Introduction
Stress is caused by a variety of stressors, that is,
harmful stimuli or challenges that lead to various physiological,
behavioral, emotional and cognitive alterations known as stress
responses. It is an adaptive response mediated by the stress
system, which includes the central nervous system and peripheral
components in various physiological and pathological states. The
primary components of the stress system are corticotropin-releasing
hormone (CRH), also known as corticotropin-releasing factor, locus
coeruleus-norepinephrine-autonomic systems and their peripheral
effectors, the hypothalamus-pituitary-adrenal (HPA) axis and the
limbs of the autonomic system (1–3). Stress
responses are highly organized and regulated, and act to reduce the
adverse impact of a stressor. By contrast with the view that the
stress response restores the stability of an organism's internal
environment (homeostasis), it is now hypothesized that stressors
cause well-organized responses with their own homeostasis, to
promote adaptive coping (1). These
adaptive changes are usually time-limited, which may improve an
individual's survivability. However, when stress is severe or
chronic, or there is a functional defect of inadequate response to
stress, the organization and regulation of stress responses may be
disrupted, resulting in various endocrine, metabolic, autoimmune
and psychiatric disorders (1,2).
Therefore, to maintain a healthy state, it is crucial to limit the
extent of harmful external stimuli, reduce the adverse effects of
severe or chronic stress and enhance the body's resistance to
stressors.
Following acute exposure to stressors, including
fatigue, heat shock, skin burn, cooling, frostbite, immobilization
and swimming under load, the amplitude and synchronization of the
CRH and arginine-vasopressin pulsations in the hypophyseal portal
system markedly increase, causing adrenocorticotropic hormone
(ACTH) and cortisol secretion levels to increase (4). CRH serves a crucial function in the
activation of the stress response. A circumscribed group of
parvocellular neurosecretory neurons in the paraventricular nuclei
(PVN) of the hypothalamus is the principal source of CRH for
delivery to the portal capillary zone of the median eminence,
although CRH is broadly expressed throughout the central nervous
system (5,6). Glucocorticoids, as the final effectors
of the HPA axis, help to maintain homeostasis and the body's
response to stress, resulting in behavioral and peripheral changes
that improve the ability to adjust to external challenges.
Adaptogens augment resistance to stress and increase
concentration, performance and fatigue endurance (7–10). In
the 1960s, on the basis of the results of numerous pharmacological
and clinical studies, three plant species, namely
Eleutherococcus senticosus, Rhodiola rosea (R.
rosea) and Schisandra chinensis (S. chinensis),
were incorporated into official medical practice in the Union of
Soviet Socialist Republics as adaptogens, and were considered to
exert stimulatory, restorative and anti-stress effects (10). These plants may be used by healthy
individuals as tonics during states of fatigue and stress, and in
sports medicine for preventing and treating injuries. In addition,
they have been employed in occupational medicine for protection
against adverse environmental factors, including extreme conditions
such as high noise levels and low temperature, and in clinical
medical practice for treating acute hepatic poisoning, ischemia due
to oxygen deprivation and for accelerating recovery following
surgery.
As medicinal plants, R. rosea and S.
chinensis appear to possess the characteristics of adaptogens
(10–13), which were described by Brekhman
(14) as follows: i) Produces a
non-specific response in an organism, for example, an increase in
power of resistance against multiple stressors, including physical,
chemical or biological agents; ii) has a normalizing influence on
physiology, irrespective of the direction of change from
physiological norms caused by the stressor; and iii) is incapable
of influencing normal body functions more than required to gain
non-specific resistance. By definition, adaptogens reinforce the
non-specific resistance against stressors, increase the capacity to
withstand stress, and thus protect against disease caused by
excessive stress. Plant adaptogens do not exert unwanted effects on
normal physiological functions (15). By contrast, stimulants such as
caffeine and nicotine that increase alertness and concentration by
enhancing the activity of the sympathetic nervous system, may
possess addictive, tolerance and abuse potential and their
long-term use may impair mental function and result in psychiatric
disorders (15).
Previous studies have shown that ADAPT-232 forte,
which comprises E. senticosus, S. chinensis and R.
rosea extracts, prolongs the period until exhaustion by ~7-fold
and repeated administration of this adaptogen may increase basal
levels of heat shock protein 72 (Hsp72) in the serum of mice
(16). A number of other
stress-related factors have been extensively studied in the process
of stress, including neuropeptide-Y (NPY) (17,18),
Hsp70, activating protein-1 (AP-1) and cytokines such as
interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α)
(19). In addition, the rapid
induction of immediate early genes such as c-Fos, Fos-related
antigen (Fra)-1, Fra-2 and c-Jun may alter gene transcription in
hormone-secreting cells in the hypothalamus, pituitary gland and
adrenal gland, which may result in changes in behavior, morphology,
and the survival or degeneration of neurons in response to
pathophysiological stimuli (19).
Previous studies have shown that c-Fos in the hypothalamus is a
reliable marker for activated cells and central nervous system
circuits that respond to stress challenges (20–25).
Furthermore, Fra-2 mRNA expression has been detected in the
hippocampus (26), and the
suprachiasmatic nucleus and PVN in the hypothalamus during stress.
These stress-related factors have been demonstrated to be crucially
involved in the mediation of the stress process.
In a previous study, we established an acute stress
model in which adult rats were subjected to water-floating and
high-intensity exercise, with the aim of simulating psychological
and physical stress, respectively (27). The aim of the present study was to
investigate the effects of S. chinensis and R. rosea
in rats subjected to acute compound stress, and to determine the
underlying mechanisms by evaluating the serum levels of ACTH, CORT
and IL-1β and the mRNA expression levels of CRH, NPY, c-Fos and
Fra-2 in the whole hypothalamus of the rats exposed to S.
chinensis and R. rosea.
Materials and methods
Animals
A total of 40 male Sprague-Dawley rats (age, 10
weeks; weight, 300±20 g) were purchased from the Shanghai SLAC
Laboratory Animal Co. Ltd. (Shanghai, China). Prior to the study,
the rats were housed for 3 days in a clean-grade animal breeding
center with an indoor temperature of 20–24°C and humidity of
50–70%, under alternate dark/light cycles. Tap water and laboratory
feed were available ad libitum. The rats were then allocated
at random into four groups: S. chinensis (n=12), R.
rosea (n=10), stress control (n=10) and quiet control (n=8).
All procedures were performed in accordance with the standards of
the Animal Research Ethics Committee of Jinling Hospital (Nanjing,
China).
Water-floating and treadmill
training
Four identical plastic containers (1.00×0.67×0.60 m)
were used as floating devices and were placed in an inflatable
swimming pool (3.8×5 m) in which a wave of 15–20° was produced
using three water pumps (Shenzhen XingRisheng Industrial Co., Ltd.,
Shenzhen, China). Three-year-old tabby cats, weighing ~5 kg, were
purchased from a local pet store, fed feline food, and maintained
in a cage in a clean-grade room with an alternate light/dark cycle
at 20–24°C and 50–70% humidity. According to the feline predator
stress model proposed by Adamec et al (28), on days 1–6 at 13:00, all rats
(including the quiet control group) were placed on the floating
container for 10 min, and simultaneously stimulated by the threat
of two cats located in cages at a distance of 0.75 m. The floating
was immediately followed by 10 min treadmill training, which
provided high-intensity exercise using an ZH-PT rat treadmill
(Huaibei Zhenghua Bioinstrumentation Co., Ltd., Huaibei, China)
with a 0° slope at a speed of 15 m/min. All rats successfully
completed the training and so none were eliminated from the study.
During this 6-day training period, S. chinensis and R.
rosea were administered by gavage twice a day to rats in the
corresponding intervention groups. The dosages of each were 1 mg/kg
each time, according to preliminary experiments. The rats in the
stress control group and quiet control group were administered
identical quantities of normal saline. S. chinensis and
R. rosea used in the study were provided by Department of
Traditional Chinese Medicine of Jinling Hospital.
Exhaustion experiment (compound stress
model)
At 7:00 a.m. on day 7, the rats in the S.
chinensis, R. rosea and stress control groups underwent
water-floating for 3 h with cats at a distance of 0.75 m, while the
quiet control rats remained in their cages. Next, the three
water-floating groups underwent high-intensity exercise on a
treadmill with a 5° slope at 26.8 m/min (27). According to the study protocol, rats
that showed signs of exhaustion during the exercise or that were
unable to maintain the required speed were immediately
anaesthetized with 50 mg/kg sodium pentobarbital and sacrificed via
decapitation. In the present study, all rats successfully completed
the 2-h treadmill exercise, with 3 exceptions; 1 rat in the S.
chinensis group, 1 rat in the R. rosea group and 1 rat
in the stress control group. All rats were deprived of food and
water following the initiation of the water-floating
experiment.
Blood sampling
Following the treadmill exercise, all rats were
anaesthetized with 50 mg/kg sodium pentobarbital dissolved in
normal saline and sacrificed via decapitation. Within 30 min, 5 ml
blood was harvested, the hair and skull at the back of the head
were carefully removed and the hypothalamus was isolated using
tweezers. Hypothalamic tissue was immediately immersed in liquid
nitrogen and stored at −80°C for subsequent reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assays to evaluate the mRNA expression levels of CRH, NPY, c-Fos
and Fra-2. Serum was isolated for the subsequent measurement of
ACTH and IL-1β concentrations.
CORT enzyme-linked immunosorbent assay
(ELISA)
CORT concentration was measured using a CORT ELISA
kit (assay sensitivity of 46.88 pg/ml; Elabscience Biotechnology
Co., Ltd., Wuhan, China) according to the manufacturers
instructions. Briefly, serum samples were added to 96-well plates
containing biotinylated primary antibody and incubated at 37°C for
45 min. Plates were washed and horseradish peroxidase-conjugated
streptavidin solution was added to the wells and incubated for a
further 30 min at 37°C. The plates were then washed,
3,3,5,5-tetramethylbenzidine substrate was added and the plates
were incubated for an additional 15 min at 37°C. Finally, stop
solution was added to the wells to terminate the reaction. The
resultant absorbance was measured at 450 nm using an iMark
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The concentration of CORT was determined using a standard
curve.
Radioimmunoassay
Serum ACTH and IL-1β concentrations were measured
using radioimmunoassay testing kits (North Institute of Biological
Technology, Beijing, China), according to the manufacturers
instructions. Assay sensitivities for ACTH and IL-1β were <0.1
ng/ml and <5 pg/ml, respectively, for a 100-µl sample. The
detection limit for ACTH was ~405 pg/ml.
RNA isolation and RT-qPCR
Frozen hypothalamic tissue was punched and ground
into pellets using a mortar and pestle so that the pellets were as
small as possible. RNA was isolated and subsequently purified from
the punched pellets of hypothalamic tissue using an RNAprep Micro
Sample RNA Extraction kit, according to the manufacturer's protocol
(BioTeke Corporation, Beijing, China). Following the isolation and
purification of the total RNA, the concentration of each individual
total RNA sample was standardized as 250 ng/ml. To generate
single-strand cDNA, total RNA was used as the starting template for
first strand cDNA synthesis, using a PCR RevertAid First strand
cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, US)
according to the manufacturer's instructions. Subsequently, qPCR
was performed using CFX connect Real-Time qPCR system (Bio-Rad
Laboratories, Inc.) and a SuperReal PreMix Plus (SYBR Green;
Qiagen, Inc., Valencia, CA, USA) kit. The PCR primers used for each
gene are presented in Table I. The
qPCR reaction was performed using a final reaction volume of 20 µl,
which included 10 µmol/µl of each primer, 10 µl SuperReal PreMix
Plus (SYBR Green) and 8 µl template. Samples were subjected to a
3-min incubation at 95°C, followed by 40 cycles of 10 sec at 95°C,
20 sec at 59°C and 30 sec at 72°C. The PCR products were subjected
to melt curve analysis to exclude the possibility of the
amplification of unwanted products. Results were analyzed using CFX
Manager software (Bio-Rad Laboratories, Inc.).
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
| Gene | Direction | Primer (5′-3′) |
|---|
| CRH | Forward |
ATCTCACCTTCCACCTTCTG |
|
| Reverse |
GCAACATTTCATTTCCCGATAATC |
| NPY | Forward |
ACAGAGATATGGCAAGAGA |
|
| Reverse |
CACAGGATGAGATGAGATG |
| c-Fos | Forward |
GGGAGGACCTTATCTGTGCG |
|
| Reverse |
TCTCCGGAAGAGGTGAGGAC |
| Fra-2 | Forward |
AACCTTGTCTTCACCTACC |
|
| Reverse |
CCACTGCTACTGCTTCTG |
Statistical analysis
All measurement data are expressed as the mean ±
standard error of the mean. Absolute measurement data of hormone
concentrations are provided; however, gene expression data are
expressed as arbitrary optical density units. One-way analysis of
variance was used to determine the significance of the effects of
the adaptogen treatment on hormone and gene expression. If a
significant interaction was detected, post hoc Dunnets t-tests were
performed. All stressed groups were compared with the control group
using the one-tailed t-test on the basis of the a priori
hypothesis that stress increases the levels of hormones and mRNA
expression. P<0.05 was considered to indicate a statistically
significant difference.
Results
HPA axis activity
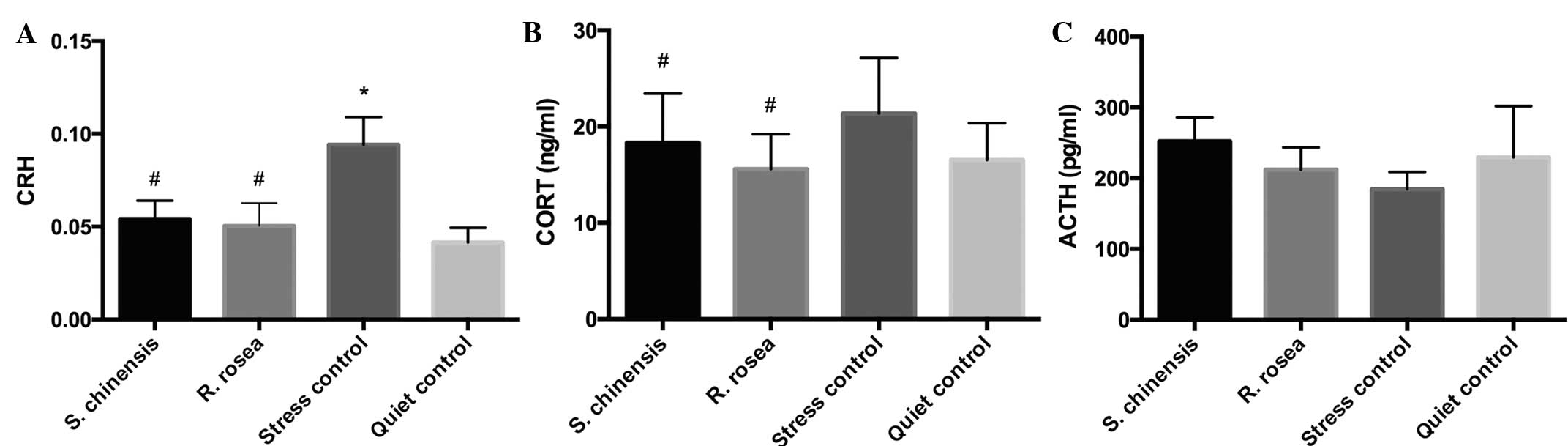
The mRNA expression levels of CRH in the
hypothalamus were significantly higher in the stress control group
compared with the quiet control group (P<0.05). No significant
differences in the serum levels of CORT and ACTH were detected
between the stress control and quiet control groups following the
water-floating and exhaustion experiments. The expression levels of
CRH mRNA in the hypothalamus and of CORT in the serum were
significantly reduced in the S. chinensis and R.
rosea groups compared with those in the stress control group
following the exhaustion experiment (P<0.05; Fig. 1).
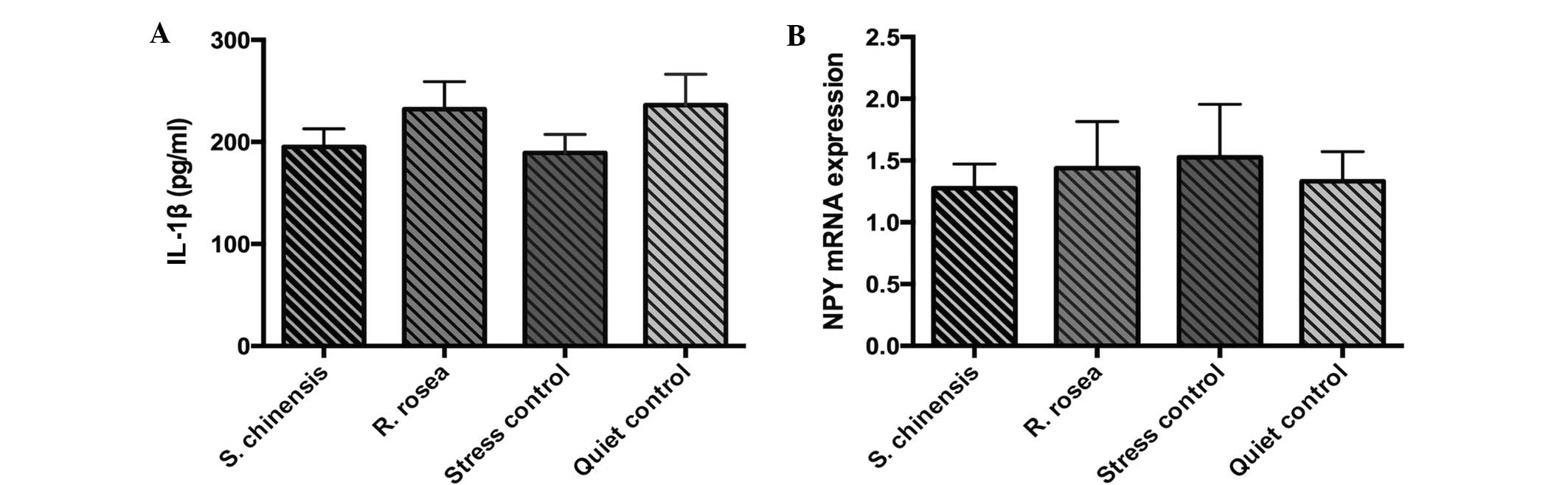
Serum IL-1β levels and hypothalamic
NPY mRNA expression
No significant differences in the serum IL-1β levels
and hypothalamic NPY mRNA expression were identified between the
four groups (P>0.05; Fig. 2).
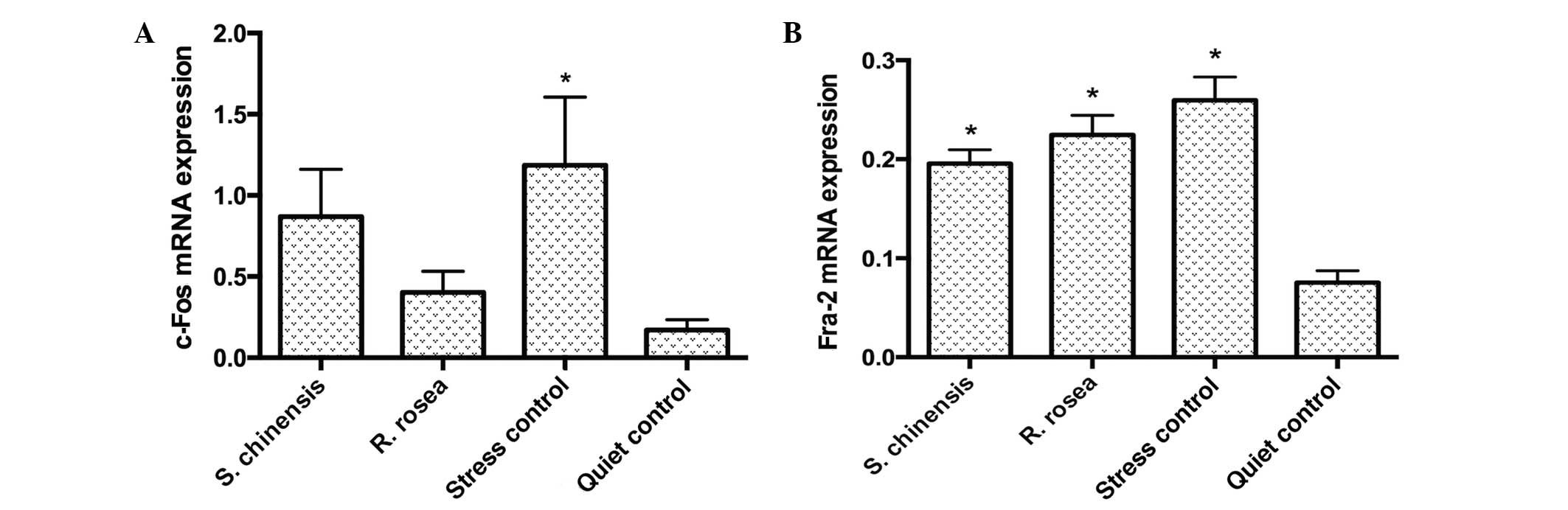
c-Fos and Fra-2 mRNA expression in the
hypothalamus
The c-Fos and Fra-2 mRNA expression levels in the
hypothalamus of the stress control group were significantly higher
compared with those of the quiet control (P<0.05). The c-Fos
mRNA expression levels in the S. chinensis and R.
rosea groups did not differ significantly compared with those
in the quiet control group (P>0.05). The Fra-2 expression level
in the S. chinensis and R. rosea groups did not
significantly differ from the stress control group, but did show a
significant difference compared with the quiet control group
(P<0.05; Fig. 3).
Discussion
In the present study, a 3-h water-floating
experiment including a feline predator stress element, and a 2-h
treadmill exercise were conducted as psychological and physical
stressors, respectively. Prior to the initiation of the experiment,
all rats underwent 6 days of training to enable them to adapt to
the long-term and intense stimulations on day 7. Following the
experiment, serum levels of CORT and ACTH were determined, in
addition to the CRH mRNA expression levels in the hypothalamus. CRH
mRNA expression was significantly increased following exposure to
prolonged compound stress; however, no significant differences in
ACTH and CORT levels were observed. Furthermore, ACTH levels
appeared to decrease slightly following the compound stress. It has
been reported that the magnitude and duration of increases in serum
ACTH concentration may be associated with the duration and
intensity of the ACTH-releasing stimulus, and that elevated levels
of ACTH persist throughout a 2-h period of ether stress (>700
pg/ml) (29). By contrast, ACTH
levels have been shown to reduce markedly under prolonged
mobilization stress, with a reduction to almost twice the basal
levels after 6 h, from >600 pg/ml to <50 pg/ml; these levels
persisted for the remainder of the stress period (30). Previous results suggest that the
elevation in plasma CORT levels in response to stress is biphasic,
whereas plasma ACTH levels exhibit only a single peak that rapidly
declines to a sustained plateau level (31). Plasma levels of CORT, but not those
of ACTH, have been found to remain elevated after 180 min restraint
(32). In addition, it has been
reported that running training attenuates the ACTH response induced
by physical or psychological stress (33,34). In
the present study, serum ACTH levels were evaluated after a total
of 5 h exposure to psychological and physical stress. On the basis
of the results of previous studies, it is not unexpected that the
ACTH levels in the present study were not markedly increased, and
decreased to a certain extent in the stress control group (<300
pg/ml on average) compared with the quiet control. In addition, a
previous study has shown that blood concentrations of adrenal
glucocorticoids increase to peak levels at 15–30 min and
subsequently decline gradually to pre-stress levels at 60–90 min
(35). This increase appears to be
attenuated by repeated exposure to stress (36), which is consistent with the present
result showing insignificant differences in CORT levels following
the water-floating and treadmill exercise experiments. Therefore,
this insignificance may be explained by the neural adaption caused
by the previous repeated stress training during the first 6 days,
indicating a possible mechanism of chronic stress characterized by
reduced sensitivity of the HPA response to similar stressors, such
as posttraumatic stress disorder (37–40).
Notably, the present results demonstrated that S.
chinensis and R. rosea significantly decreased CRH mRNA
expression levels (P<0.05) and the levels of serum CORT
(P<0.05) in the rats exposed to water-floating and exhaustion
experiments. CRH and CORT are pleiotropic, and exert effects via
ubiquitously distributed intracellular receptors (41,42).
S. chinensis increased the mildly stress-reduced ACTH
levels, and R. rosea showed a similar, but less marked
effect. These results suggest that S. chinensis and R.
rosea are able to reduce the changes in CORT and ACTH levels
caused by prolonged and intense compound stress, thus balancing the
HPA axis and maintaining homeostasis in rats.
In the present study, the effects of S.
chinensis and R. rosea on the immune system were
investigated by evaluating the serum levels of IL-1β, which is a
typical stress-associated cytokine and the most extensively studied
inflammatory cytokine. The immune inflammatory response is
considered to be closely associated with the stress system.
Activation of the HPA axis produces marked inhibitory effects on
the immune inflammatory response, as virtually all the components
of the immune response are inhibited by cortisol (43). In turn, cytokines including TNF-α,
IL-1β and IL-6 stimulate the HPA axis independently, or in
combination with one another (44).
IL-1 is a critical mediator of adaptive stress responses, in
addition to stress-associated neuropathology and psychopathology
(45). A recent study demonstrated
that the activation of the HPA axis by IL-1β is dependent on IL-1
type 1 receptors in non-hematopoietic cells, such as brain
endothelial cells, but not in perivascular macrophages (46). Furthermore, a 15-min swim stress has
been shown to provoke a significant reduction in lipopolysaccharide
(LPS)-induced IL-1β production at 120 and 240 min following LPS
challenge (<200 pg/ml), compared with the control (>600
pg/ml) (47). In the present study,
serum IL-1β levels in the rats were mildly, but not significantly,
suppressed by compound stress. Treatment with S. chinensis
and R. rosea appeared to increase the stress-reduced IL-1β
levels. However, their effect on peripheral IL-1β levels was also
mild and requires further investigation.
In addition to stress-induced changes in the HPA
axis and cytokine expression following stimulation with various
stressors, a number of ‘molecular chaperones’ appear to be
crucially involved in the regulation of the neuroendocrine system
and immune response (16).
Stress-induced CRH expression causes ACTH release from the
pituitary gland, and ACTH stimulates the release of adrenal
hormones and NPY to mobilize energy and help the body to cope with
stress, and in turn NPY activates the release of CRH (18,48).
Adaptogens are known to activate the expression of NPY in brain
cells (49). Therefore, NPY
production by brain cells has been suggested as a characteristic
marker of the adaptogenic activity of plant extracts (50). Numerous studies have reported that
acute and repeated physical stress may significantly increase the
protein and mRNA expression levels of NPY in the amygdala (17,51,52). In
the present study, NPY expression in the hypothalamus following
exposure to stress was investigated. The results showed that NPY
mRNA expression levels in the hypothalamus were relatively low,
with no statistically significant differences among the four
groups. These results suggest that NPY expression in the
hypothalamus does not have such a strong association with stress as
does NPY expression in the amygdala. Further experiments are
required to investigate the association between adaptogens and the
production of NPY (protein and mRNA) in the amygdala after
stress.
Numerous studies have identified the central
pathways mediating the stress response by mapping neuronal
activation using c-Fos as a dependable indicator (36,53).
Weinberg et al demonstrated that the kinetics of the c-Fos
response to acute stimuli is transient (32). In their study, c-Fos gene expression
in the prefrontal cortex (PFC), lateral septum (LS) and PVN peaked
at 15 min of restraint, moderately declined at 60 min of restraint
and had reduced by 180 min of continuous restraint; PVN c-Fos
expression at 180 min of restraint remained increased compared with
the expression levels in control rats; restraint for 60 min
produced a significant increase in Fra-2 mRNA expression in the
PFC, LS and PVN, and by 180 min of restraint, Fra-2 expression
returned to near-basal levels in all brain regions examined.
A previous study has demonstrated that stress
induces a marked elevation in the expression levels of the AP-1
complex in the rat hypothalamus, pituitary gland, adrenal gland and
gastric mucosa, suggesting that AP-1 binding may be a sensitive
tool for monitoring the severity of inputs of extracellular stress
signals (54). The protein Fra-2,
which is a Fos-family member, competes with Fos protein for
participation in the AP-1 transcription factor complexes. Each
protein contributes different transactivation consequences to an
AP-1 complex, leading to changes in the downstream expression of
genes containing AP-1 elements in their promoter region (24,55). The
present results demonstrated that c-Fos and Fra-2 gene expression
levels were significantly increased by stress, as demonstrated by
the c-Fos and Fra-2 mRNA expression levels remaining markedly
elevated after the 5-h stress. In a previous study of seizure
activity, Beer et al (26)
observed the rapid induction of Fra mRNA by kainic acid and
persisting levels after 6 h, with prolonged Fra-2 protein
expression for 7 days in the hippocampus. It has been reported that
Fra-2 protein levels in the adrenal medulla are significantly
elevated after 2 h of a single or repeated immobilization stress,
and are particularly pronounced at the termination of the
immobilization, demonstrating the crucial transcriptional role of
Fra-2 gene expression in the regulation of catecholamine
biosynthetic enzymes in the stress response of the adrenal medulla
(56).
In the present study, following persisting stimuli,
Fra-2 mRNA expression was significantly induced in the hypothalamus
after 5 h of physical and psychological stress. Furthermore,
following treatment with S. chinensis or R. rosea,
the compound stress-induced increase in c-Fos mRNA expression was
attenuated, and was comparable with the levels in the quiet control
rats. By contrast, Fra-2 mRNA expression remained unchanged at high
levels following the administration of S. chinensis or R.
rosea, and the effect of these herbs on the protein product of
the Fra-2 gene and Fos protein expression in the hypothalamus
requires further investigation.
The stress-associated markers investigated in the
present study are associated with each other, participating in the
complex process of the stress response. For example, a synthetic
analogue of an ACTH (4–10) fragment, known as Semax, has been
shown to reduce stress-induced Fos protein expression in the PVN
and medial septum of emotionally stressed rats (57). This may explain the observation in
the present study that ACTH levels were reduced and c-Fos levels
increased in the stress control group compared with the quiet
control group. As previously mentioned, IL-1β is able to
independently stimulate HPA activity, leading to stress,
potentially by enhancing the expression of c-Fos and CRH within the
parvocellular division of the PVN (58).
In conclusion, the present study demonstrated that
CRH, c-Fos and Fra-2 levels were significantly elevated after 5 h
of prolonged compound stress. S. chinensis and R.
rosea appear to exert anti-stress effects on stressed rats by
balancing the HPA axis, and may reduce the expression of c-Fos in
the hypothalamus. The present study had some limitations. As
laboratory conditions were limited, alterations in the hypothalamic
expression levels of proteins could not be investigated. The
present study was also limited in that the whole hypothalamus was
isolated, rather than one specific area; therefore, further studies
are required to fully characterize the stress-protective effects of
S. chinensis and R. rosea and to elucidate the
mechanisms involved. These may include the analysis of Fos protein
expression and its association with CRH production in hypothalamic
neurons following treatment with S. chinensis or R.
rosea and the potentially associated intracellular signaling
pathways. The relatively moderate side effects and marked
anti-stress effects of adaptogens indicate that they may provide an
improved therapeutic option for the treatment of acute compound
stress in the future.
Acknowledgements
The present study was supported by the Scientific
Research Foundation in the Twelfth Five-Year Plan Period of China
(no. CWS11J252). The authors thank the Department of Traditional
Chinese Medicine of Nanjing Jinling Hospital for providing S.
chinensis and R. rosea and Dr Guohong Wang in the
Department of Radioimmunity of Nanjing Jinling Hospital for
providing radioimmune analysis laboratory access.
References
|
1
|
Charmandari E, Tsigos C and Chrousos G:
Endocrinology of the stress response. Annu Rev Physiol. 67:259–284.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mills FJ: The endocrinology of stress.
Aviat Space Environ Med. 56:642–650. 1985.PubMed/NCBI
|
|
3
|
Tsigos C and Chrousos GP:
Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and
stress. J Psychosom Res. 53:865–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsigos C and Chrousos GP: Physiology of
the hypothalamic pituitary-adrenal axis in health and dysregulation
in psychiatric and autoimmune disorders. Endocrinol Metab Clin
North Am. 23:451–466. 1994.PubMed/NCBI
|
|
5
|
Zorrilla EP, Logrip ML and Koob GF:
Corticotropin releasing factor: A key role in the neurobiology of
addiction. Front Neuroendocrinol. 35:234–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koob GF: Corticotropin-releasing factor,
norepinephrine, and stress. Biol Psychiatry. 46:1167–1180. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wagner H, Nörr H and Winterhoff H: Plant
adaptogens. Phytomedicine. 1:63–76. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiegant FA, Surinova S, Ytsma E,
Langelaar-Makkinje M, Wikman G and Post JA: Plant adaptogens
increase lifespan and stress resistance in C. elegans.
Biogerontology. 10:27–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattioli L and Perfumi M: Rhodiola
rosea L. extract reduces stress- and CRF-induced anorexia in
rats. J Psychopharmacol. 21:742–750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panossian AG: Adaptogens: Tonic herbs for
fatigue and stress. Altern Complement Ther. 9:327–331. 2003.
View Article : Google Scholar
|
|
11
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: An overview of Russian research
and uses in medicine. J Ethnopharmacol. 118:183–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly GS: Rhodiola rosea: A
possible plant adaptogen. Altern Med Rev. 6:293–302.
2001.PubMed/NCBI
|
|
13
|
Kang YH and Shin HM: Inhibitory effects of
Schizandra chinensis extract on atopic dermatitis in NC/Nga
mice. Immunopharmacol Immunotoxicol. 34:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brekhman II: Chapter 3. Pharmacosanation
Medicaments. Man and Biologically Active Substances: The Effect of
Drugs, Diet and Pollution on Health (1st). (New York, NY). Pergamon
Press. 49–59. 1980. View Article : Google Scholar
|
|
15
|
Panossian A and Wagner H: Stimulating
effect of adaptogens: An overview with particular reference to
their efficacy following single dose administration. Phytother Res.
19:819–838. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panossian A, Wikman G, Kaur P and Asea A:
Adaptogens exert a stress-protective effect by modulation of
expression of molecular chaperones. Phytomedicine. 16:617–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morales-Medina JC, Dumont Y and Quirion R:
A possible role of neuropeptide Y in depression and stress. Brain
Res. 1314:194–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morgan CA III, Rasmusson AM, Wang S, Hoyt
G, Hauger RL and Hazlett G: Neuropeptide-Y, cortisol, and
subjective distress in humans exposed to acute stress: Replication
and extension of previous report. Biol Psychiatry. 52:136–142.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karanikas E and Giouzepas I:
Neuro-endocrinology of stress and immune mediated inflammation.
Psychiatriki. 19:43–51. 2008.(In Greek). PubMed/NCBI
|
|
20
|
Luckman SM, Dyball RE and Leng G:
Induction of c-fos expression in hypothalamic magnocellular neurons
requires synaptic activation and not simply increased spike
activity. J Neurosci. 14:4825–4830. 1994.PubMed/NCBI
|
|
21
|
Windle RJ, Kershaw YM, Shanks N, Wood SA,
Lightman SL and Ingram CD: Oxytocin attenuates stress-induced c-fos
mRNA expression in specific forebrain regions associated with
modulation of hypothalamo-pituitary-adrenal activity. J Neurosci.
24:2974–2982. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryan KK, Mul JD, Clemmensen C, Egan AE,
Begg DP, Halcomb K, Seeley RJ, Herman JP and Ulrich-Lai YM: Loss of
melanocortin-4 receptor function attenuates HPA responses to
psychological stress. Psychoneuroendocrinology. 42:98–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gagliano H, Delgado-Morales R, Sanz-Garcia
A and Armario A: High doses of the histone deacetylase inhibitor
sodium butyrate trigger a stress-like response. Neuropharmacology.
79:75–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kovács KJ: c-Fos as a transcription
factor: A stressful (re)view from a functional map. Neurochem Int.
33:287–297. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parrott RF and Vellucci SV: Stress-induced
changes in c-fos immunoreactivity in the porcine brain. Br Vet J.
150:355–363. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beer J, Mielke K, Zipp M, Zimmermann M and
Herdegen T: Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA
in the rat brain following seizure activity and axotomy. Brain Res.
794:255–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Zhao D, Li J, Wang G, Hu L, Shao
J, Gu P, Du H and Wang Y: The impact of water-floating and
high-intensity exercise on rat's HPA axis and interleukins
concentrations. Acta Physiol Hung. 99:261–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adamec R, Walling S and Burton P:
Long-lasting, selective, anxiogenic effects of feline predator
stress in mice. Physiol Behav. 83:401–410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cook DM, Kendall JW, Greer MA and Kramer
RM: The effect of acute or chronic ether stress on plasma ACTH
concentration in the rat. Endocrinology. 93:1019–1024. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hauger RL, Millan MA, Lorang M, Harwood JP
and Aguilera G: Corticotropin-releasing factor receptors and
pituitary adrenal responses during immobilization stress.
Endocrinology. 123:396–405. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cam GR and Bassett JR: The plasma levels
of ACTH following exposure to stress or nicotine. Arch Int
Pharmacodyn Ther. 264:154–167. 1983.PubMed/NCBI
|
|
32
|
Weinberg MS, Girotti M and Spencer RL:
Restraint-induced fra-2 and c-fos expression in the rat forebrain:
Relationship to stress duration. Neuroscience. 150:478–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe T, Morimoto A, Sakata Y, Tan N,
Morimoto K and Murakami N: Running training attenuates the ACTH
responses in rats to swimming and cage-switch stress. J Appl
Physiol (1985). 73:2452–2456. 1992.PubMed/NCBI
|
|
34
|
Keller-Wood ME and Dallman MF:
Corticosteroid inhibition of ACTH secretion. Endocr Revs. 5:1–24.
1984. View Article : Google Scholar
|
|
35
|
de Kloet ER, Joëls M and Holsboer F:
Stress and the brain: From adaptation to disease. Nat Rev Neurosci.
6:463–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X and Herbert J: Regional changes in
c-fos expression in the basal forebrain and brainstem during
adaptation to repeated stress: Correlations with cardiovascular,
hypothermic and endocrine responses. Neuroscience. 64:675–685.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dhabhar FS, McEwen BS and Spencer RL:
Adaptation to prolonged or repeated stress - comparison between rat
strains showing intrinsic differences in reactivity to acute
stress. Neuroendocrinology. 65:360–368. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miller GE, Chen E and Zhou ES: If it goes
up, must it come down? Chronic stress and the
hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull.
133:25–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seo JH, Kim TW, Kim CJ, Sung YH and Lee
SJ: Treadmill exercise during pregnancy ameliorates post-traumatic
stress disorder-induced anxiety-like responses in maternal rats.
Mol Med Rep. 7:389–395. 2013.PubMed/NCBI
|
|
40
|
O'Connor KA, Ginsberg AB, Maksimova E,
Wieseler Frank JL, Johnson JD, Spencer RL, Campeau S, Watkins LR
and Maier SF: Stress-induced sensitization of the
hypothalamic-pituitary adrenal axis is associated with alterations
of hypothalamic and pituitary gene expression. Neuroendocrinology.
80:252–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Axelrod J and Reisine TD: Stress hormones:
Their interaction and regulation. Science. 224:452–459. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Munck A, Guyre PM and Holbrook NJ:
Physiological functions of glucocorticoids in stress and their
relation to pharmacological actions. Endocr Rev. 5:25–44. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chrousos GP: Organization and integration
of the Endocrine System. Sleep Med Clin. 2:125–145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chrousos GP: The
hypothalamic-pituitary-adrenal axis and immune-mediated
inflammation. N Engl J Med. 332:1351–1362. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goshen I and Yirmiya R: Interleukin-1
(IL-1): A central regulator of stress responses. Front
Neuroendocrinol. 30:30–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsuwaki T, Eskilsson A, Kugelberg U,
Jönsson JI and Blomqvist A: Interleukin-1β induced activation of
the hypothalamus-pituitary-adrenal axis is dependent on
interleukin-1 receptors on non-hematopoietic cells. Brain Behav
Immun. 40:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Connor TJ, Brewer C, Kelly JP and Harkin
A: Acute stress suppresses pro-inflammatory cytokines TNF-alpha and
IL-1 beta independent of a catecholamine-driven increase in IL-10
production. J Neuroimmunol. 159:119–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Haas DA and George SR: Neuropeptide
Y-induced effects on hypothalamic corticotropin-releasing factor
content and release are dependent on noradrenergic/adrenergic
neurotransmission. Brain Res. 498:333–338. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Panossian A, Wikman G, Kaur P and Asea A:
Adaptogens stimulate neuropeptide Y and Hsp72 expression and
release in neuroglia cells. Front Neurosci. 6:62012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Asea A, Kaur P, Panossian A and Wikman KG:
Evaluation of molecular chaperons Hsp72 and neuropeptide Y as
characteristic markers of adaptogenic activity of plant extracts.
Phytomedicine. 20:1323–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sajdyk TJ, Fitz SD and Shekhar A: The role
of neuropeptide Y in the amygdala on corticotropin-releasing factor
receptor-mediated behavioral stress responses in the rat. Stress.
9:21–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz
SD, Dietrich A, Morin M, Gehlert DR, Urban JH and Shekhar A:
Neuropeptide Y in the amygdala induces long-term resilience to
stress-induced reductions in social responses but not
hypothalamic-adrenal-pituitary axis activity or hyperthermia. J
Neurosci. 28:893–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cullinan WE, Herman JP, Battaglia DF, Akil
H and Watson SJ: Pattern and time course of immediate early gene
expression in rat brain following acute stress. Neuroscience.
64:477–505. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hinoi E, Fujimori S, Yoneyama M and Yoneda
Y: Blockade by N-methyl-D-aspartate of elevation of activator
protein-1 binding after stress in rat adrenal gland. J Neurosci
Res. 70:161–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sabban EL, Liu X, Serova L, Gueorguiev V
and Kvetnansky R: Stress triggered changes in gene expression in
adrenal medulla: Transcriptional responses to acute and chronic
stress. Cell Mol Neurobiol. 26:845–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Umriukhin PE, Koplik EV, Grivennikov IA,
Miasoedov NF and Sudakov KV: Gene c-Fos expression in brain of rats
resistant and predisposed to emotional stress after intraperitoneal
injection of the ACTH(4–10)analog - semax. Zh Vyssh Nerv Deiat Im I
P Pavlova. 51:220–227. 2001.(In Russian). PubMed/NCBI
|
|
58
|
Rivest S and Rivier C: Stress and
interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene
expression in the hypothalamic PVN: Comparison between
Sprague-Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol.
6:101–117. 1994. View Article : Google Scholar : PubMed/NCBI
|