Introduction
Ovarian cancer (OC) is considered to be the most
lethal type of adenocarcinoma among gynecological cancers (1). Epithelial malignancy is the leading
cause of OC (2), and the 5-year
survival rate of OC patients with epithelial malignancy is
reportedly <30% (3). Although the
pathogenesis of OC is extensively studied, its precise causes
remain unknown. It has been reported that infertile women that
ovulate incessantly are at a high risk of developing OC (4). Furthermore, a number of genetic causes
have been proposed, including the specific genes BRCA1 and BRCA2,
in addition to genes for hereditary nonpolyposis colorectal cancer
(5). Symptoms of OC may be subtle
and >70% of patients have already reached a terminal stage of
cancer at diagnosis (6). Late
diagnosis also increases the likelihood of OC recurrence following
initial treatment, and increases the risk of eventual mortality
(3,7).
Numerous approaches have been suggested for the
diagnosis and management of OC. Physical examination combined with
a blood test and transvaginal ultrasound examination have been used
to detect the cancer at an early stage (8). Common methods of OC management include
surgical tumor resection, radiation therapy and chemotherapy
(9). Despite advancements in these
detection and management techniques, the prognosis of OC has not
improved substantially and an increasing rate of OC recurrence is
observed (10). The recently
proposed hypothesis of cancer stem cells (CSCs) may provide a
promising approach for the treatment of OC (11).
CSCs are a population of stem-like cells that
possess the tumorigenic capability to self-renew and differentiate
into multiple cell types within a tumor (12). CSCs were initially reported by Bonnet
and Dick (13) in 1997 and were
proposed as an explanation for the apparent heterogeneity of cancer
cells (14). As CSCs are able to
differentiate into a variety of cancer cells (15), the isolation of CSCs in tumors and
inhibition of their growth are significant for cancer therapy. To
date, a number of procedures for CSC isolation have been reported,
including fluorescence-activated cell sorting, which employs
fluorescent antibodies as markers on the cell surface (16), as well as functional approaches of
side population (SP) cell analysis based on flow cytometry (FCM),
which is performed according to the CSC characteristics (such as
possessing the tumorigenic capability to self-renew and
differentiate into multiple cell types within tumors) (17). In graphs produced by FCM analysis, SP
cells present as a distinct subpopulation, indicated by an area of
low fluorescence intensity (18). SP
cells are identified by their ability to transport Hoechst 33342
dye through verapamil-sensitive ATP-binding cassette (ABC)
transporters (19).
To date, a number of studies have investigated the
role of SP cells in OC (20–22). However, SP cells isolated from
SK-OV-3, a key epithelial OC cell line that was first identified by
Provencher et al in 2000 (23), have not been extensively studied. In
the current study, SP cells extracted from SK-OV-3 cell lines were
sorted and characterized in order to isolate CSCs associated with
OC. In isolated SP cells, the levels of adhesion and anti-apoptotic
activity, the expression of CSC-associated genes, drug
susceptibility and the capability of colony formation compared with
non-SP cells were investigated.
Materials and methods
Materials
Human SK-OV-3 OC cells were obtained from the Cell
Bank of the Chinese Academy of Sciences (Beijing, China). Fetal
bovine serum (FBS), McCoy's 5A medium, Hoechst 33342 (no. H21492)
and TRIzol reagent were purchased from Thermo Fisher Scientific,
Inc. (Rockville, MD, USA). In addition,
trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA, 0.25%),
verapamil hydrochloride (VRP) and propidium iodide (PI) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The FastQuant RT
kit (no. KR106) and Super Real PreMix Plus (SYBR Green) kit were
purchased from Tiangen Biotech Co., Ltd. (Beijing, China).
Cisplatin (CDDP) was purchased from Selleck Chemicals (Houston, TX,
USA). Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human
CD44 (cat. no. 555478) and FITC mouse IgG2b κ isotype (cat. no.
555742) antibodies were purchased from BD Biosciences (Sparks, MD,
USA). Cell Counting kit-8 (CCK-8) was obtained from Dojindo
Laboratories Co., Ltd. (Kumamoto, Japan), and the crystal violet
staining solution was obtained from Beyotime Institute of
Biotechnology (Shanghai, China).
Cell culture
SK-OV-3 cells were cultured in McCoy's 5A medium
containing 10% FBS, 100 units/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in 5% CO2 at 37°C, and subcultured every three days
according to a ratio of original medium to fresh medium of 1:3
(v:v).
SP cell sorting and analysis
SK-OV-3 cells were cultured to reach a number of
1×108 cells, and then a single-cell suspension of
1×106 cells/ml was prepared via digestion using 0.25%
trypsin-EDTA. The cells were then stained with Hoechst 33342 (3
µg/ml) with 200 µmol/l VRP (which is an inhibitor of certain
verapamil-sensitive ABC transporters) as the control group, or
without VRP as the test group (24),
respectively. Next, the two groups were cultured in a water bath at
37°C for 90 min in the dark and were homogeneously agitated every
20 min. Subsequently, the cell suspension was centrifuged at 400 ×
g for 10 min at room temperature, and the sedimentary cells were
washed using pre-cooled phosphate-buffered saline (PBS) and
suspended in PBS with 2% FBS at 4°C. PI, an intercalating agent and
fluorescent molecule, was added and used to differentiate necrotic,
apoptotic and live cells (25).
Following filtration using a 400 mesh strainer (0.0374 mm), the
cells were analyzed and sorted by FCM using a MoFlo system (Dako,
Glostrup, Denmark) with 350 nm (UV light) as the excitation
wavelength and 450/675 nm (Hoechst blue/red) as the detected
wavelength. The cells inside the area of low-Hoechst red and
low-Hoechst blue without VRP were identified as SP cells, and the
SP percentage was further calculated.
Immunocytochemical analysis
CD44 antigen is a type of homing cell adhesion
molecule involved in cell-cell interactions, adhesion and migration
(26). In order to assess the
expression of CD44 in SP and non-SP cells isolated from SK-OV-3
cells, the cells were digested using trypsin-EDTA and suspended in
McCoy's 5A medium to obtain single-cell suspension with the
concentration of 1×106/ml. Subsequently, 5 µl FITC mouse
anti-human CD44 or FITC mouse IgG2b κ isotype control antibodies
were added to 100 µl SP or non-SP cell suspension. Following a
15-min incubation at 37°C, the volume of the cell suspension was
adjusted to 500 µl by adding PBS. Finally, the mean fluorescence
intensity (MFI) of the SP and non-SP cells were recorded and
analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The SP and non-SP cells were selected and washed
twice with PBS. To isolate total RNA, 1 ml TRIzol was added and the
solution was mixed homogeneously for 10 min. The mixture was then
transferred into 1.5-ml Eppendorf (EP) tubes with 200 µl
chloroform. After 15-min agitation, the EP tubes were centrifuged
at 12,000 × g for 15 min at 4°C. The supernatant was transferred to
fresh EP tubes and mixed with isopycnic isopropanol for 15 sec.
Further centrifugation was conducted (4°C, 10 min, 12,000 × g) and
the supernatant was discarded. The precipitate was washed twice
with 75% ethanol and dissolved in 30 µl diethylpyrocarbonate after
drying for ~15 min at room temperature to obtain RNA stock
solution. Following isolation, the concentration of RNA was
assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) and the RNA solution was
stored at −80°C for further use. The isolated RNA was used as a
template and was reverse transcribed into cDNA using the FastQuant
RT kit, as previously described (27). Next, qPCR analysis was performed
using an Mx3000P qPCR system (Stratagene; Agilent Technologies,
Inc., Amsterdam, Netherlands) and the Super Real PreMix Plus (SYBR
Green) kit, as follows: 1 min at 95°C, 40 cycles of 10 sec at 95°C,
and 32 sec at 60°C.
The primers of the target genes ATP-binding cassette
sub-family G member 2 (ABCG2) and Nestin, and the reference gene
glyceraldehyde 3-phosphate dehydrogenase were designed using Primer
Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA) and are
shown in Table I. Data were compared
using the 2−ΔΔCq method.
 | Table I.Primer sequences for polymerase chain
reaction assay. |
Table I.
Primer sequences for polymerase chain
reaction assay.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| ABCG2 |
GCCTCACCTTATTGGCCTCA |
GGCTCTATGATCTCTGTGGCTT |
| Nestin |
GCGGGCTACTGAAAAGTTCC |
CCAGCTTGGGGTCCTGAAAG |
| GAPDH |
CGGAGTCAACGGATTTGGTCGTATTGG |
GCTCCTGGAAGATGGTGATGGGATTTCC |
Colony formation test
SP and non-SP cells were plated at 500 or 1,000
cells per well in 6-well plates, and cultured in McCoy's 5A medium
supplemented with 10% FBS at 37°C in 5% CO2 for 10 days.
Subsequently, the cells were washed with PBS, mixed with 4%
paraformaldehyde and fixed at room temperature for 15 min. Next,
the cells were washed three times with PBS and crystal violet was
added to stain the cells for 20 min. The cells were then washed
with PBS, dried at room temperature for 30 min and photographed
(37X–V; Shanghai 5th Optical Factory, Shanghai, China).
Cell viability assay
The sorted SP and non-SP cells at logarithmic growth
phase were seeded in a 96-well plate at an initial cell density of
3,000 cells per well and cultured overnight in McCoy's 5A medium at
150 µl per well containing 10% FBS. CDDP was then added at various
concentrations (0, 0.8, 1.6, 3.1, 6.3, 12.5, 25, 50, 100, 200 and
400 µM). The cells were cultured for 48 h and incubated with 10 µl
CCK-8 in each well for a further 4 h. After replacing the medium
with McCoy's 5A medium, the optical density (OD) values were
determined using a Cary 50 UV-Vis spectrophotometer (Varian Medical
Systems, Inc., Palo Alto, CA, USA) at 450 nm. The viability of the
SP and non-SP cells following CDDP treatment was assessed, and half
maximal inhibitory concentration (IC50) (28) was calculated using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA) as 4.32
µmol/l for SP cells and 4.26 µmol/l for non-SP cells. In addition,
SP or non-SP cells treated only with solvent (normal saline) and no
CDDP were defined as the corresponding control group, while plates
treated without CDDP or solvent were considered to be the blank
group. All groups were also treated with dimethyl sulfoxide (DMSO;
Sigma-Aldrich) at the same concentration. The 100% cell viability
was set based on the black group, which was treated with DMSO
alone. It was calculated according to the following equation: Cell
viability (%) = (ODt-ODb)/(ODc-ODb) × 100 (29), where ODt, ODb and ODc refer to the OD
value of the CDDP-treated, blank and control groups,
respectively.
Drug susceptibility of SP cells
To investigate the drug susceptibility of SP cells,
the influence of CDDP on the cell cycle progression of SP and
non-SP cells was investigated. The SP and non-SP cells were
digested by trypsin-EDTA and cultured with pre-cooled ethanol (70%)
at 4°C for 20 h. After washing with PBS, the SP and non-SP cells
were treated with 4.32 and 4.26 µmol/l CDDP for 48 h, respectively.
These CDDP concentrations corresponded to the IC50
values obtained from the CCK-8 assay. Next, 500 µl PI/RNase
staining buffer was added and the cells were incubated at 4°C for
30 min. The cell cycles of treated SP and non-SP cells, as well as
those of their respective control groups without CDDP, were
analyzed by FCM.
Statistical treatment and
analysis
The data are presented as the mean ± standard error
of the mean and were analyzed using SPSS software, version 12.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. One-way analysis
of variance was used for data analysis. The results of each group
were measured three times.
Results
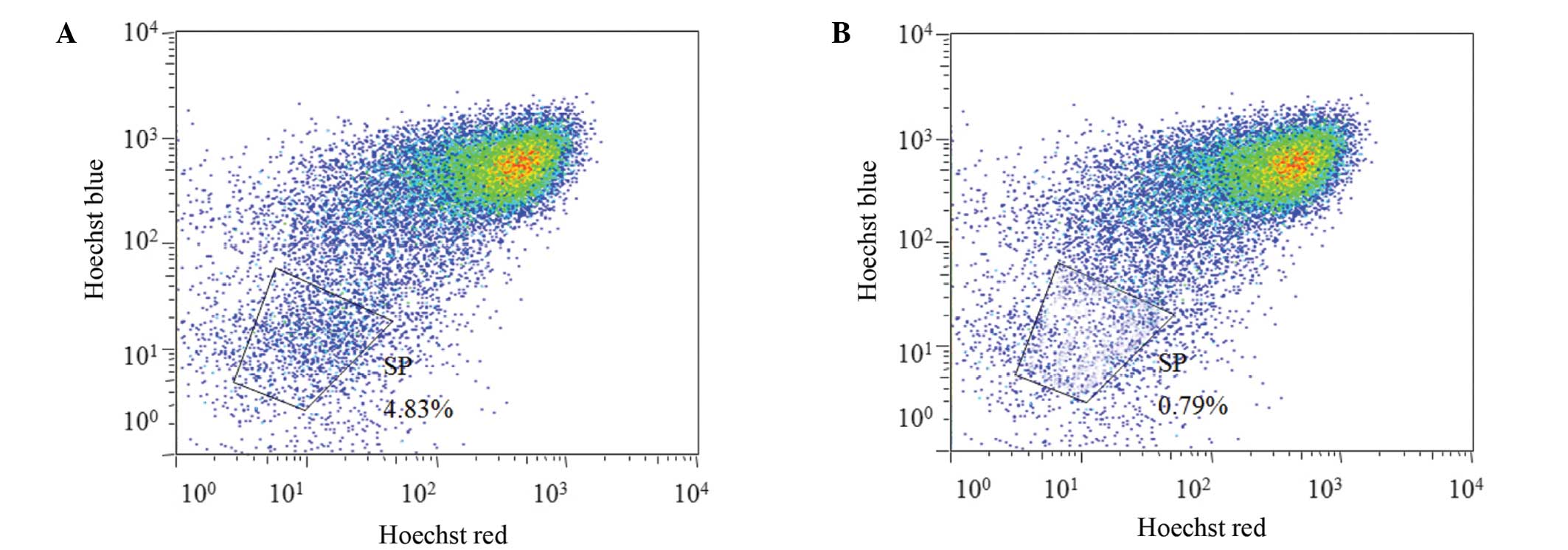
SP cell isolation
The Hoechst-low cells from the SK-OV-3 cell line
were isolated following the exclusion of apoptotic cells stained
with PI, on the basis of FCM scatter signals. A distinct
characteristic of SP cells is the ability to transport Hoechst
33342 from the cytoplasm to outside the cell; however, the Hoechst
33342 transfer is inhibited by VRP (19). Thus, the SP cells were defined as the
cell population with distinct and unstained cells in the
Hoechst-low diminished region that had been treated with VRP. The
non-SP cells were identified as the major cell population dyed by
Hoechst 33342. As shown in Fig. 1,
the fraction of potential SP cells that were eliminated following
VRP treatment among the total cell population was 4.83%.
Immunocytochemical assay
Immunofluorescence analysis uses the characteristic
fluorescence of antibody markers to detect the expression of a
target antigen in cells via the specific combination of an antigen
and its corresponding antibody (30). In the present study, the expression
of CD44 antigen, which serves a vital function in cell adhesion and
exerts an anti-apoptotic effect on SP and non-SP cells, was
investigated. FITC-conjugated mouse anti-human CD44 antibody was
used to specifically detect the expression of the CD44 antigen. The
cells were analyzed using FCM and the results are shown in Table II. Compared with the isotype, the
MFI values of the SP and non-SP cells treated with FITC mouse
anti-human CD44 were significantly increased. However, the higher
MFI values of SP cells as compared with the non-SP cells indicated
that CD44 antigen expression was more marked in the SP cells.
 | Table II.MFI of CD44 expression in SP and
non-SP cells. |
Table II.
MFI of CD44 expression in SP and
non-SP cells.
| Cell
population | Antibody
marker | MFI |
|---|
| Non-SP | FITC mouse IgG2b κ
isotype control |
4.04±2.9 |
|
| FITC mouse
anti-human CD44 | 470.3±2.6 |
| SP | FITC mouse IgG2b κ
isotype control |
4.01±3.2 |
|
| FITC mouse
anti-human CD44 | 538.9±4.0 |
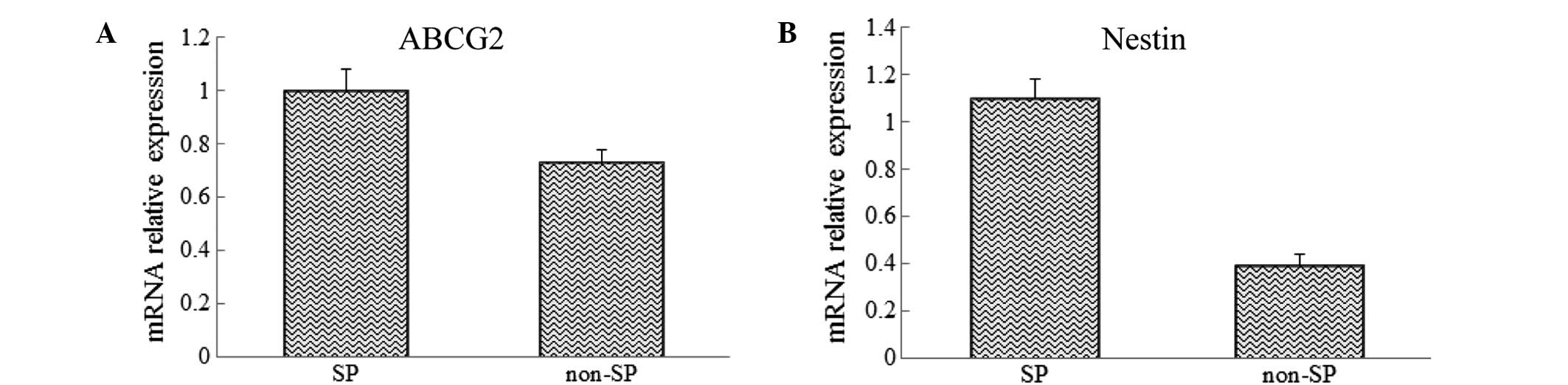
Analysis of the mRNA expression levels
of stem cell-associated genes
The mRNA of SP and non-SP cells was extracted and
analyzed using RT-qPCR to investigate the differences in the
expression levels of the CSCs genes, ABCG2 and Nestin, between the
SP and non-SP cells. The results showed that the mRNA expression
levels of ABCG2 and Nestin were significantly increased in sorted
SP cells when compared with those in the non-SP cells (Fig. 2).
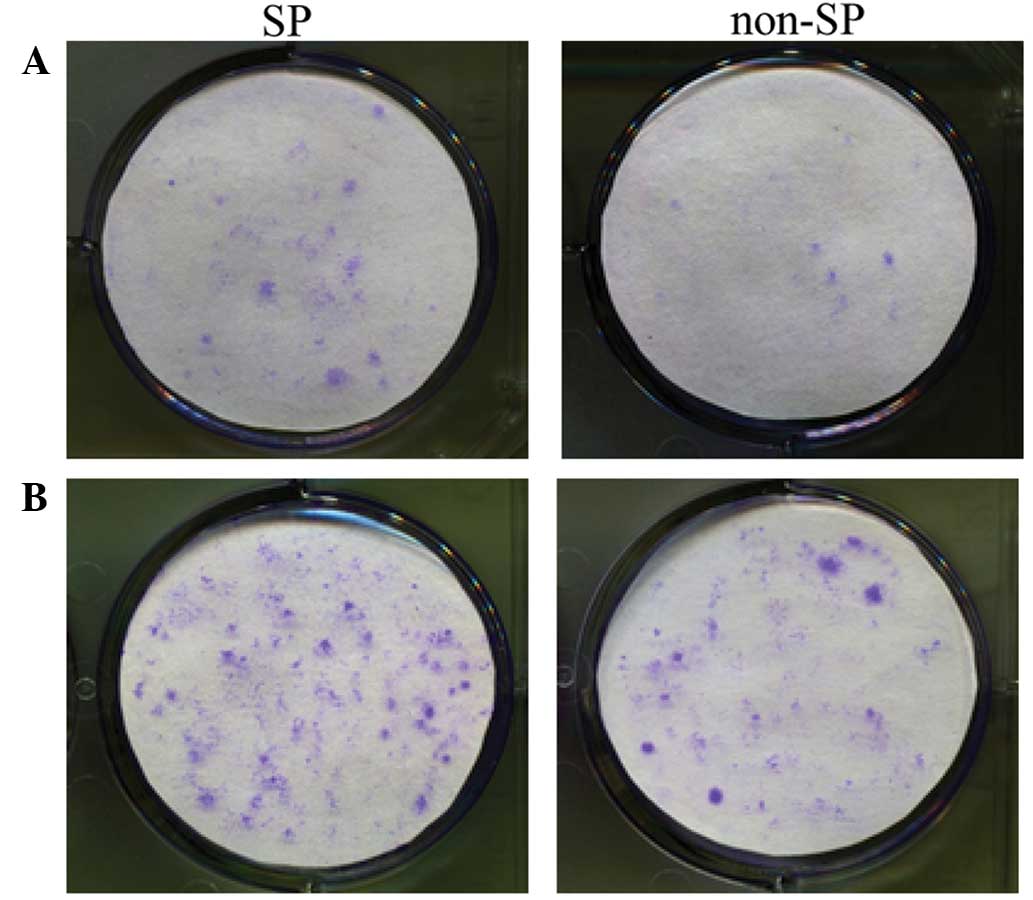
Colony formation test
The tests of colony formation were repeated thrice
in three parallel experiments. The results at day 10 of cultivation
are shown in Fig. 3. The SP cells
plated at a density of 500 or 1,000 cells per well exhibited
significantly more marked colony formation ability compared with
the non-SP cells, indicated that SP cells possessed a strong
regenerative capability.
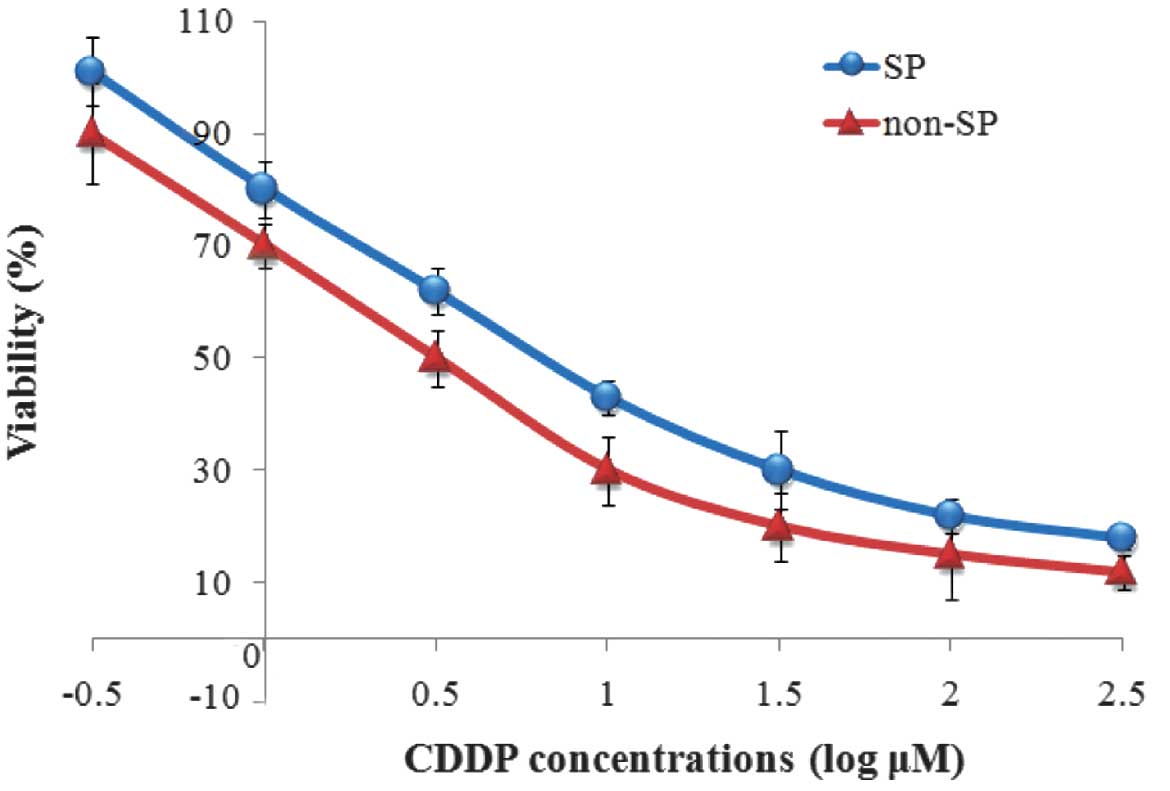
Viability of CDDP-treated SP cells and
CDDP-IC50
The viability of SP and non-SP cells treated at
different CDDP concentrations was determined by CCK-8 assay. The
results suggest that SP cells had increased viability compared with
the non-SP cells at all treated concentrations of CDDP (Fig. 4). The IC50 values were
then calculated using Graph Pad Prism 5 software based on the dose
response curve and were determined to be 4.32 and 4.26 µmol/l for
the SP and non-SP cells, respectively. These results indicate that
SP cells were more resistant to CDDP compared with the non-SP
cells.
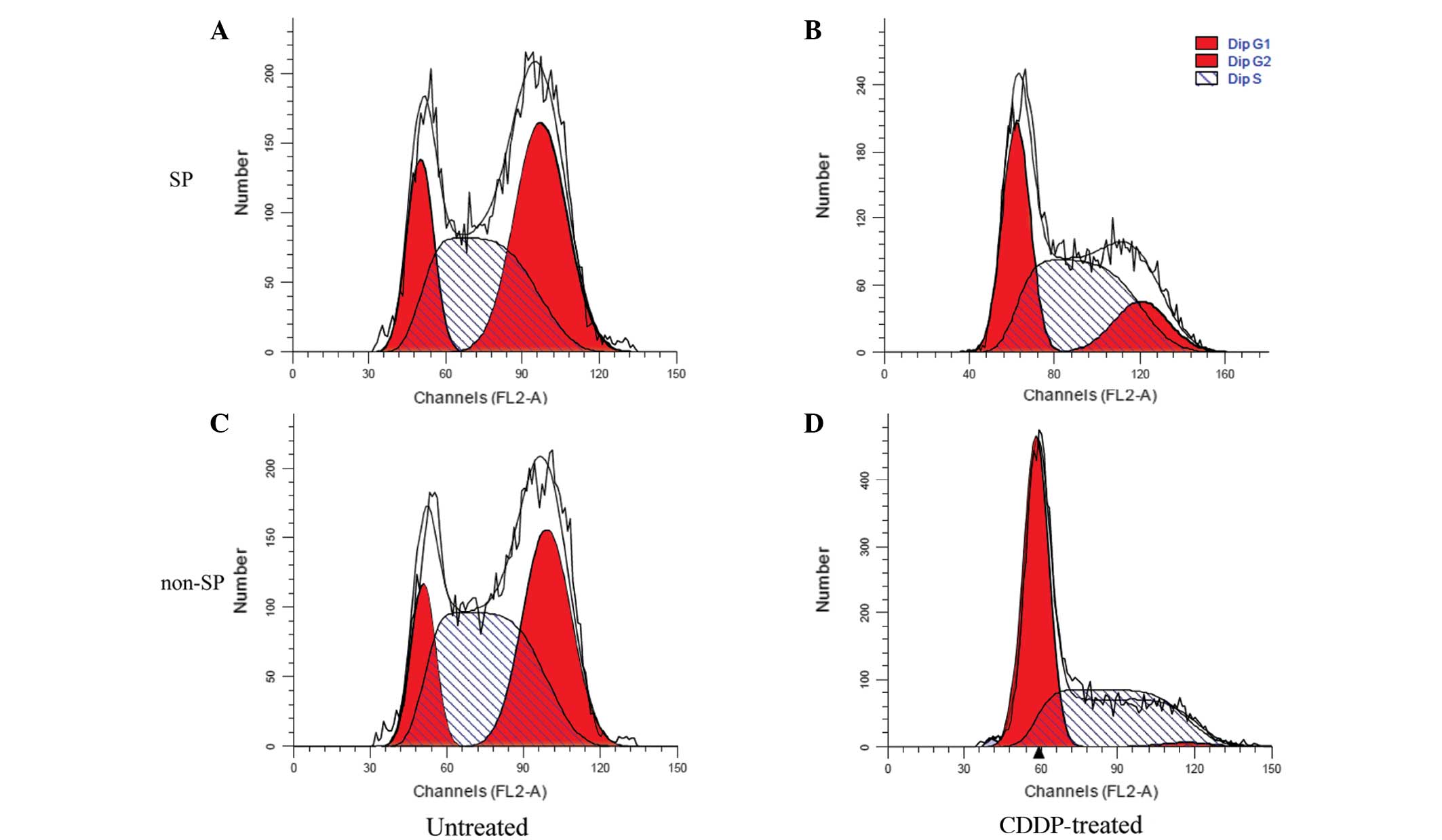
Effect of CDDP on SP and non-SP
cells
The influence of CDDP on the cell cycles of SP and
non-SP cells was investigated using FCM. The results suggested that
CDDP inhibited cell reproduction more markedly in the non-SP cells
compared with the SP cells, as the ratio of G2/M to
total cells decreased by 95.88% in the non-SP cells, but only by
63.99% in the SP cells. These results demonstrated that the sorted
SP cells had a strong proliferative capacity (Fig. 5 and Table III).
 | Table III.Effect of CDDP on the proportion of
SP and non-SP cells in each phase of the cell cycle. |
Table III.
Effect of CDDP on the proportion of
SP and non-SP cells in each phase of the cell cycle.
|
| Non-SP cells
(%) | SP cells (%) |
|---|
|
|
|
|
|---|
| Cell cycle
phase | Untreated | CDDP-treated | Untreated | CDDP-treated |
|---|
|
G0/G1 | 14.25±0.11 | 53.09±1.77 | 18.02±0.23 | 34.48±0.85 |
| S | 48.13±0.54 | 45.36±0.55 | 39.30±0.65 | 50.15±0.99 |
|
G2/M | 37.62±0.29 | 1.55±1.36 | 42.68±0.42 | 15.37±0.14 |
Discussion
OC is the leading cause of mortality among
gynecological cancer types; thus, the management and treatment of
OC patients have been widely investigated (31). Despite undergoing the typical
treatment of cytoreductive surgery followed by radiotherapy and
chemotherapy, >70% of patients with OC suffer tumor recurrence
and even succumb to the disease (32). Previous studies have suggested that
CSCs, which exhibit the characteristics of renewal, proliferation
and chemotherapy resistance, may underlie the high rate of OC
recurrence and the general failure of current therapies (33–36).
Thus, therapies targeting CSCs are required as a potential approach
for reducing the recurrence rate of OC and prolonging the lifespan
of patient. Since they were first identified by Bonnet and Dick in
1997, CSCs have been investigated and isolated using a preferred
method of identification involving the use of specific makers
(15,37–39).
However, in the majority of solid tumors, CSCs are rare and
difficult to access, and have no identifiable specific markers. In
order to isolate and enrich a population of CSCs, an SP isolation
technique based on the distinct projection pattern of SP cells that
efflux Hoechst 33342 dye via verapamil-sensitive ABC transporters
has been developed (18).
A number of previous studies have investigated the
function of CSCs in OC. Szotek et al (40) isolated SP cells in human OC cell
lines and primary ascite cancer cells. In addition, Kobayashi et
al (20) isolated SP cells from
three OC cell lines (OV2008, KF28 and TU-OM-1) and then
investigated the effect of paclitaxel (PTX) and CDDP combination
treatment on the sorted SP cells. The results demonstrated that the
SP cells proliferated despite being treated with PTX and CDDP
simultaneously, which demonstrated the chemoresistance of SP cells
(20). Furthermore, CSCs may be
enriched in SP cells, since these cells were reported to exhibit
CSC-like characteristics in OC (41). As epithelial malignancy is considered
to be the leading cause of OC, the focus of the present study was
on sorting and characterizing the SP cells in the human epithelial
OC cell line, SK-OV-3.
The SP cells were isolated using an FCM technique,
and found to account for 4.38% of the total cell population. The
CD44 antigen, a transmembrane glycoprotein, is involved in a
variety of crucial cellular processes, including cell
proliferation, differentiation, survival and migration (42). CD44 was hypothesized to be associated
with the pathology of CSC due to its ability to enhance cellular
migration and adhesion, as well as the growth and aggregation of
the tumor. According to a previous study, CD44+ cells
were demonstrated to be breast CSCs, with higher tumorigenicity and
metastatic ability (43). CD44 has
been used individually or in combination with other markers to
identify CSCs in numerous tumor types, including tumors of the
pancreas (44), colon (45), stomach (46) and ovaries (47). In the present study, the
identification of the sorted SP cells was confirmed via CD44
antigen recognition using a FITC mouse anti-human CD44 antibody.
The increased MFI values of the SP cells compared with those in the
non-SP cells indicated that the sorted SP cells migrated and
aggregated vigorously and were therefore a potential candidate
population of OC CSCs.
This result was further verified at the gene level,
by evaluating the mRNA expression levels of Nestin and ABCG2 using
RT-qPCR. ABCG2, a neural stem cell gene, encodes a
membrane-associated protein included in the ABC transporter, which
serves a crucial function in Hoechst 33342 cellular efflux
(48,49). Castillo et al (50) observed that the ABCG2 transporter is
a significant marker of chemotherapy resistance in prostate cancer.
Yanamoto et al (51)
identified a positive correlation between ABCG2 expression and
local recurrence of tongue cancer, which was reportedly associated
with CSCs. In addition, the elevation of ABCG2 expression has been
observed in a number of putative CSCs. Therefore, ABCG2 was
considered to be a significant marker of CSCs. Nestin was initially
identified as a marker of neuroepithelial stem cells, and has been
reported to be highly expressed in cells outside of the nervous
tissue, including CSCs in epithelial tumors (52), breast cancer (53) and gastric cancer (54). In the present study, the expression
levels of ABCG2 and Nestin were significantly increased in the SP
cells compared with the non-SP cells. These results suggest that
the SP cells isolated in the current study possessed properties
characteristic of CSCs.
An additional fundamental characteristic of CSCs is
their potential for proliferation and self-regeneration (55). In the present study, the capacity of
the isolated SP cells for proliferation and self-regeneration was
investigated via a colony formation test. The sorted SP and non-SP
cells were cultured under identical conditions for 10 days, and the
number of SP cells was found to be evidently increased compared
with that of the non-SP cells in plates seeded at densities of 500
or 1,000 cells. This result is consistent with the findings of a
previous study, which observed an increased colony formation
capacity in SP cells compared with non-SP cells (56).
As CSCs have been recognized to be a crucial factor
underlying the chemotherapy resistance of cancer tissues, the
chemoresistance of the sorted SP cells against CDDP was also
investigated. CDDP is a chemotherapeutic agent used in oncotherapy,
which causes DNA crosslinking and ultimately induces apoptosis
(57). The cellular viability of the
SP and non-SP cells was decreased as the concentration of CDDP was
increased. The viability of the SP cells at all treated
concentrations of CDDP, as well as the corresponding
IC50 values, were increased in the SP cells compared
with the non-SP cells, indicating that the SP cells possessed more
marked chemoresistance.
The cell cycle is defined as the entire period of
cellular development, from one cell into two daughter cells after
cell division and duplication. According to a previous study, the
cell cycle consists of five basic stages: Gap 0 (G0), a
senescent phase in which cells have stopped dividing; gap 1
(G1), the beginning of interphase in which cells
increase in size and prepare for DNA replication; synthesis (S),
the primary phase in which DNA replication occurs; gap 2
(G2), the final period of interphase in which the cell
grows continuously following DNA synthesis; and mitosis (M), the
cell division stage in which growth is complete and cells divide
(58). As the cell cycle,
particularly the M phase, is essential for the proliferation of
CSCs (59), we investigated the
effect of CDDP on the cell cycle progression of the sorted SP and
non-SP cells. Following CDDP treatment, the reduced proportion of
non-SP cells in G2/M stage compared with the SP cells
indicated that the SP cells reproduced more rapidly, and further
suggested their capacity for chemoresistance. The results indicated
that the sorted SP cells possessed a marked ability to proliferate
and resist chemotherapy, which are characteristic properties of
CSCs.
In conclusion, SP cells were isolated from the
SK-OV-3 human OC cell line using FCM, and their isolation was
verified by the immunocytochemical detection of the CD44 marker and
evaluation of the mRNA expression levels of ABCG2 and Nestin. The
sorted SP cells exhibited a high capacity for self-regeneration,
proliferation and chemotherapy resistance, which are typical
features of CSCs. Therefore, the SP cells with CSC characteristics
isolated in the present study may provide a novel insight into OC
therapy. Further studies should be performed in order to resolve
the issue of the inhibition of the growth and proliferation of SP
cells, which may be more effective in treating OC than dealing with
the non-SP cells.
References
|
1
|
Ruscito I, Dimitrova D, Vasconcelos I,
Gellhaus K, Schwachula T, Bellati F, Zeillinger R, Benedetti-Panici
P, Vergote I, Mahner S, et al: BRCA1 gene promoter methylation
status in high-grade serous ovarian cancer patients-a study of the
tumour bank ovarian cancer (TOC) and ovarian cancer diagnosis
consortium (OVCAD). Eur J Cancer. 50:2090–2098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Wang J, Zhang Y, Chen D, Yang C,
Kai C, Wang X, Shi F and Dou J: Observation of ovarian cancer stem
cell behavior and investigation of potential mechanisms of drug
resistance in three-dimensional cell culture. J Biosci Bioeng.
118:214–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purdie DM, Bain CJ, Siskind V, Webb PM and
Green AC: Ovulation and risk of epithelial ovarian cancer. Int J
Cancer. 104:228–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyd J and Rubin SC: Hereditary ovarian
cancer: Molecular genetics and clinical implications. Gynecol
Oncol. 64:196–206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goff BA, Mandel L, Muntz HG and Melancon
CH: Ovarian carcinoma diagnosis. Cancer. 89:2068–2075. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meunier L, Puiffe ML, Le Page C,
Lali-Mouhim A, Chevrette M, Tonin PN, Provencher DM and Mes-Masson
AM: Effect of ovarian cancer ascites on cell migration and gene
expression in an epithelial ovarian cancer in vitro model. Transl
Oncol. 3:230–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fishman DA, Cohen L, Blank SV, Shulman L,
Singh D, Bozorgi K, Tamura R, Timor-Tritsch I and Schwartz PE: The
role of ultrasound evaluation in the detection of early-stage
epithelial ovarian cancer. Am J Obstet Gynecol. 192:1214–1221;
discussion 1221–1222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chobanian N and Dietrich CS III: Ovarian
cancer. Surg Clin North Am. 88:285–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naora H and Montell DJ: Ovarian cancer
metastasis: Integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klonisch T, Wiechec E, Hombach-Klonisch S,
Ande SR, Wesselborg S, Schulze-Osthoff K and Los M: Cancer stem
cell markers in common cancers-therapeutic implications. Trends Mol
Med. 14:450–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dexter DL, Spremulli EN, Fligiel Z,
Barbosa JA, Vogel R, VanVoorhees A and Calabresi P: Heterogeneity
of cancer cells from a single human colon carcinoma. Am J Med.
71:949–956. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonner WA, Hulett HR, Sweet RG and
Herzenberg LA: Fluorescence activated cell sorting. Rev Sci
Instrum. 43:404–409. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi GM, Xu Y, Fan J, Zhou J, Yang XR, Qiu
SJ, Liao Y, Wu WZ, Ji Y, Ke AW, et al: Identification of side
population cells in human hepatocellular carcinoma cell lines with
stepwise metastatic potentials. J Cancer Res Clin Oncol.
134:1155–1163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ojha R, Jha V, Singh SK and Bhattacharyya
S: Autophagy inhibition suppresses the tumorigenic potential of
cancer stem cell enriched side population in bladder cancer.
Biochim Biophys Acta. 1842:2073–2086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yanamoto S, Kawasaki G, Yamada S,
Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T and Umeda
M: Isolation and characterization of cancer stem-like side
population cells in human oral cancer cells. Oral Oncol.
47:855–860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi Y, Seino K, Hosonuma S, Ohara T,
Itamochi H, Isonishi S, Kita T, Wada H, Kojo S and Kiguchi K: Side
population is increased in paclitaxel-resistant ovarian cancer cell
lines regardless of resistance to cisplatin. Gynecol Oncol.
121:390–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung WM, Chang WC, Chen L, Lin TY, Chen
LC, Hung YC and Ma WL: Ligand-independent androgen receptors
promote ovarian teratocarcinoma cell growth by stimulating
self-renewal of cancer stem/progenitor cells. Stem Cell Res.
13:24–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah MM and Landen CN: Ovarian cancer stem
cells: Are they real and why are they important? Gynecol Oncol.
132:483–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Provencher DM, Lounis H, Champoux L,
Tétrault M, Manderson EN, Wang JC, Eydoux P, Savoie R, Tonin PN and
Mes-Masson AM: Characterization of four novel epithelial ovarian
cancer cell lines. Vitro Cell Dev Biol Anim. 36:357–361. 2000.
View Article : Google Scholar
|
|
24
|
Kaur M, Ita KB, Popova IE, Parikh SJ and
Bair DA: Microneedle-assisted delivery of verapamil hydrochloride
and amlodipine besylate. Eur J Pharm Biopharm. 86:284–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lecoeur H: Nuclear apoptosis detection by
flow cytometry: Influence of endogenous endonucleases. Exp Cell
Res. 277:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prochazka L, Tesarik R and Turanek J:
Regulation of alternative splicing of CD44 in cancer. Cell Signal.
26:2234–2239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Q, Zhang J, Liu H, Wu Q, Chen J and
Chen GQ: The mechanism of anti-osteoporosis effects of
3-hydroxybutyrate and derivatives under simulated microgravity.
Biomaterials. 35:8273–8283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manoharan-Valerio M, Ortiz C, Quiñones J,
Feliciano G, Diaz A, Rivera D and Castro M: Determination of the
IC50 and LD50 of calcium sulfide (CaS)
clusters on malignant carcinoma and normal fibroblasts cell lines
(LB580). The FASEB Journal. 28:LB5802014.
|
|
29
|
Poff AM, Ari C, Arnold P, Seyfried TN and
D'Agostino DP: Ketone supplementation decreases tumor cell
viability and prolongs survival of mice with metastatic cancer. Int
J Cancer. 135:1711–1720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eissing N, Heger L, Baranska A, Cesnjevar
R, Büttner-Herold M, Söder S, Hartmann A, Heidkamp GF and Dudziak
D: Easy performance of 6-color confocal immunofluorescence with
4-laser line microscopes. Immunol Lett. 161:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barber HR: Ovarian cancer. CA Cancer J
Clin. 36:149–184. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mueller H and Hahn M: Cytoreductive
surgery plus intraperitoneal hyperthermic perfusion in the
treatment of recurrent epithelial ovarian cancer: Oral Presentation
00012. Int J Gynecol Cancer. 15:542005.
|
|
33
|
Koch U, Krause M and Baumann M: Cancer
stem cells at the crossroads of current cancer therapy
failures-radiation oncology perspective. Semin Cancer Biol.
20:116–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shigdar S, Lin J, Li Y, Yang CJ, Wei M,
Zhus Y, Liu H and Duan W: Cancer stem cell targeting: The next
generation of cancer therapy and molecular imaging. Ther Deliv.
3:227–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Espinoza LA, Albanese C and Rodriguez OC:
The potential target therapy of prostate cancer stem cells.
Prostate Cancer - From Bench to Bedside. Spiess PE: InTech.
2011.
|
|
36
|
Scatena R, Bottoni P, Pontoglio A and
Giardina B: Cancer stem cells: The development of new cancer
therapeutics. Expert Opin Biol Ther. 11:875–892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li XX, Wang J, Wang HL, Wang W, Yin XB, Li
QW, Chen YY and Yi J: Characterization of cancer stem-like cells
derived from a side population of a human gallbladder carcinoma
cell line, SGC-996. Biochem Biophys Res Commun. 419:728–734. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and mullerian
inhibiting substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu L, McArthur C and Jaffe RB: Ovarian
cancer stem-like side-population cells are tumourigenic and
chemoresistant. Br J Cancer. 102:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Spring FA, Dalchau R, Daniels GL,
Mallinson G, Judson PA, Parsons SF, Fabre JW and Anstee DJ: The Ina
and Inb blood group antigens are located on a glycoprotein of
80,000 MW (the CDw44 glycoprotein) whose expression is influenced
by the In(Lu) gene. Immunology. 64:37–43. 1988.PubMed/NCBI
|
|
43
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee CJ, Dosch J and Simeone DM: Pancreatic
cancer stem cells. J Clin Oncol. 26:2806–2812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du L, Wang H, He L, Zhang J, Ni B, Wang X,
Jin H, Cahuzac N, Mehrpour M, Lu Y and Chen Q: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weng D, Song B, Durfee J, Sugiyama V, Wu
Z, Koido S, Calderwood SK and Gong J: Induction of cytotoxic T
lymphocytes against ovarian cancer-initiating cells. Int J Cancer.
129:1990–2001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Au A, Aziz Baba A, Goh AS, Wahid Fadilah
SA, Teh A, Rosline H and Ankathil R: Association of genotypes and
haplotypes of multi-drug transporter genes ABCB1 and ABCG2 with
clinical response to imatinib mesylate in chronic myeloid leukemia
patients. Biomed Pharmacother. 68:343–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sodani K, Patel A, Anreddy N, Singh S,
Yang DH, Kathawala RJ, Kumar P, Talele TT and Chen ZS: Telatinib
reverses chemotherapeutic multidrug resistance mediated by ABCG2
efflux transporter in vitro and in vivo. Biochem Pharmacol.
89:52–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014.PubMed/NCBI
|
|
51
|
Yanamoto S, Yamada S, Takahashi H, Naruse
T, Matsushita Y, Ikeda H, Shiraishi T, Seki S, Fujita S, Ikeda T,
et al: Expression of the cancer stem cell markers CD44v6 and ABCG2
in tongue cancer: Effect of neoadjuvant chemotherapy on local
recurrence. Int J Oncol. 44:1153–1162. 2014.PubMed/NCBI
|
|
52
|
El Deeb NM and Abdelzaher E: Stem cell
markers OCT4 and nestin in laryngeal squamous cell carcinoma and
their relation to survivin expression. Pathol Res Pract.
210:751–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Krüger K, Stefansson IM, Collett K, Arnes
JB, Aas T and Akslen LA: Microvessel proliferation by co-expression
of endothelial nestin and Ki-67 is associated with a basal-like
phenotype and aggressive features in breast cancer. Breast.
22:282–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ishiwata T, Matsuda Y and Naito Z: Nestin
in gastrointestinal and other cancers: Effects on cells and tumor
angiogenesis. World J Gastroenterol. 17:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wan G, Zhou L, Xie M, Chen H and Tian J:
Characterization of side population cells from laryngeal cancer
cell lines. Head Neck. 32:1302–1309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dou J, Wen P, Hu W, Li Y, Wu Y, Liu C,
Zhao F, Hu K, Wang J, Jiang C, et al: Identifying tumor stem-like
cells in mouse melanoma cell lines by analyzing the characteristics
of side population cells. Cell Biol Int. 33:807–815. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang JL, Wang Z, Hu W, Chen SS, Lou XE
and Zhou HJ: DHA regulates angiogenesis and improves the efficiency
of CDDP for the treatment of lung carcinoma. Microvasc Res.
87:14–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fan LM and Li JM: Evaluation of methods of
detecting cell reactive oxygen species production for drug
screening and cell cycle studies. J Pharmacol Toxicol Methods.
70:40–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Matsumoto A and Nakayama KI: Role of key
regulators of the cell cycle in maintenance of hematopoietic stem
cells. Biochim Biophys Acta. 1830:2335–2344. 2013. View Article : Google Scholar : PubMed/NCBI
|