis group, hepatic CSE mRNA expression levels were
significantly decreased in the CoPP group, but significantly
increased in the ZnPP group (P<0.05) (Fig. 5).
Discussion
Hyperdynamic circulation is a key characteristic of
cirrhosis-induced portal hypertension, which is secondary to the
presence of systemic vasodilation. Numerous hypotheses have been
postulated regarding the development of systemic vasodilation,
including increased synthesis of NO and CO and the activation of
KATP channels in vascular smooth cells in the systemic
and splanchnic arterial circulation (4,20,21).
H2S has been presented as a third endogenous signaling
gasotransmitter, with similar properties to those of NO and CO.
H2S is a crucial vasodilator in the hepatic
microcirculation and causes relaxation of vascular smooth muscle
(22). H2S is produced
endogenously from cysteine by pyridoxal-5′-phosphate-dependent
enzymes, including cystathionine-β-synthase and CSE (6,23), and
induces vasorelaxation via the activation of ATP-sensitive
K+ channels in vascular smooth muscle (24). This mechanism of vasorelaxation
induction differs from that of NO and CO (7,8). CSE is
the primary enzyme in mammalian hepatic tissue for catalyzing the
endogenous production of H2S (13,19), and
>50% of the volume of gas in the liver appears to be derived
from CSE (13,19). To the best of our knowledge, the
present study is the first to investigate whether the
H2S/CSE pathway is involved in the formation of liver
cirrhosis.
The results of the present study demonstrated that
serum levels of ALT, AST and TBIL were significantly higher in the
cirrhosis group than those in the sham group, indicating that BDL
caused marked liver injury. The presence of liver cirrhosis was
confirmed by H&E staining in the livers of 4-week BDL rats.
Additionally, the PVP was significantly higher in rats that
underwent BDL compared with that in time-matched sham rats,
indicating the presence of portal hypertension. These results
suggested that the BDL model had been successfully established. The
levels of H2S were significantly lower in the cirrhosis
group than those in the sham group. Furthermore, CSE protein and
mRNA levels were significantly lower in the cirrhosis group than
those in the sham group. A recent study by Wang et al
(25) generated a similar result; it
was observed that rats with portal hypertension had lower
endogenous H2S concentrations in comparison with healthy
control rats and that the concentration was inversely associated
with disease severity.
Previous studies have shown that H2S is
endogenously generated by vascular smooth muscle cells, while CO is
primarily formed in vascular smooth muscle cells and partially in
vascular endothelial cells. These observations suggest that
H2S and CO may interact in the regulation of biological
functions (26–28); however, the interaction between the
CO/HO and H2S/CSE pathways remains unclear. A secondary
aim of the present study was to investigate the effect of
endogenous CO on the H2S/CSE pathway in the livers of
cirrhotic rats by manipulating HO-1 enzyme activity via an
intraperitoneal injection of either ZnPP, a specific HO-1 enzyme
inhibitor, or CoPP, a specific HO-1 enzyme inducer. The levels of
COHb in the rat arterial blood were significantly higher in the
cirrhosis group compared with those in the sham group, suggesting
that overproduction of CO occurs in the rats with cirrhosis, as CO
is predominately found bound to hemoglobin in the form of COHb in
the circulation (29). Compared with
the cirrhosis group, COHb levels were significantly lower in the
ZnPP group and significantly higher in the CoPP group. HO-1 is the
primary source of circulating CO (30), and the results of the present study
showed that the levels of COHb were in accordance with HO-1
expression. Furthermore, the present results demonstrated that the
serum levels of H2S were significantly higher in the
ZnPP group and significantly lower in the CoPP group, compared with
the levels observed in the rats in the cirrhosis group. In
addition, in comparison with the cirrhosis group, CSE protein and
mRNA expression levels were significantly higher in the ZnPP group
and significantly lower in the CoPP group. Collectively, these
results suggest that endogenous CO is able to downregulate hepatic
CSE expression in the livers of rats with cirrhosis.
The serum levels of AST, ALT and TBIL in the CoPP
group were significantly higher than those in the cirrhosis group.
Furthermore, compared with the cirrhosis group, more fibrous
hyperplasia and fibrotic extensions with fibroblast proliferation
were observed in the CoPP group. These differences indicated that
the liver damage was more severe in the CoPP group than that in the
cirrhosis group. In contrast to the levels of liver damage, the
production of H2S and hepatic CSE expression were
reduced in the CoPP group compared with those in the cirrhosis
group. The ZnPP group, however, exhibited decreased liver damage
and increased H2S production and hepatic CSE expression
compared with the cirrhosis group. These results suggest that
H2S may serve a crucial function in protecting liver
cells against the progression of liver fibrosis. Poliakova et
al (31) observed that
H2S was able to induce biochemical restructuring of the
rat liver, with long-term exposure (>2 weeks) to a low dose and
short-term exposure to a high dose of an H2S-containing
gaseous mixture both leading to reversible changes in the liver. A
potential mechanism by which H2S inhibits liver fibrosis
involves the induction of apoptosis (32) and inhibition of HSC activation
(22,33). In the present study, the increased
severity of liver damage observed in the CoPP group may have been
partially due to the lower production of H2S, as a
result of inhibition by endogenous CO.
Although previous reports have indicated that HO-1
performs a protective function in various liver diseases (34) and that the upregulation of HO-1
prevents the progression of liver fibrosis in Mdr2-knockout mice
(35), our previous study suggested
that the overexpression of HO-1 is harmful to liver function and
aggravates liver fibrosis in rats subjected to BDL (36). A potential reason underlying these
conflicting results may be that HO-1 plays various roles in the
progression of liver fibrosis (37),
and the protection of HO-1 is restricted to a narrow threshold of
expression (38). In the early
stages of liver fibrosis, low HO-1 induction may be protective in
liver cells (39); however, in the
end stage of cirrhosis with portal hypertension, excessive HO-1
expression may deteriorate liver function and aggravate liver
cirrhosis (36).
In conclusion, the present study indicates that
vascular function cannot be independently regulated by a single
molecule or its pathway (40). In
addition to NO and CO, the H2S/CSE pathway is involved
in the formation of liver cirrhosis, and H2S may be
involved in protecting liver cells against the progression of liver
fibrosis. Endogenous CO downregulated the mRNA and protein
expression levels of hepatic CSE, in addition to the production of
H2S in rats with liver cirrhosis. As CO and
H2S are produced in vascular smooth muscle cells, and
have comparable characteristics and biological function to
vasodilation, the dynamic interplay between CO and H2S
may have a significant role in the maintenance of homeostasis;
however, the specific underlying mechanisms and interaction of
these molecules with NO require further study.
Acknowledgements
This study was supported by grants from the National
Natural and Science Foundation of China (no. 30970886) and the
Initial Doctoral Foundation of Liaoning Province (no.
20121110).
References
|
1
|
Montaño-Loza A and Meza-Junco J:
Pathogenesis of portal hypertension. Rev Invest Clin. 57:596–607.
2005.(In Spanish). PubMed/NCBI
|
|
2
|
Guo SB, Li Q, Duan ZJ, Wang QM, Zhou Q and
Sun XY: Octreotide attenuates liver fibrosis by inhibiting hepatic
heme oxygenase-1 expression. Mol Med Rep. 11:83–90. 2015.PubMed/NCBI
|
|
3
|
Li Volti G, Sacerdoti D, Di Giacomo C,
Barcellona ML, Scacco A, Murabito P, Biondi A, Basile F, Gazzolo D,
Abella R, et al: Natural heme oxygenase-1 inducers in hepatobiliary
function. World J Gastroenterol. 14:6122–6132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tarquini R, Masini E, La Villa G, Barletta
G, Novelli M, Mastroianni R, Romanelli RG, Vizzutti F, Santosuosso
U and Laffi G: Increased plasma carbon monoxide in patients with
viral cirrhosis and hyperdynamic circulation. Am J Gastroenterol.
104:891–897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiorucci S, Antonelli E, Mencarelli A,
Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V and Morelli A: The
third gas: H2S regulates perfusion pressure in both the
isolated and perfused normal rat liver and in cirrhosis.
Hepatology. 42:539–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stipanuk MH and Beck PW: Characterization
of the enzymic capacity for cysteine desulphhydration in liver and
kidney of the rat. Biochem J. 206:267–277. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J
and Tang C: H2S generated by heart in rat and its
effects on cardiac function. Biochem Biophys Res Commun.
313:362–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartsfield CL: Cross talk between carbon
monoxide and nitric oxide. Antioxid Redox Signal. 4:301–307. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Altaany Z, Yang G and Wang R: Crosstalk
between hydrogen sulfide and nitric oxide in endothelial cells. J
Cell Mol Med. 17:879–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li RN, Zeng XJ, Chen YH, Lu LQ and Hao G:
Interaction between hydrogen sulfide and nitric oxide on cardiac
protection in rats with metabolic syndrome. Zhong Guo Yi Xue Ke Xue
Yuan Xue Bao. 33:25–32. 2011.(In Chinese).
|
|
12
|
Jin HF, Du JB, Li XH, Wang YF, Liang YF
and Tang CS: Interaction between hydrogen sulfide/cystathionine
gamma-lyase and carbon monoxide/heme oxygenase pathways in aortic
smooth muscle cells. Acta Pharmacol Sin. 27:1561–1566. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kabil O, Vitvitsky V, Xie P and Banerjee
R: The quantitative significance of the transsulfuration enzymes
for H2S production in murine tissues. Antioxid Redox
Signal. 15:363–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pereira RM, dos Santos RA, Oliveira EA,
Leite VH, Dias FL, Rezende AS, Costa LP, Barcelos LS, Teixeira MM
and Simoese Silva AC: Development of hepatorenal syndrome in bile
duct ligated rats. World J Gastroenterol. 14:4505–4511. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amersi F, Buelow R, Kato H, Ke B, Coito
AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, et al:
Upregulation of heme oxygenase-1 protects genetically fat Zucker
rat livers from ischemia/reperfusion injury. J Clin Invest.
104:1631–1639. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown KE, Poulos JE, Li L, Soweid AM, Ramm
GA, O'Neill R, Britton RS and Bacon BR: Effect of vitamin E
supplementation on hepatic fibrogenesis in chronic dietary iron
overload. Am J Physiol. 272:G116–G123. 1997.PubMed/NCBI
|
|
17
|
Makino N, Suematsu M, Sugiura Y, Morikawa
H, Shiomi S, Goda N, Sano T, Nimura Y, Sugimachi K and Ishimura Y:
Altered expression of heme oxygenase-1 in the livers of patients
with portal hypertensive diseases. Hepatology. 33:32–42. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei CL, Lee KH, Khoo HE and Hon WM:
Expression of haem oxygenase in cirrhotic rat liver. J Pathol.
199:324–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii K, Sakuragawa T, Kashiba M, Sugiura
Y, Kondo M, Maruyama K, Goda N, Nimura Y and Suematsu M: Hydrogen
sulfide as an endogenous modulator of biliary bicarbonate excretion
in the rat liver. Antioxid Redox Signal. 7:788–794. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung TM, Fung ML, Liong EC, Lau TY, Nanji
AA and Tipoe GL: Role of nitric oxide in the regulation of
fibrogenic factors in experimental liver fibrosis in mice. Histol
Histopathol. 26:201–211. 2011.PubMed/NCBI
|
|
21
|
Matei V, Rodríguez-Vilarrupla A, Deulofeu
R, García-Calderó H, Fernández M, Bosch J and Garcia-Pagán JC:
Three-day tetrahydrobiopterin therapy increases in vivo hepatic NOS
activity and reduces portal pressure in CCl4 cirrhotic rats. J
Hepatol. 49:192–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Li Y, Yang W and Cao G:
H2S inhibits the activation of hepatic stellate cells
and downregulates the expression of urotensin II. Hepatol Res.
43:670–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamoun P: Endogenous production of
hydrogen sulfide in mammals. Amino Acids. 26:243–254. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng Y, Ndisang JF, Tang G, Cao K and
Wang R: Hydrogen sulfide-induced relaxation of resistance
mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol.
287:H2316–H2323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Han J, Xiao L, Jin CE, Li DJ and
Yang Z: Role of hydrogen sulfide in portal hypertension and
esophagogastric junction vascular disease. World J Gastroenterol.
20:1079–1087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebrahimkhani MR, Mani AR and Moore K:
Hydrogen sulphide and the hyperdynamic circulation in cirrhosis: A
hypothesis. Gut. 54:1668–1671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang CY, Li XH, Zhang T, Fu J and Cui XD:
Hydrogen sulfide upregulates heme oxygenase-1 expression in rats
with volume overload-induced heart failure. Biomed Rep. 1:454–458.
2013.PubMed/NCBI
|
|
28
|
Zhang QY, Du JB, Zhou WJ, Yan H, Tang CS
and Zhang CY: Impact of hydrogen sulfide on carbon monoxide/heme
oxygenase pathway in the pathogenesis of hypoxic pulmonary
hypertension. Biochem Biophys Res Commun. 317:30–37. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo SB, Duan ZJ, Li Q and Sun XY: Effect
of heme oxygenase-1 on renal function in rats with liver cirrhosis.
World J Gastroenterol. 17:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naik JS, O'Donaughy TL and Walker BR:
Endogenous carbon monoxide is an endothelial-derived vasodilator
factor in the mesenteric circulation. Am J Physiol Heart Circ
Physiol. 284:H838–H845. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Poliakova VS, Shakhlamov VA, Stadnikov AA
and Solnyshkova TG: Structural-biochemical reorganization of rat
liver caused by hydrogen sulfide-containing gas mixture.
Morfologiia. 124:84–87. 2003.(In Russian). PubMed/NCBI
|
|
32
|
Fan HN, Wang HJ, Yang-Dan CR, Wang C, Li
YF and Deng Y: Protective effects of hydrogen sulfide on oxidative
stress and fibrosis in hepatic stellate cells. Mol Med Rep.
7:247–253. 2013.PubMed/NCBI
|
|
33
|
Lu F, Xing J, Zhang X, Dong S, Zhao Y,
Wang L, Li H, Yang F, Xu C and Zhang W: Exogenous hydrogen sulfide
prevents cardiomyocyte apoptosis from cardiac hypertrophy induced
by isoproterenol. Mol Cell Biochem. 381:41–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang H, Zhao LF, Zhao ZF, Wang Y, Zhao JJ
and Zhang L: Heme oxygenase-1 prevents liver fibrosis in rats by
regulating the expression of PPARγ and NF-κB. World J
Gastroenterol. 18:1680–1688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barikbin R, Neureiter D, Wirth J, Erhardt
A, Schwinge D, Kluwe J, Schramm C, Tiegs G and Sass G: Induction of
heme oxygenase 1 prevents progression of liver fibrosis in Mdr2
knockout mice. Hepatology. 55:553–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang QM, Du JL, Duan ZJ, Guo SB, Sun XY
and Liu Z: Inhibiting heme oxygenase-1 attenuates rat liver
fibrosis by removing iron accumulation. World J Gastroenterol.
19:2921–2934. 2013.PubMed/NCBI
|
|
37
|
Wang QM, Duan ZJ, Du JL, Guo SB, Sun XY
and Liu Z: Heme oxygenase/carbon monoxide pathway inhibition plays
a role in ameliorating fibrosis following splenectomy. Int J Mol
Med. 31:1186–1194. 2013.PubMed/NCBI
|
|
38
|
Geuken E, Buis CI, Visser DS, Blokzijl H,
Moshage H, Nemes B, Leuvenink HG, de Jong KP, Peeters PM, Slooff MJ
and Porte RJ: Expression of heme oxygenase-1 in human livers before
transplantation correlates with graft injury and function after
transplantation. Am J Transplant. 5:1875–1885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan ZJ, Yang D, Wang F, Sun YJ, Sun XY
and Zheng ML: Heme oxygenase-1 regulates the major route involved
in formation of immune hepatic fibrosis in rats. Chin Med J (Engl).
123:3304–3308. 2010.PubMed/NCBI
|
|
40
|
Weston AD and Hood L: Systems biology,
proteomics and the future of health care: Toward predictive,
preventative, and personalized medicine. J Proteome Res. 3:179–196.
2004. View Article : Google Scholar : PubMed/NCBI
|