Introduction
The metabolic function of brown adipose tissue (BAT)
contributes to the maintenance of body temperature during cold
exposure, and to the elevated core temperature during various
behavioral states, including the acute phase response and stress
(1). BAT contains numerous cell
types in addition to adipocytes, including pericytes, monocytes and
macrophages; therefore, it has a critical role in the immune
response (2). Numerous studies have
investigated the thermal regulation and immunological functions of
BAT (3,4).
The transient receptor potential vanilloid 1 (TRPV1)
channel, which is a member of a large family of transient receptor
potential ion channels (5), is a
ligand-gated, non-selective cation channel that is permeable to
Ca2+. Numerous studies have investigated the function of
TRPV1 and have proposed various sensory mechanisms. Tékus et
al (6) demonstrated that
blocking TRPV1 with various antagonists resulted in acute
hyperthermia in rodents; thus suggesting that TRPV1 may be involved
in regulating body temperature in vivo (9). However, this effect was not observed
for TRPV1-knockout mice (7,8). TRPV1 is activated by noxious heat,
protons and various endogenous factors in vitro (10), and capsaicin and capsazepine have
previously been demonstrated to be specific ligands of TRPV1
(11). Capsaicin activates TRPV1,
whereas capsazepine inhibits TRPV1 (11,12).
Capsaicin is the predominant constituent of hot
chilli peppers, and is responsible for their spicy and strong
flavor. In a previous study, treatment of neonatal rats with
capsaicin was associated with neurotoxic effects, including the
destruction of a subset of small-diameter primary afferents
(13); thus suggesting that
capsaicin may be a useful tool for investigating TRPV1-mediated
sensory fiber functions, including taste, pain and thermosensation
(14,15). Hypersensitivity associated with
immunoglobulin (Ig)E mediates pathological pruritus; however, the
exact etiology remains unknown. The pathogenesis of
hypersensitivity involves a complex immunologic cascade, including
disruption of the epidermal barrier. The major elements in immune
dysregulation are Langerhans' cells, inflammatory dendritic
epidermal cells and mast cells, all of which interact through an
intricate cascade of cytokines leading to a predominance of
Th2 cells. The Th2 cytokines: Interleukin
(IL)-4, IL-5, IL-10 and IL-13, are therefore increased in the skin
(16). Leptin is an
adipocyte-derived hormone. Recently, leptin has been shown to
modulate innate immune responses such as cytokine synthesis, in
vitro, and has been shown to have a role in the innate host
response against bacteria in vivo (17)
In our previous study, we investigated the effects
of capsaicin on neonatal Sprague-Dawley rat pups, and consistently
demonstrated long-lasting hyperthermia and severe cutaneous lesions
on their heads, necks and backs, associated with vigorous
scratching behavior. The present study evaluated the effects of
capsaicin-induced hyperthermia on the immune function of rat
neonates, including their ability to resist bacterial
infections.
Materials and methods
Rats
The rat facilities were approved by the Association
of Assessment and Accreditation of Laboratory Animal Care, and
animal experiments were performed according to the institutional
guidelines outlined by the Institutional Animal Care and Use
Committee at Gachon University (LCDI-2014-0082; Incheon, Republic
of Korea). Pregnant Sprague-Dawley rats (Samtako, Seong-nam,
Republic of Korea) were obtained 1 week prior to parturition,
housed individually in plastic cages with soft bedding, and allowed
to deliver. Pups from each litter were randomly assigned to an
experimental group, weaned 21 days postnatally, separated on the
basis of gender, and housed in groups of 3–5 pups until the end of
the experiment. Only the male pups were used in the present study,
including 10 in the capsaicin-treated (cap-treated) group and 5 in
the vehicle-treated group. All female rats were sacrificed by
CO2 inhalation. All of the rats were maintained in a 12
h light/dark cycle (light on, 8:00 AM) at 22–25°C, with free access
to food and water.
TRPV1 antagonist
Capsazepine (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in phosphate-buffered saline (PBS), and 50 mg/kg
capsazepine was injected intraperitoneally into 6-week-old rats.
Untreated 6-week-old naïve rats were used as untreated
controls.
Neonatal capsaicin treatment to induce
hyperthermia
Capsaicin (Sigma-Aldrich) was suspended in PBS
containing 10% Tween 80 (Sigma-Aldrich) and 10% ethanol, using the
method outlined in Kim et al (18). Subsequently, capsaicin (50 mg/kg,
cap-treated) or an equal volume of saline containing 10% Tween 80
and 10% ethanol (vehicle-treated), were systemically administered
to SD rat pups within 48 h of birth.
Measurement of body temperature
The body temperatures of rat pups were measured
using small implantable transponders (PDT-4000; Mini-Mitter, Co.,
Inc., Bend, OR, USA) that were implanted into the abdominal cavity
of the rats, following anesthetization using isoflurane (0.5–2%;
Hana Pharm. Co., Ltd, Seoul, South Korea). Temperature data were
constantly received using an ER4000 receiver (56×29×7 cm; RS 232
serial; Mini-Mitter, Co., Inc.), and automatically recorded onto a
main computer using PDT-4000 software (Mini-Mitter, Co., Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Rat L5 dorsal root ganglion (DRG) and skin samples
were obtained following sacrifice of the rat pups with
CO2 inhalation. Total RNA from each tissue was extracted
using an RNeasy® Micro kit (Qiagen, Venlo, Limburg, Netherlands),
according to the manufacturer's instructions. Subsequently, total
RNA was reverse transcribed to cDNA using a reverse transcription
system (Promega Corporation, Madison, WI, USA). qPCR was performed
for the rat L5 DRG total RNA sample, using a total reaction volume
of 20 µl containing 10 µl SYBR® Green PCR Master mix (Applied
Biosystems, Grand Island, NY, USA), a primer pair (1 µl each of 10
pmol/µl primers), and 8 µl diluted cDNA (500 ng/µl). qPCR was
performed for the skin total RNA sample using PCR pre-mixture
(Bioneer, Seong-Nam, Korea), a primer pair (1 µl each of 10 pmol/µl
primers) and 8 µl diluted cDNA (500 ng/µl). The PCR cycling
conditions were as follows: 95°C for 10 sec, 55°C for 20 sec and
72°C for 30 sec, for 40 cycles and the initial denaturation and
final extension conditions were 95°C (5 mins) and 72°C (10 mins).
Relative expression levels were determined in comparison with the
GAPDH gene, or using the 2−ΔΔCt method (19). The primer pairs for rat TRPV1, IL-4,
IL-13 and GAPDH are listed in Table
I.
 | Table I.Primer pairs for polymerase chain
reaction. |
Table I.
Primer pairs for polymerase chain
reaction.
| Gene | Forward | Reverse |
|---|
| TRPV1 |
GGCTCCGGTACTTCTCTTTC |
AATAGGGGAGTGGTCAAAGG |
| IL-4 |
CATGGCCAGTGTGCAGAGAG |
GAGGCCACCAAACAGACAGG |
| IL-13 |
AACCCGTGGACCAAGGAAGT |
GTGAGCTGTGGGAAGGTTGG |
| GAPDH |
AACCCGTGGACCAAGGAAGT |
GTGAGCTGTGGGAAGGTTGG |
ELISA
Blood samples were collected from rats following gas
anesthetization using isoflurane (0.5%-2%). The samples were
centrifuged at 7,500 × g for 30 min and the supernatants,
corresponding to the blood serum, were collected. Total protein
concentrations for each serum sample were determined using a
bicinchoninic acid (BCA) assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Protein expression levels of leptin, IL-4,
IL-13, and IgE, were measured using a Rat ELISA Quantitation kit
(Bethyl Laboratories, Inc., Montgomery, TX, USA), according to the
manufacturer's instructions.
Western blot
Skin samples were collected from cap- and
vehicle-treated rats, following anesthesia with intraperitoneally
injected pentobarbital (50 mg/kg; Sigma-Aldrich). The skin samples
(2 µg) were homogenized in T-per tissue lysis buffer (20 µl; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing protease
inhibitors (Thermo Fisher Scientific, Inc.), the homogenates were
centrifuged at 10,000 × g for 5 min and the protein supernatant was
collected. Total protein concentrations for each sample were
determined using a BCA assay (Pierce Biotechnology, Inc.). Protein
extracts (30 mg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis at 100 V and 25 mA for 2
h (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The separated
proteins were transferred onto a protran-nitrocellulose membrane
(GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), blocked for 1 h
in blocking buffer (5% non-fat powdered milk in Tris-buffered
saline containing Tween 20), and then incubated for 24 h in 1:500
diluted rabbit anti-rat β-defensin 3 (RBD3) polyclonal antibody
(1:500; cat. no. NB200-117; Novus Biologicals, LLC, Littleton, CO,
USA) and rabbit anti-rat GAPDH polyclonal antibodies (1:5,000; cat.
no. NB100-56875; Novus Biologicals, LLC). The membranes were
incubated in 1:1,000 diluted horseradish peroxidase-conjugated goat
anti-rabbit IgG (bs-0295G-HRP) Bioss Antibodies, Woburn, MA, USA)
for 1 h at room temperature. Antibody complexes were detected using
a chemiluminescent peroxidase substrate (Sigma-Aldrich), and
developed using X-ray film and developer (Agfa, Mortsel, Belgium).
Densitometry measurements were made using Image J software
(National Institutes of Health, Bethesda, MD, USA).
Bacterial colonization
Skin samples from lesional or non-lesional epidermis
were obtained from cap- or vehicle-treated rats via punching
biopsies, following anesthetization using intraperitoneally
administered pentobarbital (50 mg/kg). Bacterial colonies from the
skin samples were grown on blood agar plates (Thermo Fisher
Scientific, Inc.), after which the colonies were suspended in 100
µl distilled water, inoculated onto Müller-Hinton agar plates and
incubated at 37°C for 48 h at 5% CO2. The number of
colonies were counted as colony-forming units/cm2.
Bacterial identification was cross-checked using a conventional
method (coagulase and mannitol fermentation tests) (20) and an automated identification system,
VITEK® 2 (bioMérieux, Durham, NC, USA). Methicillin resistance was
monitored using the Clinical and Laboratory Standards Institute
antimicrobial susceptibility method (21). Briefly, cefoxitin disks (30 µg) were
placed on Müller-Hinton agar, and an inhibition zone diameter of
≤21 mm was considered to indicate methicillin resistance.
Scratching behavior
Rats were placed into separate plastic chambers
(room temperature; 200×300×200 mm; Daihan Bio, Seongnam, South
Korea), equipped with a mirror behind the chamber, which allowed an
unobstructed view. Following habituation, scratching behavior was
recorded using an unmanned digital video camera (DCR-SR300; Sony,
Tokyo, Japan). A bout of consecutive scratching strokes using the
hind paw was regarded as one scratch.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical significance was analyzed using the
Student's t-test or the Mann-Whitney rank sum test, depending on
normality. P<0.05 was considered to indicate statistically
significant differences. All statistical analyses were conducted
using SigmaStat software (version 3.5; Systat Software Inc., San
Jose, IL, USA).
Results
Neonatal capsaicin treatment induces
TRPV1 knockdown-associated chronic hyperthermia in rats
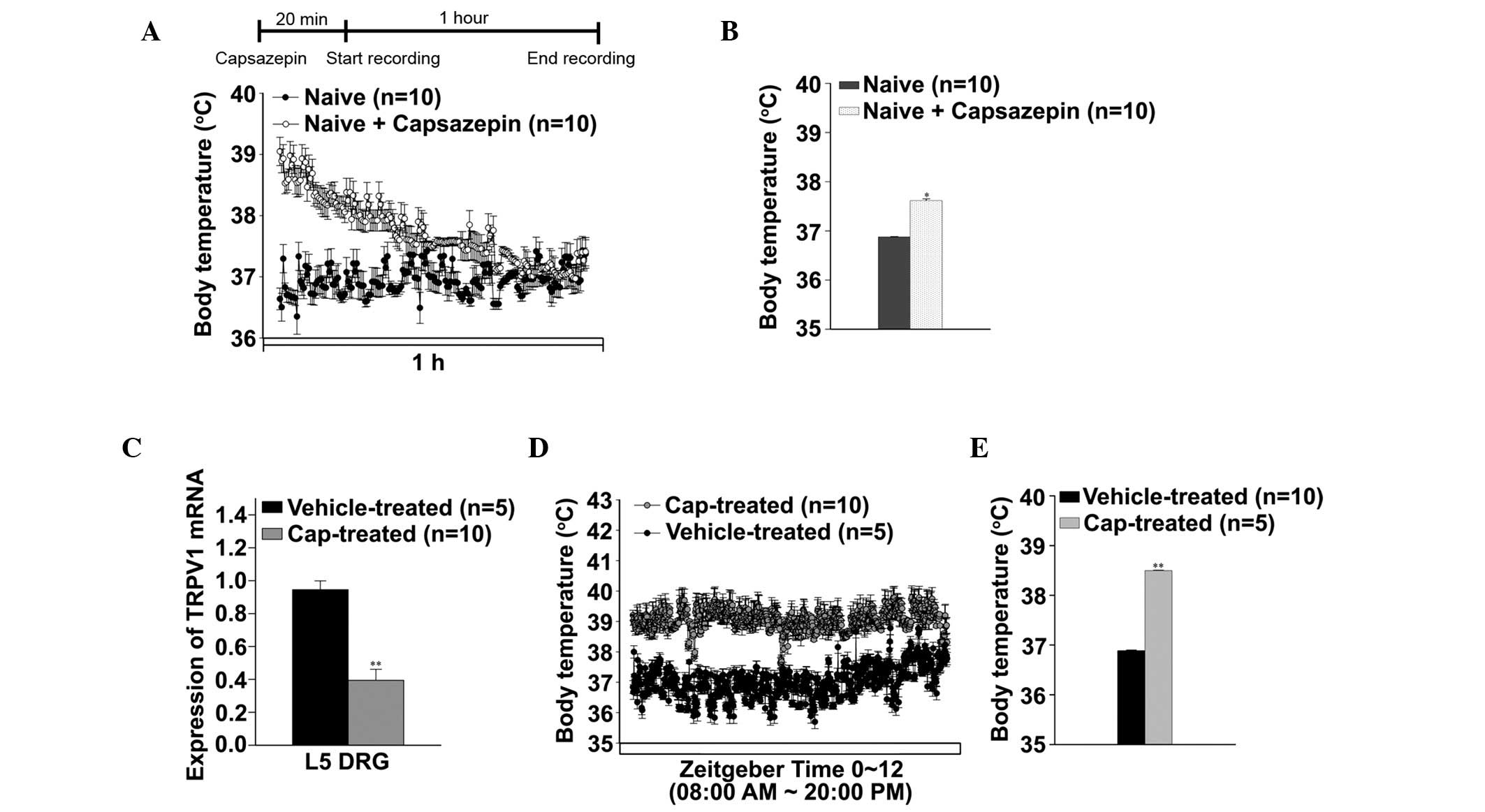
Rat pups were treated with capsaicin (age, 48 h) and
capsazepine (age, 6 weeks), and alterations in body temperature
were evaluated (Fig. 1). The
capsazepine-treated rats demonstrated hyperthermic symptoms for 1
h, and the core body temperature was markedly increased in these
rats, as compared with the naïve rats (37.61±0.03 and 36.8±0.01°C,
respectively; Fig. 1A and B). The
expression levels of TRPV1 mRNA significantly decreased by ~40% in
the rat L5 DRG following neonatal capsaicin treatment, compared
with the vehicle-treated rats (P<0.001; Fig. 1C). Neonatal capsaicin treatment was
associated with chronic hyperthermia; the body temperature
significantly increased to 38.47±0.04°C, compared with the
vehicle-treated rats (body temperature, 36.86±0.01°C; P<0.001;
Fig. 1D and E).
Chronic hyperthermia disrupts the
immune defense against bacterial infection
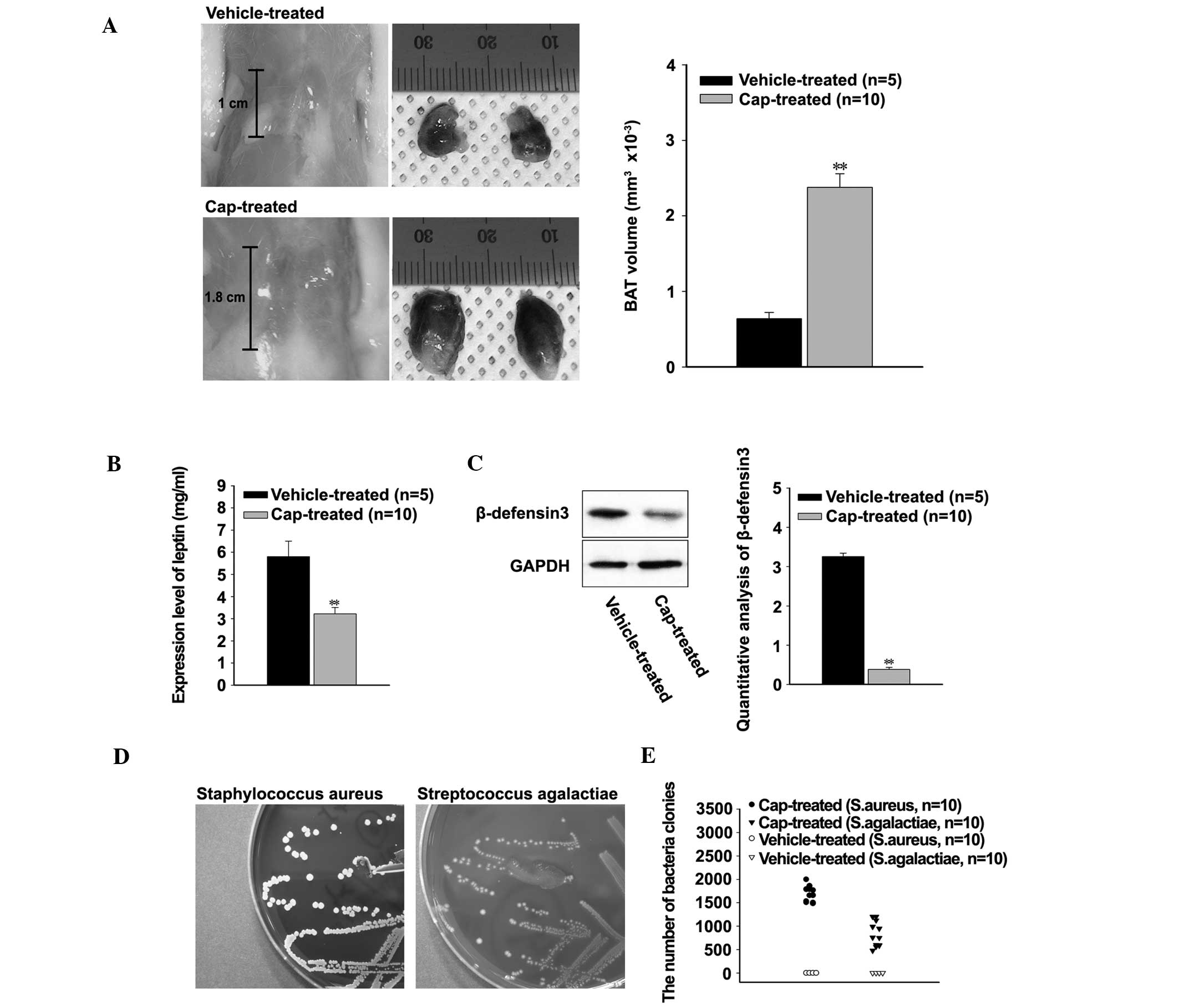
In order to investigate the effects of hyperthermia
on the immune systems of the rats, the sizes of interscapular BAT
were compared. The mean length of BAT was 1 cm in the
vehicle-treated rats and 1.8 cm in the cap-treated rats (Fig. 2A, left). Furthermore, the mean volume
of BAT significantly increased in the cap-treated rats, compared
with the vehicle-treated rats (P<0.001; Fig. 2A, right). Conversely, the expression
levels of leptin were significantly decreased in the cap-treated
rats, compared with the vehicle-treated rats (P<0.001; Fig. 2B). The expression levels of RBD3 were
investigated in order to understand the effects of decreased levels
of leptin on the host defense system. According to the western
blot, expression levels of RBD3 were significantly decreased in the
cap-treated rats (P<0.001; Fig.
2C). Bacterial infection was confirmed by growth on blood agar
plates, and the number of colonies were ascertained using
conventional and automated colony counting assays (Fig. 2D and E). Up to 2,000 colonies of
Staphylococcus aureus and 1,200 colonies of Streptococcus
agalactiae were identified in the cap-treated rats. Conversely,
no bacterial infection could be identified in the vehicle-treated
rats (Fig. 2E).
Bacterial infection induces
dysregulation of pruritus-associated cytokines
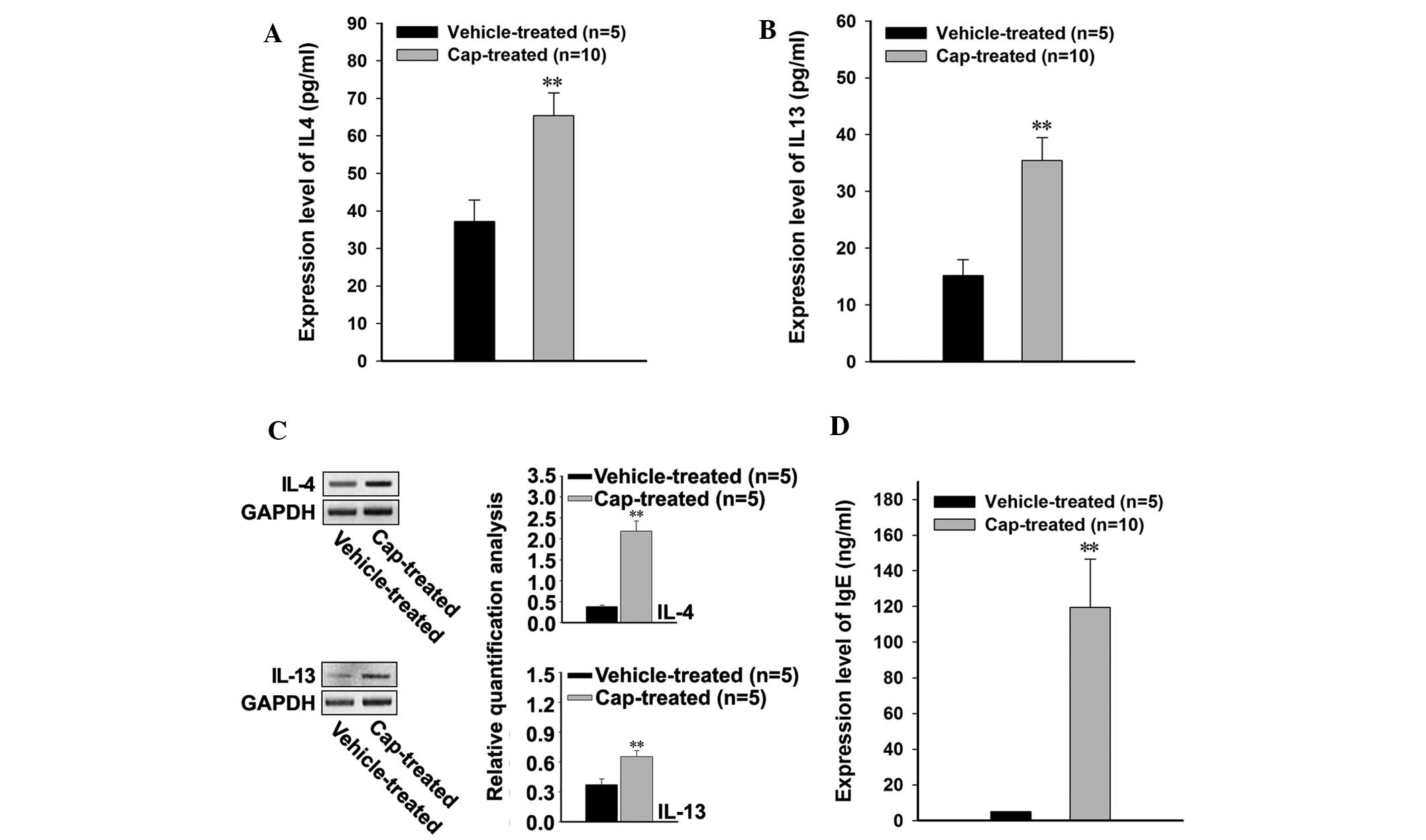
In order to investigate the effects of bacterial
infection on the levels of Th2-associated cytokines, the
blood serum protein expression levels of IL-4 and IL-13 were
measured, and were demonstrated to have significantly increased in
the cap-treated rats following bacterial infection, as compared
with the vehicle-treated rats (P<0.001; Fig. 3A and B). The endogenous expression
levels of IL-4 and IL-13 mRNA were investigated in lesional and
non-lesional skin samples from the rats, and both cytokines were
significantly increased in the cap-treated rats, compared with the
vehicle-treated rats (P<0.001; Fig.
3C). In addition, upregulation of the Th2-associated
cytokines was associated with significantly increased expression
levels of IgE in the cap-treated rats (P<0.001; Fig. 3D).
Increased expression levels of
pruritic-associated cytokines evoke scratching behavior and
dermatitis
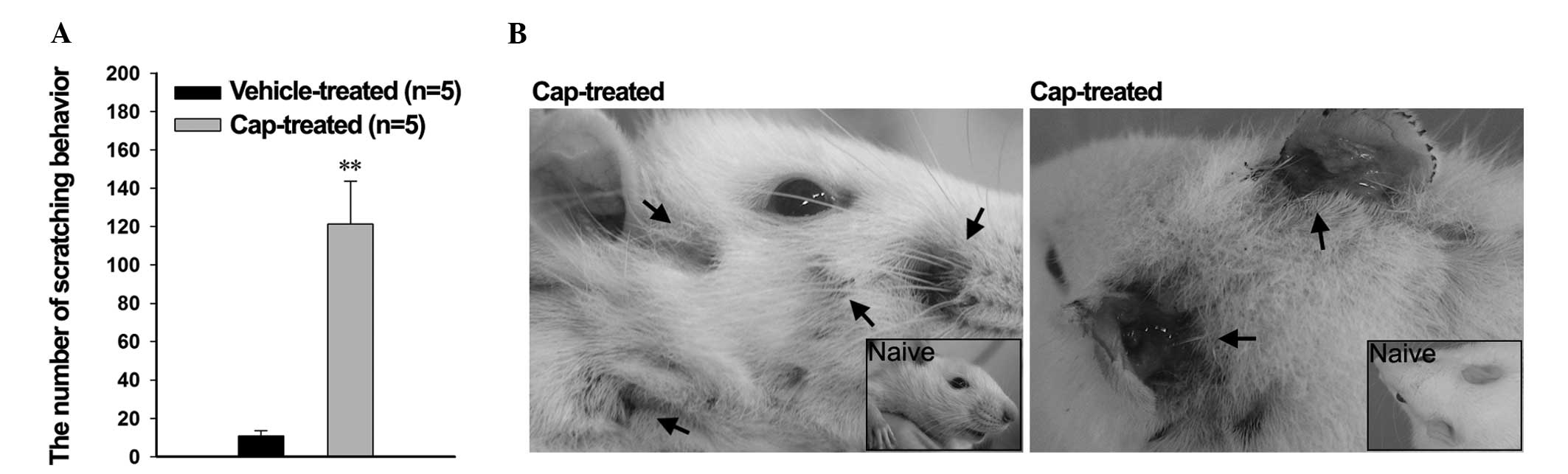
In order to investigate the effects of the
pruritic-associated cytokines, the scratching behavior of the rats
was observed using a digital video camera. Pruritus-induced
scratching behavior was significantly increased in the cap-treated
rats after 1 h, compared with the vehicle-treated rats
(121.29±22.48 and 10.81±2.76 times, respectively; P<0.001;
Fig. 4A). Concordantly, nail marks
and signs of inflammatory bleeding were detected on the face,
behind the ears, and on the nape of the neck of the cap-treated
rats, whereas the vehicle-treated rats exhibited a normal
appearance (Fig. 4B); these regions
are easily accessible to the rat hind paws.
Discussion
In our previous study, treatment of rats with
capsaicin was associated with long-lasting hyperthermia and severe
cutaneous lesions; therefore, the present study aimed to
investigate the effects of capsaicin-induced hyperthermia on the
susceptibility of neonatal rats to bacterial infections, in
particular pruritic dermatitis.
In the present study, treatment with capsazepine
markedly increased the core body temperature of naïve rats, in line
with a previous study in which blocking TRPV1 was associated with
an increased body temperature (9);
thus suggesting that TRPV1 may have a role in thermoregulation. The
present study demonstrated that the use of capsazepine in the
generation of a rat hyperthermia model is limited, as it is only
able to increase the core body temperature of rats for a short
period of time, due to its limited duration of activity. By
contrast, treatment of neonatal rats with capsaicin initiated
long-lasting hyperthermia, and reduced the expression of TRPV, in a
manner that mimicked the effects of TRPV1 antagonists (22).
BAT is present and active in human newborns, in
which it is responsible for maintaining body temperature, and is
essential for classical non-shivering thermogenesis (1); therefore, BAT activity may be affected
by an abnormal increase in core body temperature. It is generally
accepted that BAT is rapidly lost postnatally, and that this
process is normally concluded within the first few years of life
(23); however, in the present
study, the size of BAT increased in cap-treated adult rats. This
abnormal increase in the size of BAT may have been indicative of
problems with thermogenic regulation.
BAT thermogenesis is important for the maintenance
of normothermia when small animals are exposed to a cold
environment (24). Therefore, we
hypothesized that the BAT of cap-treated rats may be affected by a
thermogenic regulation disorder, such as chronic hyperthermia. As
the regulation of in vivo metabolism is an additional
function of the BAT, the body weight of the rats was expected to
alter in response to hyperthermia (25); however, there was no significant
difference in body weight between the vehicle-treated and
cap-treated rats (data not shown).
Leptin is synthesized exclusively by adipocytes and
acts to regulate the balance of energy. Previous studies
investigating mRNA expression levels of leptin demonstrated that
leptin is expressed in the skeletal muscle, particularly in BAT
(26). The most important biological
activities attributed to leptin include effects on feeding,
metabolism and the neuroendocrine axis (27). Numerous studies have detected
elevated serum expression levels of leptin in humans and mice
during the early phase of sepsis, following systemic endotoxin
administration, and during the acute phase response (28). Furthermore, a deficiency in leptin
has been associated with an increased frequency of infection
(29); therefore, in the present
study, decreased leptin expression levels may have increased the
susceptibility of the rats to bacterial infections.
Leptin contributes to cutaneous antimicrobial
defense systems by upregulating the expression of defensins,
although the underlying mechanism of this is yet to be elucidated
(30,31). Defensins are a family of
antimicrobial peptides secreted by epidermal keratinocytes, in
particular in response to cutaneous infections or in inflammatory
diseases (32). Defensins have been
demonstrated to contribute to the innate host defense via direct
bacteriocidal activity (31). In
particular, BD3 exhibits antibacterial activity towards
gram-positive bacteria under physiological salt concentrations, and
has significant involvement in adaptive immunity, compared with
other defensins (33); thus
suggesting that the leptin-associated decreased expression levels
of BD3 in the cap-treated rats may have initiated immune
dysfunction of the skin barrier, leading to a decline in the host
defense and enhanced susceptibility to bacterial infections,
including S. aureus and S. agalactiae.
It has been reported that the acute skin lesions of
pruritus patients contain increased numbers of cells expressing
IL-4 and IL-13 mRNA. IL-4 and IL-13 are pleiotropic cytokines that
have a central role in IgE-dependent inflammatory reactions
(34). IL-4 has an important role in
stimulating B cells to produce IgE antibodies, and in the
differentiation of Th cells into the Th2 phenotype.
IL-13 similarly induces B cells to produce IgE, and IL-4 and IL-13
operate through the IL-4R and IL-13R receptors, respectively
(34). In the present study,
hyperthermia-induced bacterial infections in the cap-treated rats
were associated with elevated expression levels of the
Th2 cytokines, IL-4 and IL-13, which may have resulted
in the occurrence of pruritic dermatitis (35).
In conclusion, the results of the present study
suggest that treatment of neonatal rats with capsaicin induces
chronic hyperthermia, which may have increased the susceptibility
of the rats to bacterial infections. Bacterial infections in turn
were associated with upregulated expression of the
Th2-associated cytokines, which may have resulted in
pruritus-induced scratching behavior and dermatitis in the
cap-treated rats. Therefore, a capsaicin-induced chronic
hyperthermia rat model may be useful for investigating the
association between hyperthermia and infectious disease (36,37).
Acknowledgements
The present study was supported by the Gachon
Institute of Pharmaceutical Sciences Research Fund 2014, Gachon,
Gachon University, Seongnam, South Korea.
Glossary
Abbreviations
Abbreviations:
|
BAT
|
brown adipose tissue
|
|
cap-treated
|
capsaicin-treated
|
|
IL
|
interleukin
|
|
Ig
|
immunoglobulin
|
|
TRPV1
|
transient receptor potential vanilloid
1
|
|
RBD3
|
rat β-defensin 3
|
References
|
1
|
Morrison SF, Madden CJ and Tupone D:
Central neural regulation of brown adipose tissue thermogenesis and
energy expenditure. Cell Metab. 19:741–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desruisseaux MS, Trujillo Nagajyothi ME,
Tanowitz HB and Scherer PE: Adipocyte, adipose tissue, and
infectious disease. Infect Immun. 75:1066–1078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lateef DM, Abreu-Vieira G, Xiao C and
Reitman ML: Regulation of body temperature and brown adipose tissue
thermogenesis by bombesin receptor subtype-3. Am J Physiol
Endocrinol Metab. 306:E681–E687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen JJ, Nagajyothi F, Machado FS, Weiss
LM, Scherer PE, Tanowitz HB and Garg NJ: Markers of oxidative
stress in adipose tissue during Trypanosoma cruzi infection.
Parasitol Res. 113:3159–3165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szallasi A, Cortright DN, Blum CA and Eid
SR: The vanilloid receptor TRPV1: 10 years from channel cloning to
antagonist proof-of-concept. Nat Rev Drug Discov. 6:357–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tékus V, Bölcskei K, Kis-Varga A, Dézsi L,
Szentirmay E, Visegrády A, Horváth C, Szolcsányi J and Petho G:
Effect of transient receptor potential vanilloid 1 (TRPV1) receptor
antagonist compounds SB705498, BCTC and AMG9810 in rat models of
thermal hyperalgesia measured with an increasing-temperature water
bath. Eur J Pharmacol. 641:135–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gavva NR, Bannon AW, Hovland DN Jr, Lehto
SG, Klionsky L, Surapaneni S, Immke DC, Henley C, Arik L, Bak A, et
al: Repeated administration of vanilloid receptor TRPV1 antagonists
attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp
Ther. 323:128–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szelényi Z, Hummel Z, Szolcsányi J and
Davis JB: Daily body temperature rhythm and heat tolerance in TRPV1
knockout and capsaicin pretreated mice. Eur J Neurosci.
19:1421–1424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gavva NR, Bannon AW, Surapaneni S, Hovland
DN Jr, Lehto SG, Gore A, Juan T, Deng H, Han B and Klionsky L: The
vanilloid receptor TRPV1 is tonically activated in vivo and
involved in body temperature regulation. J Neurosci. 27:3366–3374.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lambert DG: Capsaicin receptor
antagonists: A promising new addition to the pain clinic. Br J
Anaesth. 102:153–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valenzano KJ and Sun Q: Current
perspectives on the therapeutic utility of VR1 antagonists. Curr
Med Chem. 11:3185–3202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiura A: Neuroanatomical effects of
capsaicin on the primary afferent neurons. Arch Histol Cytol.
63:199–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benham CD, Davis JB and Randall AD:
Vanilloid and TRP channels: A family of lipid-gated cation
channels. Neuropharmacology. 42:873–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tominaga M: Function of TRP channel as a
thermal receptor. Nihon Seirigaku Zasshi. 65:130–137. 2003.(In
Japanese). PubMed/NCBI
|
|
16
|
Leung DY and Bieber T: Atopic dermatitis.
Lancet. 361:151–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mancuso P, Gottschalk A, Phare SM,
Peters-Golden M, Lukacs NW and Huffnagle GB: Leptin-deficient mice
exhibit impaired host defense in Gram-negative pneumonia. J
Immunol. 168:4018–4024. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YI, Na HS, Han JS and Hong SK:
Critical role of the capsaicin-sensitive nerve fibers in the
development of the causalgic symptoms produced by transecting some
but not all of the nerves innervating the rat tail. J Neurosci.
15:4133–4139. 1995.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raymond EA and Traub WH: Identification of
staphylococci isolated from clinical material. Appl Microbiol.
19:919–922. 1970.PubMed/NCBI
|
|
21
|
Cuenca-Estrella M, Gomez-Lopez A,
Alastruey-Izquierdo A, Bernal-Martinez L, Cuseta I and Buitrago MJ:
Comparison of the Vitek 2 antifungal susceptibility system with the
clinical and laboratory standards institute (CLSI) and European
Committee on Antimicrobial Susceptibility Testing (EUCAST) Broth
Microdilution Reference Methods and with the Sensititre YeastOne
and Etest techniques for in vitro detection of antifungal
resistance in yeast isolates. J Clin Microbiol. 48:1782–1786. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brandt MR, Beyer CE and Stahl SM: TRPV1
Antagonists and chronic pain: Beyond thermal perception.
Pharmaceuticals (Basel). 5:114–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nedergaard J, Bengtsson T and Cannon B:
Unexpected evidence for active brown adipose tissue in adult
humans. Am J Physiol Endocrinol Metab. 293:E444–E452. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
King VL, Dwoskin LP and Cassis LA: Cold
exposure regulates the norepinephrine uptake transporter in rat
brown adipose tissue. Am J Physiol. 276:R143–R151. 1999.PubMed/NCBI
|
|
25
|
Williamson JR, Prusiner S, Olson MS and
Fukami M: Control of metabolism in brown adipose tissue. Lipids.
5:1–14. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dessolin S, Schalling M, Champigny O,
Lönnqvist F, Ailhaud G, Dani C and Ricquier D: Leptin gene is
expressed in rat brown adipose tissue at birth. FASEB J.
11:382–387. 1997.PubMed/NCBI
|
|
27
|
Bennett BD, Solar GP, Yuan JQ, Mathias J,
Thomas GR and Matthews W: A role for leptin and its cognate
receptor in hematopoiesis. Curr Biol. 6:1170–1180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sarraf P, Frederich RC, Turner EM, Ma G,
Jaskowiak NT, Rivet DJ III, Flier JS, Lowell BB, Fraker DL and
Alexander HR: Multiple cytokines and acute inflammation raise mouse
leptin levels: Potential role in inflammatory anorexia. J Exp Med.
185:171–175. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lord GM, Matarese G, Howard JK, Baker RJ,
Bloom SR and Lechler RI: Leptin modulates the T-cell immune
response and reverses starvation-induced immunosuppression. Nature.
394:897–901. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wieland CW, Stegenga ME, Florquin S,
Fantuzzi G and van der Poll T: Leptin and host defense against
Gram-positive and Gram-negative pneumonia in mice. Shock.
25:414–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanda N and Watanabe S: Leptin enhances
human beta-defensin-2 production in human keratinocytes.
Endocrinology. 149:5189–5198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Jongh GJ, Zeeuwen PL, Kucharekova M,
Pfundt R, van der Valk PG, Blokx W, Dogan A, Hiemstra PS, van de
Kerkhof PC and Schalkwijk J: High expression levels of keratinocyte
antimicrobial proteins in psoriasis compared with atopic
dermatitis. J Invest Dermatol. 125:1163–1173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dhople V, Krukemeyer A and Ramamoorthy A:
The human beta-defensin-3, an antibacterial peptide with multiple
biological functions. Biochim Biophys Acta. 1758:1499–1512. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Namkung JH, Lee JE, Kim E, Kim HJ, Seo EY,
Jang HY, Shin ES, Cho EY and Yang JM: Association of polymorphisms
in genes encoding IL-4, IL-13 and their receptors with atopic
dermatitis in a Korean population. Exp Dermatol. 20:915–919. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Varin A, Mukhopadhyay S, Herbein G and
Gordon S: Alternative activation of macrophages by IL-4 impairs
phagocytosis of pathogens but potentiates microbial-induced
signalling and cytokine secretion. Blood. 115:353–362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janković BD, Popesković L, Janezić A and
Lukić ML: Brown adipose tissue: Effect on immune reactions in the
rat. Naturwissenschaften. 61:361974. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Himms-Hagen J: Brown adipose tissue
thermogenesis: Interdisciplinary studies. FASEB J. 4:2890–2898.
1990.PubMed/NCBI
|