Introduction
The intestinal walls contain heterogeneous
populations of stromal cells, including one of the most important
stromal cells, the interstitial cells of Cajal (ICCs) (1). ICCs are a class of cells located
between the autonomic nerve endings and smooth muscle cells in the
gastrointestinal tract (GIT). ICCs produce a direct impact on the
contraction of smooth muscle, such as peristalsis and segmentation
in the GIT. The pacemaker activity of ICC cells may initiate
myoelectric slow waves, a basal electrical rhythm leading to
contraction of the smooth muscle (1–3). In the
last decade, ICCs were investigated from a number of aspects,
including ultrastructure, immunoreactivity, electrophysiology and
pathology (4–6). In a 2008 study (7), the intestinal wall cell population was
re-examined; consequently, a novel cell type was observed by
transmission electron microscopy (TEM) and immunohistochemistry.
This novel type of interstitial cell, previously known as
interstitial Cajal-like cells (ICLCs), are now established to be
telocytes (TCs) (8). Characteristics
of TCs include long, projected prolongations termed telopodes (Tps)
measuring tens to hundreds of µm long, as determined from TEM
images (free access data are available at www.telocytes.com). Tps are podomeres with dilated
portions termed podoms. TCs present no similarity to ICCs on the
basis of structural differences.
TCs have been described in the interstitium of
certain parenchimatous organs in mammals (9–22) and,
although ICCs have been identified in the digestive tract of
turkeys (23) and in the intestine
of chickens (24), the presence of
ICLCs or TCs in the digestive system of the chicken remains
unclear. In addition, Cantarero Carmona et al (25) described TCs at the intestinal level,
in the lamina propria of rat duodenum; however, to the best of our
knowledge, studies on TCs in the intestinal muscularis have not
previously been conducted.
Poultry has great economical importance as it is a
major source of meat to fulfill the human requirement. The present
study aimed to provide further details on the identification and
features of TCs in the muscularis of chicken ileum. Their
relationships with neighboring cells, including nerve cells and
smooth muscle cells (SMC) in the muscularis of adult chicken ileum,
were investigated.
Materials and methods
A total of 10 healthy adult Chinese Three Yellow
broilers (English translation of the local Chinese chicken breed),
between 7 and 9 weeks of age and weighing 1.8–2.0 kg/chicken, were
used in the current study. The feeding was conducted with
commercially available feeds (New Hope Group, Chengdu, China) for
adult broiler chickens. They were housed in temperature-controlled
rooms (20±1°C) with natural light (light/dark cycle, 12/12 h), and
had free access to food and tap water. The chickens were sacrificed
by cervical dislocation, under 3% ether anesthesia through
inhalation. The experiment was approved by the Science and
Technology Agency of Jiangsu Province [license number, SYXK (SU)
2011-0036]. Tissue samples were collected from the ileum and
immersed in 2.5% glutaraldehyde fixative (Sigma-Aldrich, Saint
Louis, USA) in 0.1 M phosphate-buffered saline (4°C, pH 7.4) for 1
h, then washed in the same buffer overnight. The tissues were
postfixed with a mixture of 1% osmiumtetroxide (Sigma-Aldrich) and
1.25% potassium ferrocyanide (Sigma-Aldrich) for 1.5–2.0 h, washed
in the buffer, dehydrated in increasing concentrations of
ethylalcohol (75 and 85%, for 1 h each; 95 and 100%, two cycles for
1 h each), infiltrated with a propylene oxide/Araldite mixture
(Boster, Wuhan, China), and embedded in Araldite (Boster). Semithin
sections (1 µm) of the intestine were labeled with 1% toluidine
blue (Sigma-Aldrich) for light microscope examination, and specific
areas were selected for ultrathin sectioning. Sections were cut
using a Reichert Jung Ultracut E ultramicrotome (Reichert, Vienna,
Austria). Ultrathin sections (50 nm) were collected on copper
grids, counterstained for 10 min with 1% uranyl acetate
(Sigma-Aldrich) and Reynold's lead citrate (Sigma-Aldrich), then
observed with a JEM-1200EX transmission electron microscope (Jeol,
Tokyo, Japan).
Results
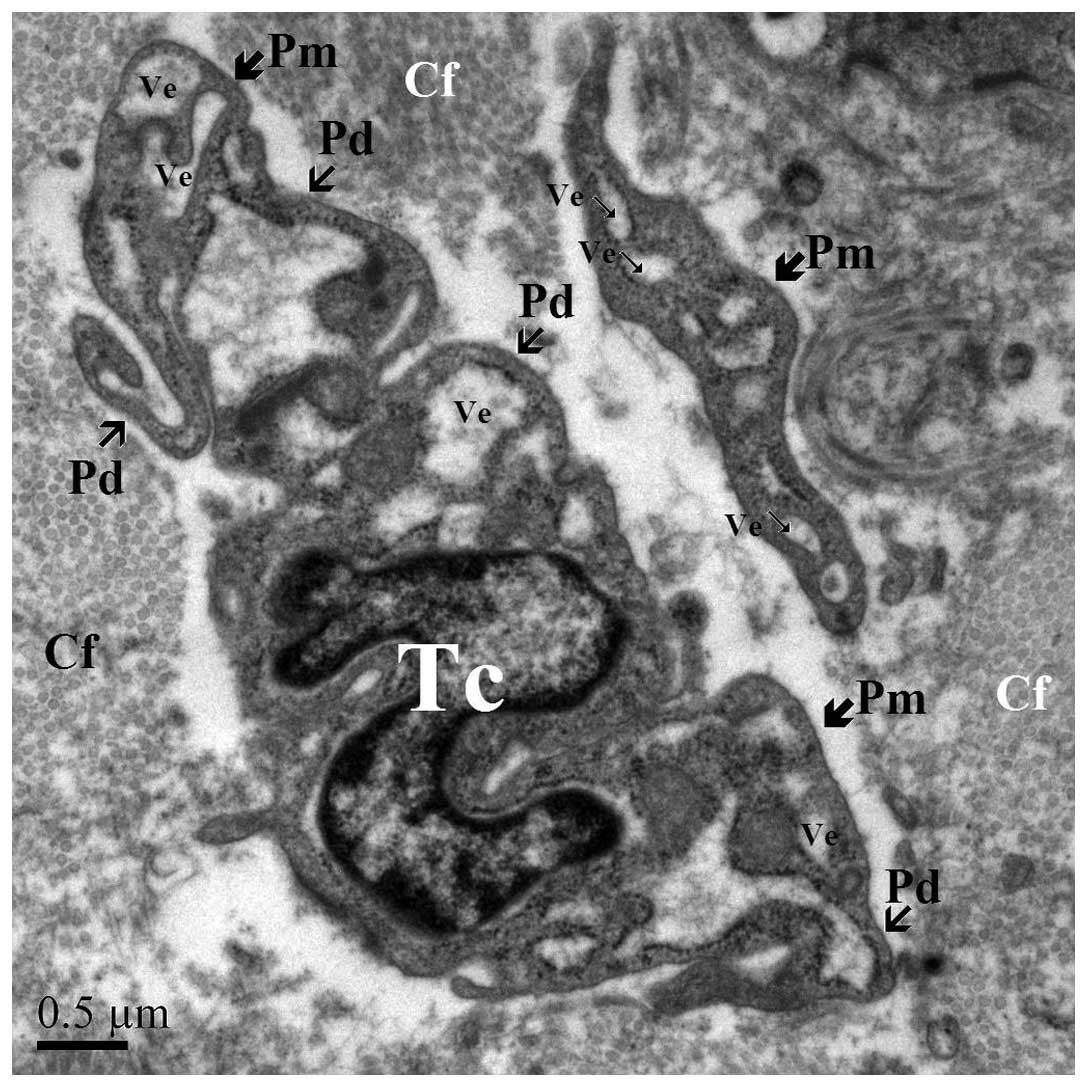
TEM examination is fundamental for identifying TCs.
TCs are cells with a small body and a variable number of Tps,
containing a nucleus, and are surrounded by a small volume of
cytoplasm (Fig. 1). Tps have a
particular ultrastructural signature, consisting of alternating
thin segments (podomers) and dilations (podoms). Each TC has 1–5
Tps, and the shape of the TC is determined by the number of their
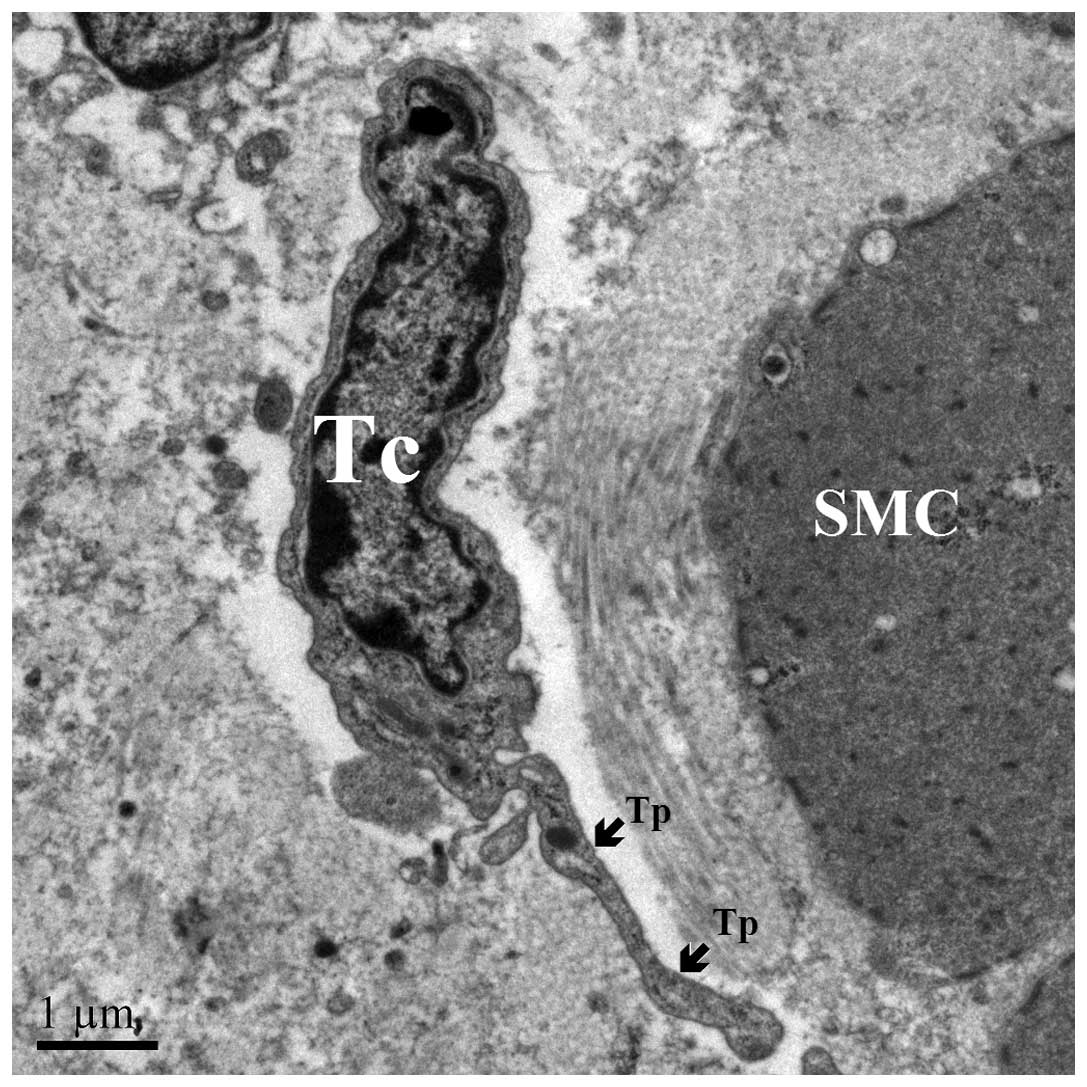
Tps: Piriform for 1 prolongation (Fig.
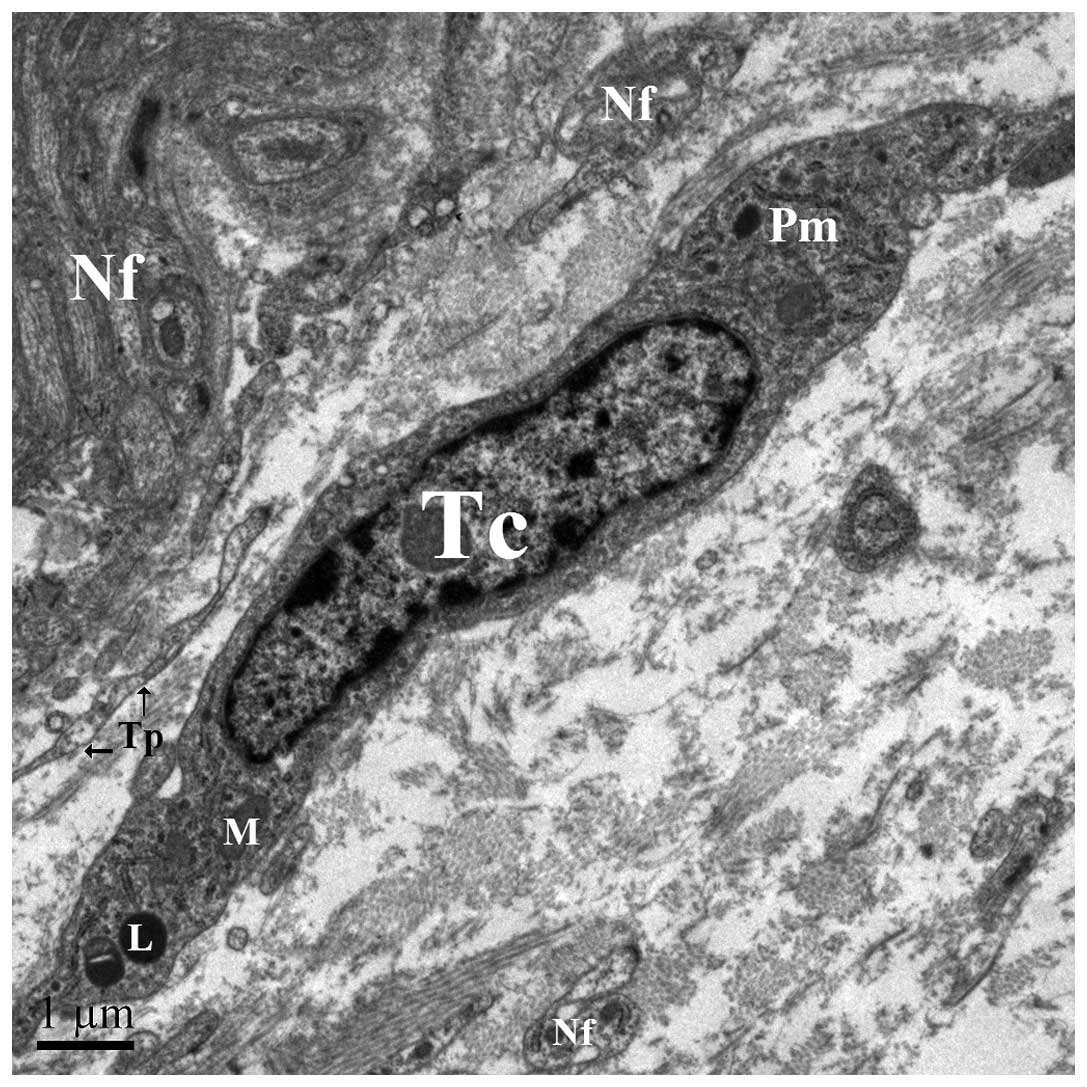
2), spindle for 2 Tps (Fig. 3),
triangular for 3 and stellate for ≥4 Tps (Fig. 4). The thin rim of cytoplasm
surrounding the nucleus contains a small Golgi apparatus, a number
of mitochondria, and few cisternae of rough and smooth endoplasmic
reticulum (Fig. 5).
In the myenteric plexus, TCs were identified
according to a particular ultrastructural signature. They often
surround blood vessels (Fig. 6) and
certain TCs are connected to each other (Fig. 7) and neighboring SMCs (Fig. 6) via gap junctions. Bundles of
enteric nerve fibers lay close to TCs (Fig. 8), although intimate relationships
were not observed. In the current study, TCs were predominantly
spindle-shaped, with 2 Tps.
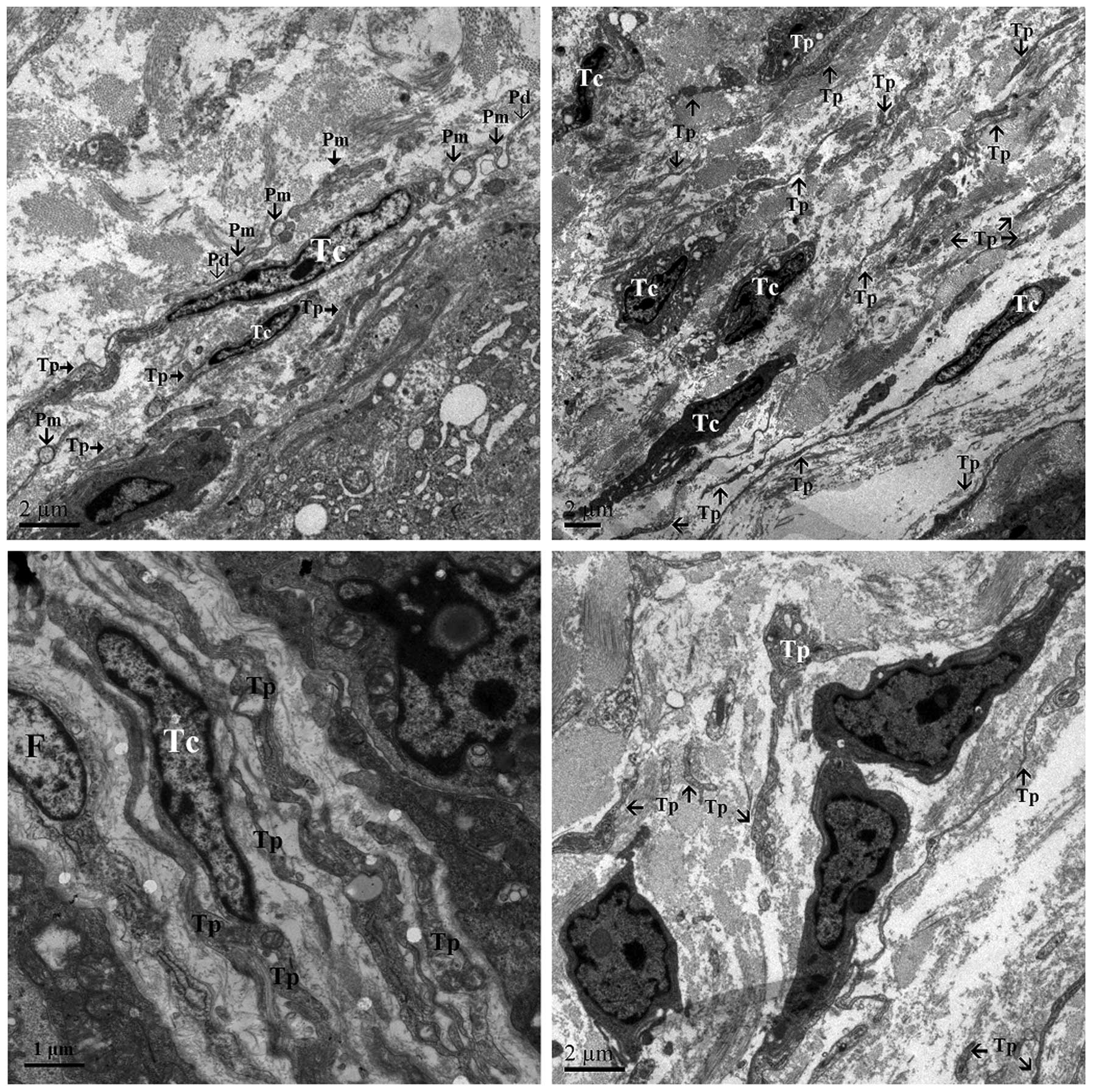
Other TCs were located within the longitudinal and
circular muscle layers, containing numerous mitochondria, caveolae
and rough endoplasmic reticulum. These TCs displayed similar
ultrastructural features, and exhibited a predominantly stellate
shape with 2 long processes and multiple short processes (Fig. 4). The ends of the processes often
formed large caveolae (Fig. 4). The
TCs of the longitudinal layer formed distinct close contacts, not
gap junctions, with adjacent SMCs (Fig.
4).
Numerous TCs were observed along the submucosal
surface of the circular muscle layer. These TCs were arranged
around the ganglia of the submucosal plexus (Fig. 9) and occasionally surrounded
neighboring SMC (Fig. 10).
TCs were located in the lamina propria of the ileum.
These cells presented a stellate morphology with multiple branches.
The branches made frequent close contacts with each other, forming
a network and close contacts with neighboring cells (Fig. 11). In addition, these TCs displayed
longer Tps than the TCs in muscularis.
Discussion
The current study demonstrated the morphological and
ultrastructural characteristics of TCs in the in the muscularis and
the lamina propria of the chicken ileum, revealing characteristic
ultrastructural features according to the TEM diagnostic criteria
for TCs (8). Although the term
‘telocyte’ is relatively new, a review of the literature indicated
various studies reporting the presence of TCs in other organs,
including the epicardium (20),
myocardium (21), endocardium
(26), placenta (27) and skin (28). The role of TCs is unclear; however,
based on the available data, numerous potential and relevant roles
have been proposed. Taking into account the 3D network of Tps, and
their strategic position between target cells, nerve endings and
blood capillaries, Popescu and Faussone-Pellegrini (8) suggested that TCs may be involved in
intercellular signaling. They considered at least two mechanisms
that may be involved: i) Aparacrine and/or juxtacrine secretion of
small signal molecules; and ii) shedding microvesicles, which serve
unique roles in the horizontal transfer of important macromolecules
among neighboring cells (e.g. proteins or RNAs, such as microRNA).
The result of the present study also demonstrates a close
connection of TCs with blood capillaries and nerve endings. It is
suggested that TCs may have the following functions in the
myocardium: i) Involvement in intercellular signaling at a
distance, as they are situated close to blood capillaries and nerve
endings; ii) mechanoreceptors/transducers, due to their extremely
long Tps and their ability to form attachment plaques connecting
them to the extracellular matrix; and iii) cardiac renewal and
cardiac repair (29).
Cretoiu and Popescu described the close contacts
that establish TCs with various types of immunoreactive cells,
including lymphocytes, plasma cells, eosinophils, basophils,
macrophages and mast cells in human mammary gland and myometrium,
rat stomach, gut, bladder, and uterus (30). The current study demonstrated these
contacts with immune cells in the lamina propria of chicken ileum;
on the basis of contact distribution and morphology, these cells
may be involved in the immune response.
The present study indicated that TCs form contacts
with stem cells (SCs) in discrete sites that appear to be SC niches
in the myenteric plexus. The tandem TC-SC has previously been
observed in SC niches in a number of organs, including the
epicardium, lungs, skeletal muscle, choroid plexus and skin
(31). SC niches are highly
organized interactive structural units that commonly occur at
tissue intersections or transition zones, and coordinate tissue
repair and renewal (32,33). The functionality of a SC niche relies
on the physical contact and signaling interactions of SCs with
neighboring nurse cells, in addition to the paracrine and endocrine
signals from local or distant sources, neural input and metabolic
products of tissue (33). TCs have a
strategic position in the ileum between blood capillaries and SC,
and are in close contact with nerve endings. TCs may be nurse
cells, integrating local, short-distance signals (direct contacts,
exosomes and shedding vesicles) and long-distance signals through
the long TPs, due to their 3D network. Recent studies have
demonstrated a particular immunophenotype (34,35),
distinct microRNA expression (36),
specific gene-expression profiles (37) and peculiar electrophysiological
properties of TCs in various organs (38). Close contact between TCs and
fibroblasts was indicated in the present study. Fibroblasts can be
differentiated from TCs on the basis of their short cell processes
with thick protrusions from the cell body. Furthermore, they
exhibit a different phenotype from Tps, which are long, moniliform
and convoluted (34). A comparative
proteomic analysis of human lung TCs versus fibroblasts showed that
TCs are different from fibroblasts (39). The results described here are in
agreement with these findings as there are clear long, moniliform
and convoluted Tps.
The function of TCs in chicken ileum may be
associated with the reparation of injured tissues during disease,
as in skeletal muscle (18). This
hypothesis is supported by the present observation that TCs and Tps
were located in close vicinity to blood capillaries, nerve endings
and smooth muscle cells. However, there is a requirement for the
investigation of TC-specific biomarkers in order to clarify TC cell
functionality and network biomarkers, to improve understanding of
the interaction between proteins, genes, signaling pathways and
dynamic networks, and to define and predict time-dependent TC
function and morphological features (40,41). TCs
also function in the vascular system, nervous system, immune
system, interstitium and SC/progenitors (42). Manole et al (36) demonstrated that TCs are involved in
neo-angiogenesis in experimental acute myocardial infarction. TCs
may further be involved in tissue regeneration and reparation
(28,18); it is suggested that TCs are involved
in angiogenesis, as it has been demonstrated that TCs express CD34
and VEGF (27).
The present study provided ultrastructural evidence
for the existence of TCs in the muscularis and the lamina propria
of the chicken ileum. Tps connect with immune cells, smooth muscle
cells, nerve fibers and blood vessels. These data demonstrate the
existence of TCs in birds. Future studies should explore the
potential biological functions of TCs in certain pathological
conditions of the intestine, and investigate the mechanisms of
interaction between TCs and other cells.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (Youth Project, no.
31402155 and no. 31172282), the Natural Science Foundation of
Jiangsu Province of China (youth project, no. BK20130681), the
Fundamental Research Funds for the Central Universities of China
(no. KJ201302), and the Priority Academic Program Development of
Jiangsu Higher Education Institutions of China.
References
|
1
|
Faussone-Pellegrini MS and Thuneberg L:
Guide to the identification of interstitial cells of Cajal. Microsc
Res Tech. 47:248–266. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daniel EE: Physiology and pathophysiology
of the interstitial cell of Cajal: From bench to bedside. III.
Interaction of interstitial cells of Cajal with neuromediators: an
interim assessment. Am J Physiol Gastrointest Liver Physiol.
281:G1329–G1332. 2001.PubMed/NCBI
|
|
3
|
Ward SM and Sanders KM: Interstitial cells
of Cajal: Primary targets of enteric motor innervation. Anat Rec.
262:125–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vanderwinden JM, Rumessen JJ, Bernex F,
Schiffmann SN and Panthier JJ: Distribution and ultrastructure of
interstitial cells of Cajal in the mouse colon, using antibodies to
Kit and Kit(W-lacZ) mice. Cell Tissue Res. 302:155–170. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farrugia G: Interstitial cells of Cajal in
health and disease. Neurogastroenterol Motil. 20(Suppl 1): 54–63.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mikkelsen HB: Interstitial cells of Cajal,
macrophages and mast cells in the gut musculature: Morphology,
distribution, spatial and possible functional interactions. J Cell
Mol Med. 14:818–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pieri L, Vannucchi MG and
Faussone-Pellegrini MS: Histochemical and ultrastructural
characteristics of an interstitial cell type different from ICC and
resident in the muscle coat of human gut. J Cell Mol Med.
12:1944–1955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Popescu LM and Faussone-Pellegrini MS:
Telocytes - a case of serendipity: The winding way from
interstitial cells of Cajal (ICC), via interstitial Cajal-like
cells (ICLC) to telocytes. J Cell Mol Med. 14:729–740. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicolescu MI and Popescu LM: Telocytes in
the interstitium of human exocrine pancreas: Ultrastructural
evidence. Pancreas. 41:949–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz-Flores L, Gutiérrez R, Sáez FJ,
Díaz-Flores L Jr and Madrid JF: Telocytes in neuromuscular
spindles. J Cell Mol Med. 17:457–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milia AF, Ruffo M, Manetti M, Rosa I,
Conte D, Fazi M, Messerini L and Ibba-Manneschi L: Telocytes in
Crohn's disease. J Cell Mol Med. 17:1525–1536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manetti M, Rosa I, Messerini L, Guiducci
S, Matucci-Cerinic M and Ibba-Manneschi L: A loss of telocytes
accompanies fibrosis of multiple organs in systemic sclerosis. J
Cell Mol Med. 18:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Lin M, Li L, Wang R, Zhang C, Qi G,
Xu M, Rong R and Zhu T: Renal telocytes contribute to the repair of
ischemically injured renal tubules. J Cell Mol Med. 18:1144–1156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Zhang H, Yang L, Lu S and Ge J:
Telocytes in mice bone marrow: Electron microscope evidence. J Cell
Mol Med. 18:975–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicolescu MI and Manole CG: Telocytes and
stem cells in regenerative medicine. FASEB J. 27:752–754. 2013.
|
|
16
|
Chen X, Zheng Y, Manole CG, Wang X and
Wang Q: Telocytes in human oesophagus. J Cell Mol Med.
17:1506–1512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Popescu BO, Gherghiceanu M, Kostin S,
Ceafalan L and Popescu LM: Telocytes in meninges and choroid
plexus. Neurosci Lett. 516:265–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Popescu LM, Manole E, Serboiu CS, Manole
CG, Suciu LC, Gherghiceanu M and Popescu BO: Identification of
telocytes in skeletal muscle interstitium: implication for muscle
regeneration. J Cell Mol Med. 15:1379–1392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Popescu LM, Gherghiceanu M, Suciu LC,
Manole CG and Hinescu ME: Telocytes and putative stem cells in the
lungs: Electron microscopy, electron tomography and laser scanning
microscopy. Cell Tissue Res. 345:391–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Popescu LM, Manole CG, Gherghiceanu M,
Ardelean A, Nicolescu MI, Hinescu ME and Kostin S: Telocytes in
human epicardium. J Cell Mol Med. 14:2085–2093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kostin S: Myocardial telocytes: A specific
new cellular entity. J Cell Mol Med. 14:1917–1921. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bani D, Formigli L, Gherghiceanu M and
Faussone-Pellegrini MS: Telocytes as supporting cells for
myocardial tissue organization in developing and adult heart. J
Cell Mol Med. 14:2531–2538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reynhout JK and Duke GE: Identification of
interstitial cells of Cajal in the digestive tract of turkeys
(Meleagris gallopavo). J Exp Zool. 283:426–440. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang P, Wang S, Gandahi J, Bian X, Wu L,
Liu Y, Zhang L, Zhang Q and Chen Q: Ultrastructural identification
of different subtypes of interstitial cells of Cajal in the chicken
ileum. Poult Sci. 91:1936–1940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cantarero Carmona I, Luesma Bartolomé MJ
and Junquera Escribano C: Identification of telocytes in the lamina
propria of rat duodenum: transmission electron microscopy. J Cell
Mol Med. 15:26–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gherghiceanu M, Manole C and Popescu L:
Telocytes in endocardium: Electron microscope evidence. J Cell Mol
Med. 14:2330–2334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suciu L, Popescu LM, Gherghiceanu M,
Regalia T, Nicolescu MI, Hinescu ME and Faussone-Pellegrini MS:
Telocytes in human term placenta: Morphology and phenotype. Cells
Tissues Organs. 192:325–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ceafalan LM, Gherghiceanu M, Popescu L and
Simionescu O: Telocytes in human skin-are they involved in skin
regeneration? J Cell Mol Med. 16:1405–1420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kostin S and Popescu L: A distinct type of
cell in myocardium: Interstitial Cajal-like cells (ICLCs). J Cell
Mol Med. 13:295–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cretoiu SM and Popescu LM: Telocytes
revisited. Biomol concepts. 5:353–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Popescu LM and Nicolescu MI: Telocytes and
stem cells. Resident Stem Cells and Regenerative Therapy.
Goldenberg and de Carvalho Campos: (Elsevier, Amsterdam). 205–231.
2013. View Article : Google Scholar
|
|
32
|
Ordonez P and Di Girolamo N: Limbal
epithelial stem cells: Role of the niche microenvironment. Stem
Cells. 30:100–107. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scadden DT: The stem-cell niche as an
entity of action. Nature. 441:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Faussone-Pellegrini MS and Popescu LM:
Telocytes. Biomol Concepts. 2:481–489. 2011.PubMed/NCBI
|
|
35
|
Koh BH, Roy R, Hollywood MA, Thornbury KD,
McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM and Koh SD:
Platelet-derived growth factor receptor-α cells in mouse urinary
bladder: a new class of interstitial cells. J Cell Mol Med.
16:691–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Manole CG, Cismaşiu V, Gherghiceanu M and
Popescu LM: Experimental acute myocardial infarction: Telocytes
involvement in neo-angiogenesis. J Cell Mol Med. 15:2284–2296.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng Y, Zhang M, Qian M, Wang L, Cismasiu
VB, Bai C, Popescu LM and Wang X: Genetic comparison of mouse lung
telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol
Med. 17:567–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cretoiu SM, Cretoiu D, Marin A, Radu BM
and Popescu LM: Telocytes: Ultrastructural, immunohistochemical and
electrophysiological characteristics in human myometrium.
Reproduction. 145:357–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng Y, Cretoiu D, Yan G, Cretoiu SM,
Popescu LM and Wang X: Comparative proteomic analysis of human lung
telocytes with fibroblasts. J Cell Mol Med. 18:568–589. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen H and Wang X: Significance of
bioinformatics in research of chronic obstructive pulmonary
disease. J Clin Bioinforma. 1:352011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu D, Rice CM and Wang X: Cancer
bioinformatics: A new approach to systems clinical medicine. BMC
Bioinformatics. 13:712012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gherghiceanu M and Popescu LM: Cardiac
telocytes - their junctions and functional implications. Cell
Tissue Res. 348:265–279. 2012. View Article : Google Scholar : PubMed/NCBI
|