Introduction
Psoriasis is a common type of chronic inflammatory
dermatosis and a long-lasting autoimmune disease characterized by
patches of abnormal skin, that is characterized by skin lesions
including red, scaly patches, papules and plaques that usually
itch. The exact cause and mechanism of action of psoriasis are
unclear. It is not entirely a skin disorder and can have a negative
impact on many organ systems. They may vary in severity from small
and localized to complete body coverage. There are five main types
of psoriasis: plaque, guttate, inverse, pustular, and
erythrodermic. A previous study indicated that the levels of the
typical inflammatory signal nuclear factor-κB (NF-κB) are increased
in the lymphocytes of the skin lesions and peripheral blood of
patients with psoriasis (1). This
suggests that NF-κB plays an important role as a regulator in the
pathological mechanism of psoriasis (1). The zinc finger protein A20, also termed
tumor necrosis factor (TNF)-α induced protein-3 (TNFAIP3), is a
negative regulating protein of the NF-κB signaling pathway and
inhibits the activity of NF-κB; the expression of TNFAIP3 mRNA has
been found to be negatively associated with the severity of
psoriasis (2). It has been confirmed
that the vitamin D3 derivative calcipotriol acts as an effective
treatment for psoriasis by inhibiting the NF-κB signaling pathway
(3). However, the detailed mechanism
of this and the association between calcipotriol and zinc finger
protein A20 in psoriasis are not clear. Therefore, the aim of the
present study was to analyze the changes in the expression levels
of zinc finger protein A20 and NF-κB in the skin lesions of
patients with psoriasis following topical treatment with
calcipotriol in order to investigate the possible mechanism of the
zinc finger protein A20 in psoriasis and the effect of treatment
with calcipotriol.
Materials and methods
Materials
Calcipotriol ointment (0.05 mg/g) was purchased from
Bright Future Pharmaceutical Laboratories Ltd. (Hong Kong, China).
The rabbit anti-human A20 and NF-κB p65 polyclonal primary
antibodies were obtained from Abcam (cat no. ab16502, Cambridge,
MA, USA) and the horseradish-peroxidase (HRP)-labeled goat
anti-rabbit IgG secondary antibody was from Pierce Biotechnology,
Inc. (cat no. G-21234, Rockford, IL, USA). The quantitative BCA
protein kit and electrochemical luminescence (EasyBlot ECL)
developing system were also purchased from Pierce Biotechnology,
Inc. Protease inhibitor cocktail tablets were obtained from Roche
Diagnostics (Indianapolis, IN, USA). The MaxVision™
immunohistochemical staining kit and DAB staining solution were
purchased from Maixin Biotechnology Development Co., Ltd. (Fuzhou,
China). The upright Olympus BX53 microscope was from Olympus
Corporation (Tokyo, Japan).
Patients
A total of 26 patients with psoriasis vulgaris,
including 15 males and 11 females with an age range of 20–58 years
(mean, 30.56±9.78 years) and a disease history of 3–42 months
(mean, 10.32±4.61 months), were recruited for the present study.
All patients had a skin lesion area <30% of the total body
surface area and had not been treated with a systemic or topical
application of calcipotriol or any other anti-psoriasis medication
for the previous 4 weeks. Control samples were collected from the
normal skin at 0.5 cm from the edge of the pigmented nevus in 18
patients (11 males and 7 females) undergoing resection of pigmented
nevi in the outpatient department of the Third Affiliated Hospital
of Suzhou University (Changzhou, China). Erythema, scaling,
infiltrating hypertrophy and itch were measured by a clinician. The
age, gender and locations of the skin lesions were matched between
the patient and control groups. The present study was approved by
the Ethics Committee of the Third Affiliated Hospital of Suzhou
University and informed consent was obtained from the patients.
Calcipotriol administration
procedure
Calcipotriol ointment was topically administered to
the patients twice a day for 6 weeks. The Psoriasis Area and
Severity Index (PASI) was used to evaluate the severity of the skin
lesions (4) by sampling the skin
with a skin trephine prior to, during and following the
calcipotriol therapy.
Immunohistochemical staining
Following regular dewaxing, the slices were stained
using the MaxVision™ immunohistochemical staining kit
according to the manufacturer's instructions. The primary
antibodies used were rabbit anti-human A20 and NF-κB p65 polyclonal
antibodies at a dilution of 1:200 and the samples were incubated at
37°C for 12 h. They were washed with phosphate-buffered saline and
the slices were subsequently developed in DAB staining solution
followed by hematoxylin counterstaining at 37°C in a water bath for
2 h, differentiation with 0.1% hydrochloric acid, regular
dehydration and vitrification, and mounting with neutral balsam.
The cytoplasms and nuclei of the stained cells were observed under
an Olympus BX53 microscope for semi-quantitative analysis using a
combination of scores allocated for the percentage of positive
cells and the staining intensity. The staining intensity was
defined as follows: 0, negative; 1, light yellow; 2, tan; and 3,
dark brown. The percentage of positive cells was defined as
follows: 0, no positive cells; 1, ≤25%; 2, ~26–50%; 3, ~51–75% and;
4, >75%. The product of staining intensity and percentage of
positive cells was used to assess the overall staining result:
negative (−), ≤1; weak positive (+), 2 or 3; middle positive (++),
4 or 5; strong positive (+++), ≥6 and a result of + to +++ was
considered as positive expression (5,6).
Western blot analysis
The expression levels of A20 and NF-κB proteins were
measured with western blot analysis, using an improved version of a
previously used technique and glyceraldehyde 3-phosphate
dehydrogenase as a control (7). The
tissue samples were placed in 200 µl lysis buffer containing 0.1 M
pH 7.5 Tris-HCl, 1% NP-40 and 0.01% SDS, and fully ground in a
glass homogenizer following the addition of a protease inhibitor
cocktail tablet. The proteins were measured with a quantitative BCA
protein kit according to the manufacturer's instructions. The
extracted proteins were denatured by heating at 95°C for 5 min,
cooled on ice and loaded (10 µg/well) for electrophoresis on a 10%
SDS-PAGE gel. They were subsequently transferred to a PVDF membrane
(25 V for 1 h) that was soaked in 5% skimmed milk, and incubated in
0.1% Tris-buffered saline and Tween 20 (TBS-T) blocking buffer
(Pierce Biotechnology, Inc.) at 4°C overnight. The membrane was
incubated in a buffer containing the primary antibodies against A20
and NF-κB p65 (dilution, 1:1,000) for 1 h and rinsed with TBS-T
buffer 5 times, at 3–5 min each time. A buffer containing goat
anti-rabbit IgG labeled with HRP was subsequently added (1:5,000
dilution) and the solution was incubated at room temperature for 1
h. Following rinsing with TBS-T buffer and drying, the protein was
measured with the an ECL developing system according to the
manufacturer's instructions.
Briefly, the membrane was incubated with ECL
developing solution for 10–15 min and then a film was placed on the
PVDF membrane in a darkroom. The film was then developed using a
developing machine (Bio-RAD ChemiDox XRS, Bio-RAD, Hercules, CA,
USA) and the exposure time that created a well-developed film was
noted down for further runs of this procedure. The developed film
was scanned on a computer and the bands were analysed using
BandScan5.0 (http://soft.bio1000.com/show-149.html) (8).
Statistical analysis
All data are expressed as the mean ± standard
deviation and were analyzed with SPSS software, version 13.0 (SPSS,
Inc., Chicago, IL, USA). The intergroup means, rates and
correlations were compared with the Student's t-test, χ2
test and Spearman rank-order correlation analysis, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of the clinical
effectiveness rate and PASI score
At the end of week 6 of treatment with calcipotriol,
the erythema, scaling, infiltrating hypertrophy, itch and area of
the skin lesions was markedly improved; the effectiveness rate
(57.69%, 15/26) was significantly higher compared with that at the
end of week 4 (38.46%, 10/26) and week 2 (19.23%, 5/26;
χ2=8.12 and 9.06, respectively; P<0.05). Compared
with that prior to treatment (10.92±1.72), the PASI score at the
end of week 2 (8.86±2.42), week 4 (6.57±2.25) and week 6
(4.92±1.79) of treatment with calcipotriol was significantly
reduced (t=9.37, 10.54 and 12.43; P<0.05, 0.05 and 0.001,
respectively).
Effect of calcipotriol on the
expression levels of A20 and NF-κB in the skin lesions of patients
with psoriasis as evaluated by immunohistochemistry
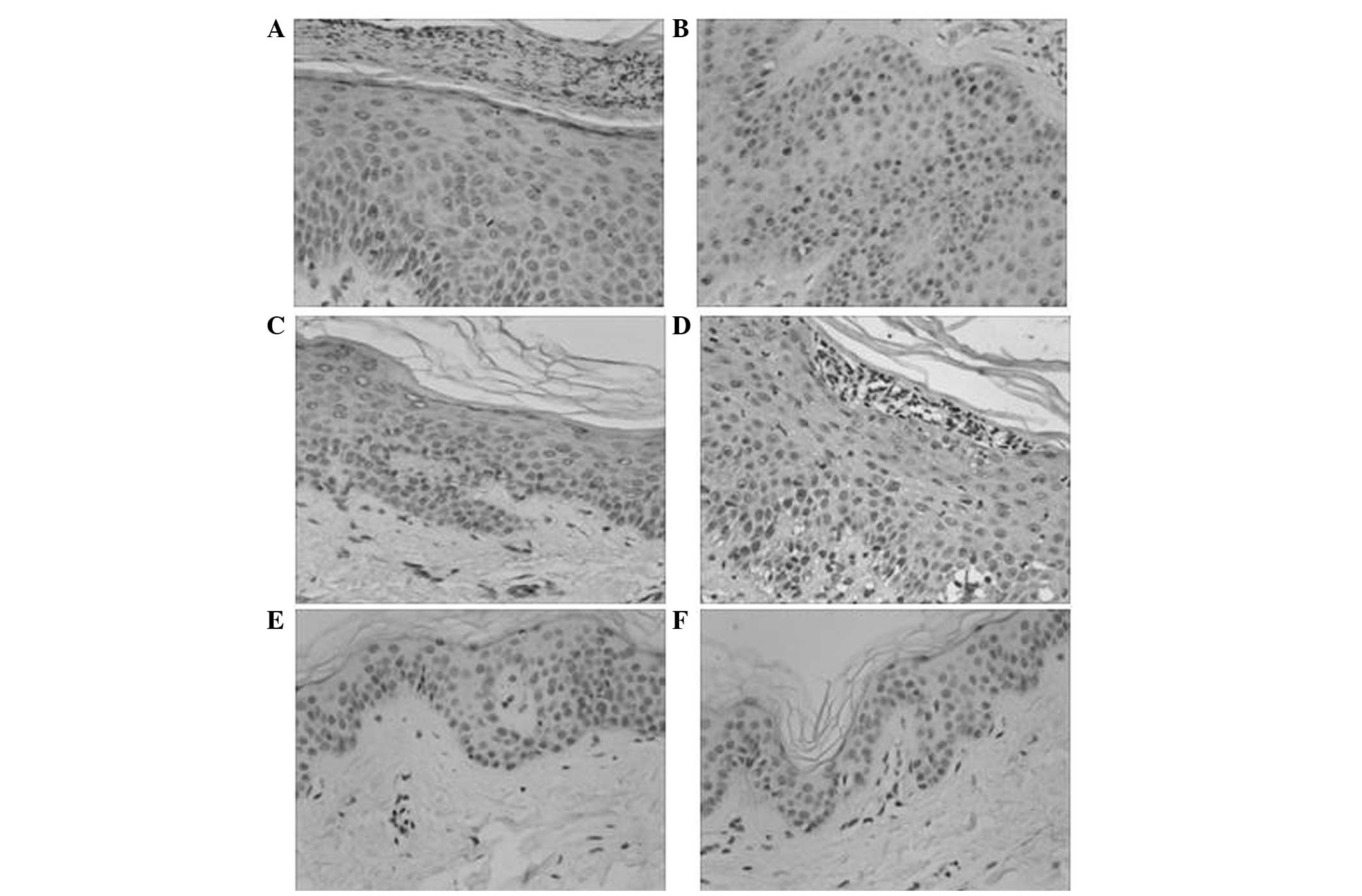
Immunohistochemical staining showed that A20 was
expressed in the layers of basal and prickle cells (Fig. 1), and was mainly located in the
membranes and cytoplasms of keratinocytes as tan or dark brown
colors. The expression levels of A20 in psoriasis skin lesions
prior to treatment with calcipotriol were elevated compared with
those in normal skin tissue (χ2=8.34; P<0.001). At
the end of week 6 of treatment, A20 expression levels were
significantly lower in the psoriasis skin lesions compared with the
same lesions prior to treatment (χ2=3.65; P<0.01).
NF-κB p65 was expressed in the entire epithelium layer, mainly in
the cytoplasms and nuclei of keratinocytes, which was exhibited as
dark brown staining in the nuclei and strong tan staining in the
cytoplasm. In normal skin tissue, the majority of cellular nuclei
were negative for NF-κB p65 although the cytoplasms were partially
positive (χ2=9.15; P<0.001). Following the 6-week
treatment with calcipotriol, NF-κB p65 expression levels were
significantly decreased in the psoriasis skin lesions when compared
with those in the lesions prior to treatment (χ2=4.17;
P<0.01).
Effect of calcipotriol on the
expression levels of A20 and NF-κB in the skin lesions of patients
with psoriasis as evaluated by western blotting
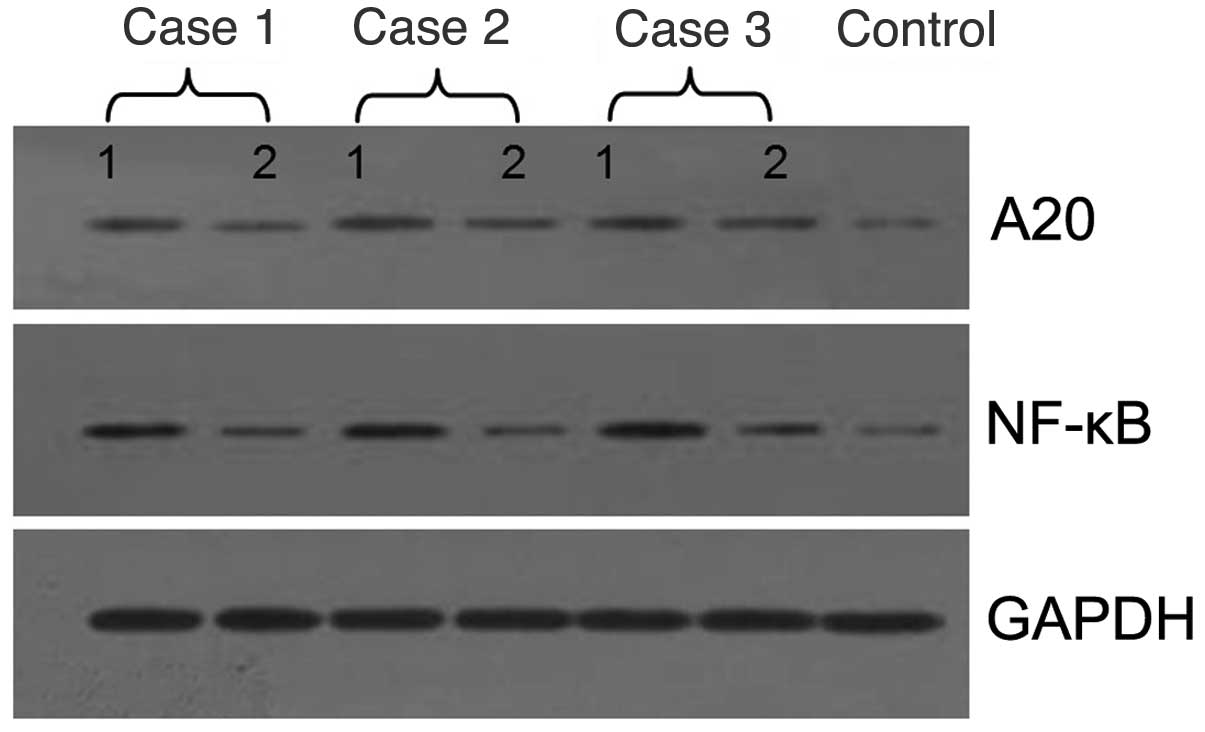
The results of the western blot analysis indicated
that the expression levels of A20 and NF-κB in the skin lesions of
patients with psoriasis prior to treatment were significantly
higher compared with those in the normal tissue (1:3.2 and 1:5.4,
respectively; Fig. 2), where 1 is
the intensity of the protein band in normal tissue and 3.2 and 5.4
are the intensities of the bands from the patients. However, they
were significantly decreased following 6 weeks of treatment with
calcipotriol (1:1.6 and 1:1.2, respectively; P<0.01).
Discussion
Psoriasis is a type of immune-mediated chronic
inflammatory dermatosis. It affects 1–3% of the global population
and is presented pathologically as hyperkeratosis, akeratosis,
hypertrophy in the prickle-cell layer and invasion of the skin by
inflammatory cells (9). A previous
study indicated that there is an upregulation of phosphorylated
NF-κB in psoriasis skin lesions when compared with normal skin
(1). As the key regulator in
inflammation, cellular proliferation, differentiation and
apoptosis, NF-κB is also considered to be the key regulator in the
pathology of psoriasis; multiple types of cells, chemokines and
cytokines associated with psoriasis are dependent on the activation
of NF-κB signaling (10). As an
example, the innate immune response protein toll-like receptor 2
(11) and caspase-5 (12) are able to recognize the upregulation
of pathogen-associated molecular patterns in psoriasis and promote
the fine regulation of proinflammatory cytokines through activation
of the downstream signaling of NF-κB.
Zinc finger protein A20 is a type of intracellular
zinc finger protein that is coded by the TNFAIP3 gene. There are
two κB motifs at the −54 and −66 bps of the A20 gene, whose
expression is mediated by NF-κB. The basal expression level of A20
is low in the majority of cells; NF-κB is activated by multiple
stimulations and is translocated into the nucleus where it binds
with the κB component of the TNFAIP3 promoter and enhances the
transcription of the TNFAIP3 gene. The expressed A20 induces the
destruction of the inhibitor of κB kinase γ (also known as the
NF-κB essential modulator) to modulate the NF-κB pathway through
ubiquitylation by negative feedback and also modulates TNF
signaling through deubiquitylation (13,14). The
features of ubiquitylation and deubiquitylation allow A20 to
specifically modulate the functional status of cells in order to
ensure the self-preservation of organs during inflammation, thereby
demonstrating dual anti-inflammatory and anti-apoptotic effects
(15). Notably, genome-wide
association studies found that the gene polymorphism of A20 and
ABIN-1 (also known as TNFAIP-3 interacting protein 1, TNIP1) was
closely associated with not only the development of psoriasis but
also the efficiency of TNF inhibitors, including infliximab,
adalimumab and etanercept, in the treatment of psoriasis (16,17).
Since A20 has ubiquitylation and deubiquitylation
functions, the present study investigated the expression changes of
A20 and NF-κB in the skin lesions of patients with psoriasis
vulgaris during treatment with calcipotriol. The results indicated
that the clinical effectiveness rate was 57.69% (15/26), the PASI
scores and the staining of A20 and NF-κB at the end of 6 weeks of
treatment were significantly lower compared with those prior to
treatment and the corresponding expression levels of proteins
decreased. These results suggest that A20 regulates NF-κB through
negative feedback and plays an important role in the treatment of
psoriasis with calcipotriol. The mechanism of A20 in the regulation
of apoptosis-associated factors or the signaling transduction of
keratinocytes in psoriasis requires further study, which may
uncover new therapeutic regimens and strategies.
Acknowledgements
This study was supported by the Youth Foundation of
the Natural Science Research Project of Jiangsu Province (no.
BK2012153) and the Psoriasis Vulgaris Foundation of Bright Future
Pharmaceutical Laboratories Ltd, Chinese Society of
Dermatology.
References
|
1
|
Lizzul PF, Aphale A, Malaviya R, Sun Y,
Masud S, Dombrovskiy V and Gottlieb AB: Differential expression of
phosphorylated NF-κB/RelA in normal and psoriatic epidermis and
downregulation of NF-κB in response to treatment with etanercept. J
Invest Dermatol. 124:1275–1283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang X, Tian H, Fan Y, Chen J, Song Y,
Wang S, Zhu F, Guo C, Zhang L and Shi Y: Expression of tumor
necrosis factor α-induced protein 3 mRNA in peripheral blood
mononuclear cells negatively correlates with disease severity in
psoriasis vulgaris. Clin Vaccine Immunol. 19:1938–1942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peric M, Koglin S, Dombrowski Y, Gross K,
Bradac E, Büchau A, Steinmeyer A, Zügel U, Ruzicka T and Schauber
J: Vitamin D analogs differentially control antimicrobial
peptide/‘alarmin’ expression in psoriasis. PLoS One. 4:e63402009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van de Kerkhof PC: The psoriasis area and
severity index and alternative approaches for the assessment of
severity persisting areas of confusion. Br J Dermatol. 137:661–662.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou XD, Yu JP, Liu J, Luo HS, Chen HX and
Yu HG: Overexpression of cellular FLICE-inhibitory protein (FLIP)
in gastric adenocarcinoma. Clin Sci (Lond). 106:397–405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massi D, Tarantini F, Franchi A,
Paglierani M, Di Serio C, Pellerito S, Leoncini G, Cirino G,
Geppetti P and Santucci M: Evidence for differential expression of
Notch receptors and their ligands in melanocytic nevi and cutaneous
malignant melanoma. Mod Pathol. 19:246–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan J, Liu XM, Lei TC and Xu SZ: Effects
of mutation in dopachrome tautomerase on melanosome maturation and
anti-oxidative potential in cultured melanocytes. Zhonghua Yi Xue
Za Zhi. 89:1707–1710. 2009.(In Chinese). PubMed/NCBI
|
|
8
|
BandScan5.0. http://soft.bio1000.com/show-149.htmlAccessed.
September 27–2013
|
|
9
|
Colombo GL, Di Matteo S, Bruno G,
Girolomoni G and Vena GA: Calcipotriol and betamethasone
dipropionate in the treatment of mild-to-moderate psoriasis: a
cost-effectiveness analysis of the ointment versus gel formulation.
Clinicoecon Outcomes Res. 4:261–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuruta D: NF-κB links keratinocytes and
lymphocytes in the pathogenesis of psoriasis. Recent Pat Inflamm
Allergy Drug Discov. 3:40–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Begon E, Michel L, Flageul B, Beaudoin I,
Jean-Louis F, Bachelez H, Dubertret L and Musette P: Expression,
subcellular localization and cytokinic modulation of Toll-like
receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation
in psoriatic skin. Eur J Dermatol. 17:497–506. 2007.PubMed/NCBI
|
|
12
|
Salskov-Iversen ML, Johansen C, Kragballe
K and Iversen L: Caspase-5 expression is upregulated in lesional
psoriatic skin. J Invest Dermatol. 131:670–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harhaj EW and Dixit VM: Deubiquitinases in
the regulation of NF-κB signaling. Cell Res. 21:22–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vereecke L, Beyaert R and van Loo G: The
ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of
immunopathology. Trends Immunol. 30:383–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wertz IE, O'Rourke KM, Zhou H, Eby M, et
al: De-ubiquitination and ubiquitin ligase domains of A20
downregulate NF-κB signalling. Nature. 430:694–699. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nair RP, Duffin KC, Helms C, Ding J,
Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, et
al: Collaborative Association Study of Psoriasis: Genome-wide scan
reveals association of psoriasis with IL-23 and NF-κB pathways. Nat
Genet. 41:199–204. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tejasvi T, Stuart PE, Chandran V, Voorhees
JJ, Gladman DD, Rahman P, Elder JT and Nair RP: TNFAIP3 gene
polymorphisms are associated with response to TNF blockade in
psoriasis. J Invest Dermatol. 132:593–600. 2012. View Article : Google Scholar : PubMed/NCBI
|