Introduction
Alzheimer's disease (AD) is a progressive
neurodegenerative disorder that is predominantly characterized by
senile plaques (SP), neurofibrillary tangles and regional neuronal
loss (1,2). The primary component of SP is β-amyloid
(Aβ), which has previously been demonstrated to damage synaptic
structures and induce neuronal cell death (1–4). In
addition, oxidative stress and ageing promote Aβ production, which
has been associated with the occurrence of AD (5). The p66shc adaptor protein is important
for the regulation of cellular senescence and oxidative stress
(6–12); under oxidative stress, the brain is
more susceptible to damage, as compared with other tissues and
organs. In the early stages of AD, oxidative stress occurs prior to
the appearance of pathological characteristics, and accelerates
neurodegeneration and Aβ formation; thus suggesting that oxidative
stress may be involved in the neuropathological process of AD
(1–6).
Anisomycin is an antibiotic produced by
Streptomyces griseolus, which is capable of binding to the
60S ribosomal subunit and blocking polypeptide chain elongation,
thereby inhibiting protein synthesis (13–17). As
a result, anisomycin exhibits some degree of toxicity towards all
cells, including inducing inflammatory responses and oxidative
stress injuries that are associated with neuron oxidation and
ageing (13–17). As such, anisomycin-induced cell
damage may be used to explore the relationship between
p66shc-mediated oxidative stress responses and the development and
progression of AD. By treating SH-SY5Y cells with anisomycin, Guo
et al (13) demonstrated that
anisomycin was able to enhance the production of Aβ and increase
the expression of presenilin-1 and other ageing-associated
proteins.
Astragaloside IV, cinnamic acid, paeoniflorin, and
gallic acid are small-molecule compounds with antioxidant
properties (2). In the present
study, a cell model was established in which Aβ deposition was
induced via exposure of SH-SY5Y cells to anisomycin. Subsequently,
the cell model was used to verify whether downregulation of p66shc
expression via small molecule compounds was able to alleviate
anisomycin-induced damage to SH-SY5Y human neuroblastoma cells.
Materials and methods
Cell culture
The SH-SY5Y human neuroblastoma cells obtained from
the Cell Bank of Shanghai Institutes for Biological Sciences
(Shanghai, China) were cultured in a routine manner using
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum at 37°C in a humidified incubator containing 5%
CO2. The culture medium was changed every 3 days. Upon
reaching confluence, the cells were trypsinized (Gibco; Thermo
Fisher Scientific, Inc.) and passaged. Cells in the logarithmic
growth phase were selected for subsequent experiments.
Model construction
The SH-SY5Y cells were treated with 0, 1, 3, 6, 12,
25, 50 or 100 µmol/l anisomycin (Shanghai Yuanye Bio Co., Ltd.,
Shanghai, China) for 0, 12, 24 or 48 h. The anisomycin
concentration and treatment duration for optimizing SH-SY5Y cell
damage and Aβ deposition were selected for construction of the AD
model. The cells were trypsinized and seeded into cell culture
plates. Four replicate wells were set up for each group. Upon
reaching 70 to 80% confluence, the culture medium was discarded and
the cells were incubated with either dimethyl sulfoxide (DMSO;
Gibco; Thermo Fisher Scientific, Inc.) or one of the various
concentrations of medium-diluted anisomycin. In addition, a control
group and blank control group were constructed: The control group
was treated with equal volumes of DMSO, and the blank control group
was cultured in equal volumes of medium for an additional 24 hours.
Cell morphology was observed under a microscope (DMI3000; Leica
Microsystems, Inc., Buffalo Grove, IL, USA). At specified times,
the cells were centrifuged (Allegra 64R; Beckman Coulter, Inc.,
Miami, FL, USA) at 1,000 × g for 5 min at 10°C, and the resulting
supernatant and cell pellets were collected for subsequent assays.
After examining relevant indices, an anisomycin concentration of 3
µmol/l was selected. Subsequently, cells were cultured for 0, 12,
24 and 48 h in the presence of anisomycin prior to being harvested
and centrifuged. The resulting supernatant and cell pellets were
collected for assays.
Screening of small molecule
compounds
The SH-SY5Y cells were treated with 500 µmol/l of
the small molecule compounds astragaloside IV, cinnamic acid,
paeoniflorin and gallic acid (all Shanghai Yuanye Bio Co., Ltd.).
Initially, SH-SY5Y cells cultured in petri dishes were subjected to
tryptic digestion, and subsequently harvested and seeded into cell
culture plates at a density of 1×104 cells/ml, after
which the cells were incubated with 3 µmol/l anisomycin for 48 h in
order to establish the AD model. Subsequently, the cells were
treated with 50 µmol/l small molecule compounds, which had been
diluted in culture medium. In addition, the control group
(DMSO-treated group) and blank control group (non-treated group)
were constructed. Neither the control group nor the blank control
group were treated with small molecule compounds, and the blank
control group did not receive anisomycin treatment; instead, an
equal volume of culture medium was added to the blank control
group, and the cells were cultured for another 24 h. Cell
morphology was observed under a phase contrast microscope (DMI3000;
Leica Microsystems, Inc.). At predetermined times, the cells were
centrifuged, and the resulting supernatant and cell pellets were
collected for future assays.
Real time-quantitative polymerase
chain reaction (RT-qPCR)
qPCR was conducted according to the method outlined
in previous studies (18,19). Briefly, the cell pellets were
collected following treatment, after which total RNA was extracted
from the cells grown in culture flasks using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and the concentration
(>10 µg/µl) and purity (OD260/280>1.80) of the
total RNA were determined. All RNA samples were stored at −80°C
prior to analysis. The RNA was reverse transcribed into cDNA using
a Reverse Transcription kit (TOYOBO, Co., Ltd., Osaka, Japan),
according to the manufacturer's instructions. The resulting cDNA
was stored at −20°C. RT-qPCR was performed using a MasterCycler
RealPlex4 RT-qPCR detection system from Eppendorf (Hamburg,
Germany), with the SYBR-Green RealTime PCR Master mix (TOYOBO Co.,
Ltd.) as the detection dye. RT-qPCR amplification was performed
over 40 cycles, with denaturation at 95°C for 15 sec and annealing
at 58°C for 45 sec, and a final extension step at 72°C for 42 sec.
The target cDNA was quantified using the relative quantification
method. Briefly, a comparative quantification cycle (Cq) was used
to determine gene expression levels relative to the 18S ribosomal
RNA (rRNA), which served as an internal control. The steady state
mRNA levels were reported as an n-fold difference relative to the
internal control. For each sample, the Cq values of the marker
genes were normalized using the following equation: ΔCq =
Cq(genes)-Cq(18S rRNA). The relative expression levels were
determined using the equation: ΔΔCq = ΔCq(sample
groups)-ΔCt(control group). The values used to plot the relative
expression of markers were calculated using the 2−ΔΔCq
values. The primers used were as follows: Forward
5′-GTAACCCGTTGAACCCCATT-3′, and reverse 5′-CCATCCAATCGGTAGTAGCG-3′
for 18S rRNA; and forward 5′-GTATGTGCTCACTGGCTTGC-3′, and reverse
5′-CTGACACTTTCAAAGCGGTG-3′ for p66.
Western blot analysis
Western blotting was performed according to a method
outlined in previous studies (18,19).
Briefly, the SH-SY5Y cells were removed from culture flasks using
cell scrapers and lysed in precooled (4°C) cell lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). The protein
concentration (50 µg/µl) was measured using the bicinchoninic acid
assay. Total protein extract (15 µl) was separated by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were then blocked with a solution
containing 10% calf serum, followed by four times washing for 15
min with Tris-buffered saline containing Tween 20 (TBST;
Sigma-Aldrich, St. Louis, MO, USA) at room temperature.
Subsequently, the membranes were incubated with primary rabbit
anti-human p66 (1:1,000; ab87633; Abcam, Shanghai, China) and
glyceraldehyde-3-phosphate dehydrogenase (1:1,000; 5174, Cell
Signaling Technology, Inc., Danvers, MA, USA) polyclonal antibodies
at 4°C overnight. Following extensive washing, the membranes were
incubated with secondary peroxidase-conjugated goat anti-rabbit
immunoglobulin G antibodies (1:1,000; sc-2004; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h. Following further
washing steps (15 min each) with TBST at room temperature, the
target proteins were visualized by enhanced chemiluminescence (ECL
kit; Pierce Biotechnology, Inc., Rockford, IL, USA) and exposure to
Kodak Biomax XAR-5 films (Sigma-Aldrich).
Quantification of Aβ1–42 levels by
ELISA
The cell pellets were suspended in cell lysis buffer
and a double-antibody sandwich ELISA was performed, according to
the manufacturer's instructions (Human Aβ1–42 ELISA kit; cat no.
h022931; Westang Bio, Shanghai, China). The primary antibody
working solution, enzyme-labeled antibody working solution,
substrate working solution and stop solution, were added
sequentially to the cell lysates. The Aβ1–42 concentration was
calculated according to the absorbance values of a standard
curve.
Quantification of levels of superoxide
dismutase (SOD), malondialdehyde (MDA), and acetylcholine
(Ach)
The SH-SY5Y cells and their supernatants were
collected, and cell pellets were suspended in buffer solution
(phosphate-buffered saline or physiological saline; 0.3–0.5 ml per
106 cells). Cells were lysed in an ice bath using
sonication, in which the tip of an ultrasonic probe was immersed in
the liquid. Enzyme and substrate working solutions were then added
sequentially. The absorbance of various concentrations of the
standard compounds was determined. The SOD, MDA and Ach
concentrations were calculated by comparing the absorbance of the
samples to those of the standards. The SOD Assay kit, MDA Assay
kit, and Ach Assay kit were all purchased from Westang Bio
(Shanghai, China).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences were evaluated using the Student's t-test and GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Anisomycin increases the expression
levels of Aβ1–42 in SH-SY5Y cells
The levels of Aβ1–42 in the SH-SY5Y cell pellets and
culture supernatants were determined using an ELISA. The Aβ1–42
levels in the SH-SY5Y cells reached a maximum value following
treatment of the cells with 3 µmol/l anisomycin (Fig. 1A and B), and this occurred after 48 h
of stimulation (Fig. 1C and D).
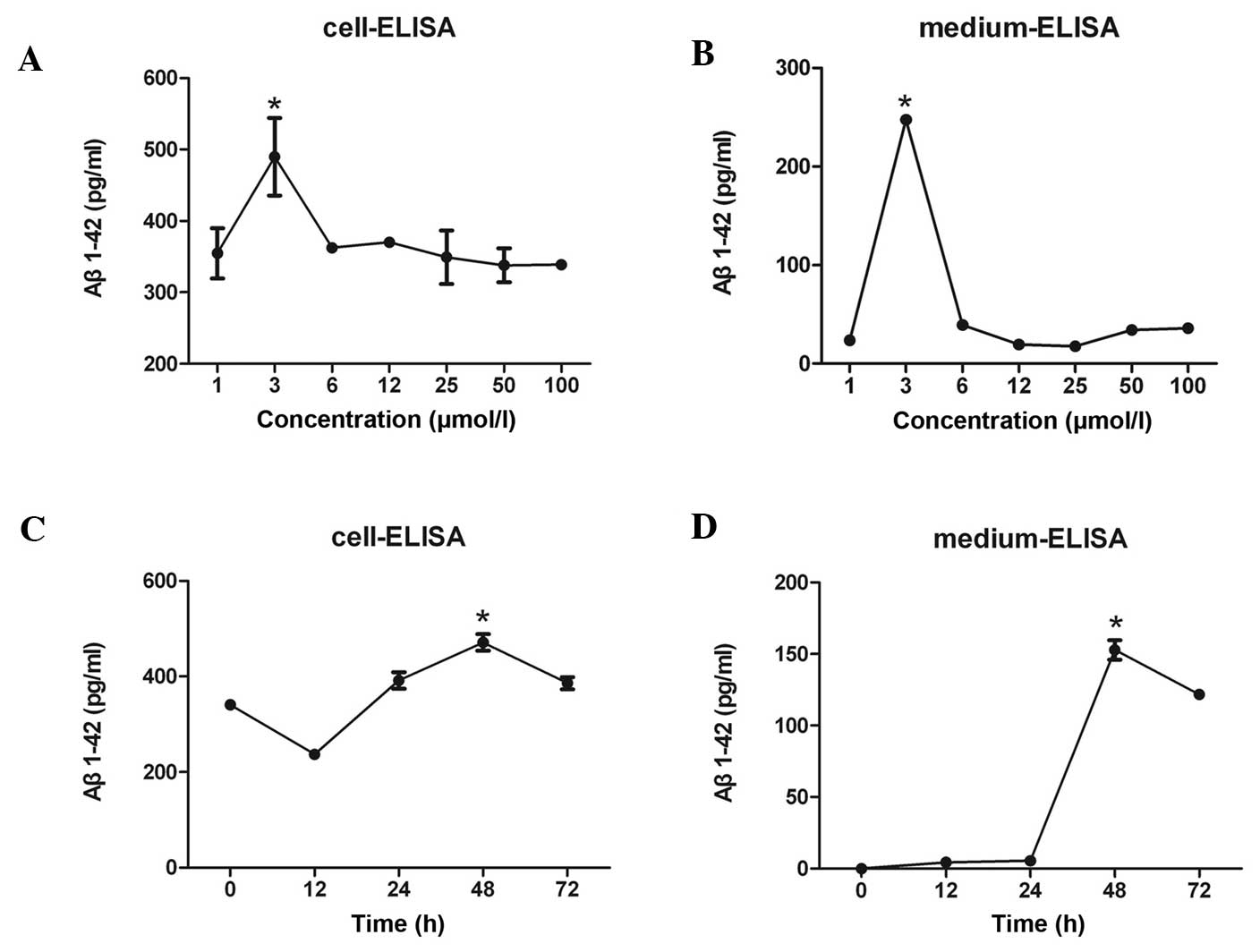 | Figure 1.(A) Various concentrations of Aβ1–42
were detected in SH-SY5Y human neuroblastoma cells following
treatment of the cells with 1, 3, 6, 12, 25, 50 or 100 µmol/l
anisomycin. Aβ1–42 levels in SH-SY5Y cells reached a maximum value
following treatment of the cells with 3 µmol/l anisomycin. (B)
Various concentrations of secreted Aβ1–42 were detected in the cell
culture medium following treatment of SH-SY5Y cells with 1, 3, 6,
12, 25, 50 or 100 µmol/l anisomycin. The level of secreted Aβ1–42
in cell culture supernatant reached a peak following treatment of
SH-SY5Y cells with 3 µmol/l anisomycin. (C) Treatment of SH-SY5Y
cells with 3 µmol/l anisomycin for 0, 12, 24, 48 or 72 h resulted
in various Aβ1–42 concentrations in the cells. The Aβ1–42
concentration in SH-SY5Y cells reached a maximum value following
stimulation with anisomycin for 48 h. (D) Stimulation of SH-SY5Y
cells with 3 µmol/l anisomycin for 0, 12, 24, 48 or 72 h resulted
in various levels of secreted Aβ1–42 into the cell culture
supernatant. The level of secreted Aβ1–42 in cell culture
supernatant reached a peak following 48 h of treatment with 3
µmol/l anisomycin. Data are presented as the mean ± standard
deviation of triplicate experiments. *P<0.05 vs. 0 h. Aβ,
β-amyloid. |
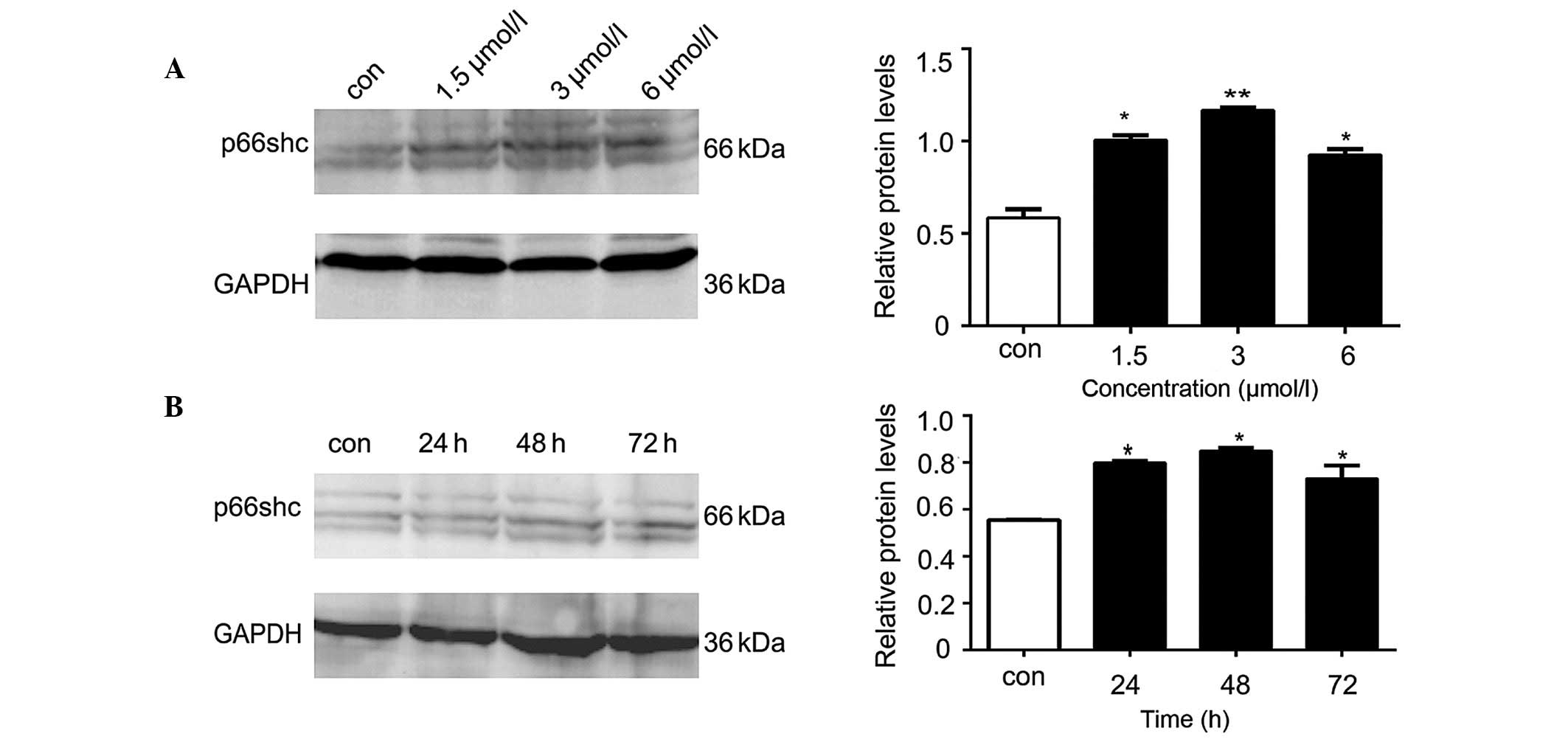
Anisomycin increases the expression
levels of p66shc in SH-SY5Y cells
The western blot analysis demonstrated that the
protein expression levels of p66shc in the SH-SY5Y cells
significantly increased following treatment with various
concentrations of anisomycin (1.5, 3 or 6 µmol/l), as compared with
the control group (P<0.05; Fig.
2A). The increase in p66shc protein expression levels was most
significant when the cells were treated with 3 µmol/l anisomycin
(P<0.01; Fig. 2A). Compared with
the p66shc levels at 0 h, the protein expression levels of p66shc
were significantly increased at various time points (24, 48 and 72
h) following treatment of SH-SY5Y cells with 3 µmol/l anisomycin
(P<0.05; Fig. 2B). The p66shc
protein expression levels peaked after 48 h of treatment with 3
µmol/l anisomycin (P<0.01; Fig.
2B).
Anisomycin increases the mRNA
expression levels of p66shc in SH-SY5Y cells
The results of the qPCR demonstrated that the mRNA
expression levels of p66shc were significantly increased in the
SH-SY5Y cells following treatment with 3 µmol/l anisomycin, in a
time-dependent manner (P<0.01; Fig.
3A), as compared with the mRNA expression levels at 0 h. The
differences in the threshold cycle (Ct) values between p66shc mRNA
and 18S rRNA were compared. The mRNA expression levels of p66shc
increased 2.5 fold following treatment of SH-SY5Y cells with 3
µmol/l anisomycin for 48 h, as compared with the mRNA expression
levels at 0 h. (P<0.01; Fig. 3A).
These results suggest that anisomycin-induced damage in SH-SY5Y
cells may be associated with significantly increased p66shc
expression.
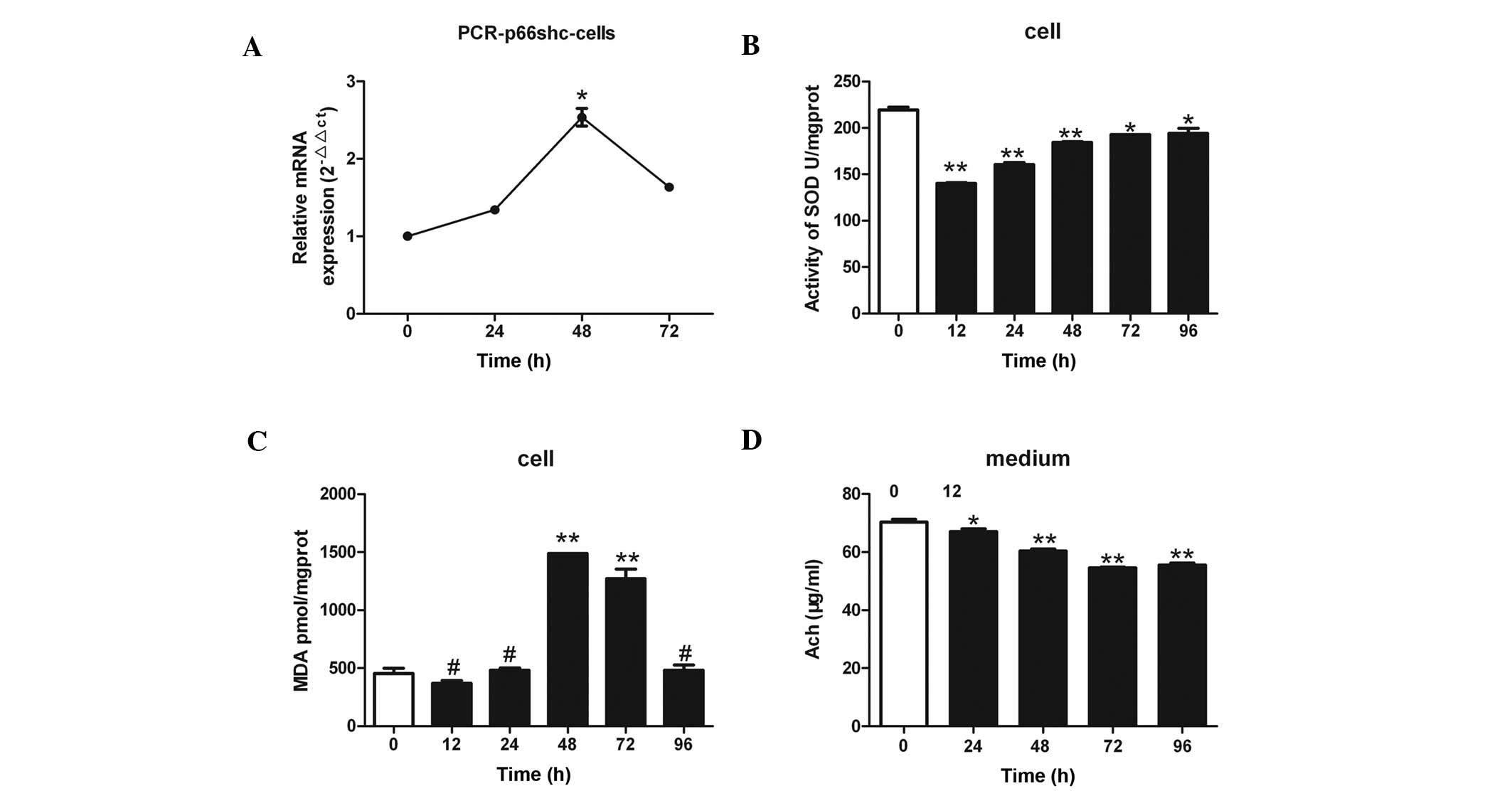 | Figure 3.(A) Treatment of SH-SY5Y human
neuroblastoma cells with 3 µmol/l anisomycin was associated with
significant changes in p66shc mRNA expression levels. The p66shc
mRNA expression levels increased by 2.5-fold after 48 h of
anisomycin treatment, as compared with 0 h. (B) Treatment of
SH-SY5Y cells with 3 µmol/l anisomycin for various lengths of time
(0, 12, 24, 48, 72 and 96 h) resulted in significantly reduced SOD
activity in the cells, as compared with 0 h of treatment. The
decrease in SOD activity was more evident after 12, 24 and 48 h of
anisomycin treatment. (C) SH-SY5Y cells were treated with 3 µmol/l
anisomycin for various lengths of time (0, 12, 24, 48, 72 and 96
h). The protein expression levels of MDA were significantly
increased after 48 and 72 h of treatment, as compared with 0 h. (D)
SH-SY5Y cells were treated with 3 µmol/l anisomycin for various
lengths of time (0, 24, 48, 72 and 96 h) and the levels of Ach
secreted from the cells were significantly reduced after 24, 48, 72
and 96 h, as compared with 0 h. Data are presented as the mean ±
standard deviation of triplicate experiments. #P>0.05
vs. 0 h; *P<0.05 vs. 0 h; **P<0.01 vs. 0 h. SOD, superoxide
dismutase; MDA, malondialdehyde; Ach, acetylcholine. |
Anisomycin decreases the activity of
SOD and increases the levels of MDA in SH-SY5Y cells
SOD activity was significantly reduced in a
time-dependent manner in the SH-SY5Y cells following treatment with
3 µmol/l anisomycin, as compared with SOD activity at 0 h
(P<0.01; Fig. 3B), and this was
most pronounced after 12, 24, and 48 h of anisomycin treatment
(P<0.01). In addition, the protein expression levels of MDA in
the SH-SY5Y cells were significantly increased after 48 and 72 h of
anisomycin treatment, as compared with 0 h of treatment (P<0.01;
Fig. 3C).
Anisomycin reduces the levels of Ach
in cell culture supernatants
The levels of Ach secreted by the SH-SY5Y cells
significantly decreased over time following treatment of the cells
with 3 µmol/l anisomycin, as compared with the levels at 0 h
(P<0.01; Fig 3D). The most
significant decreases in Ach secretion occurred following 48, 72
and 96 h treatment with anisomycin (P<0.01).
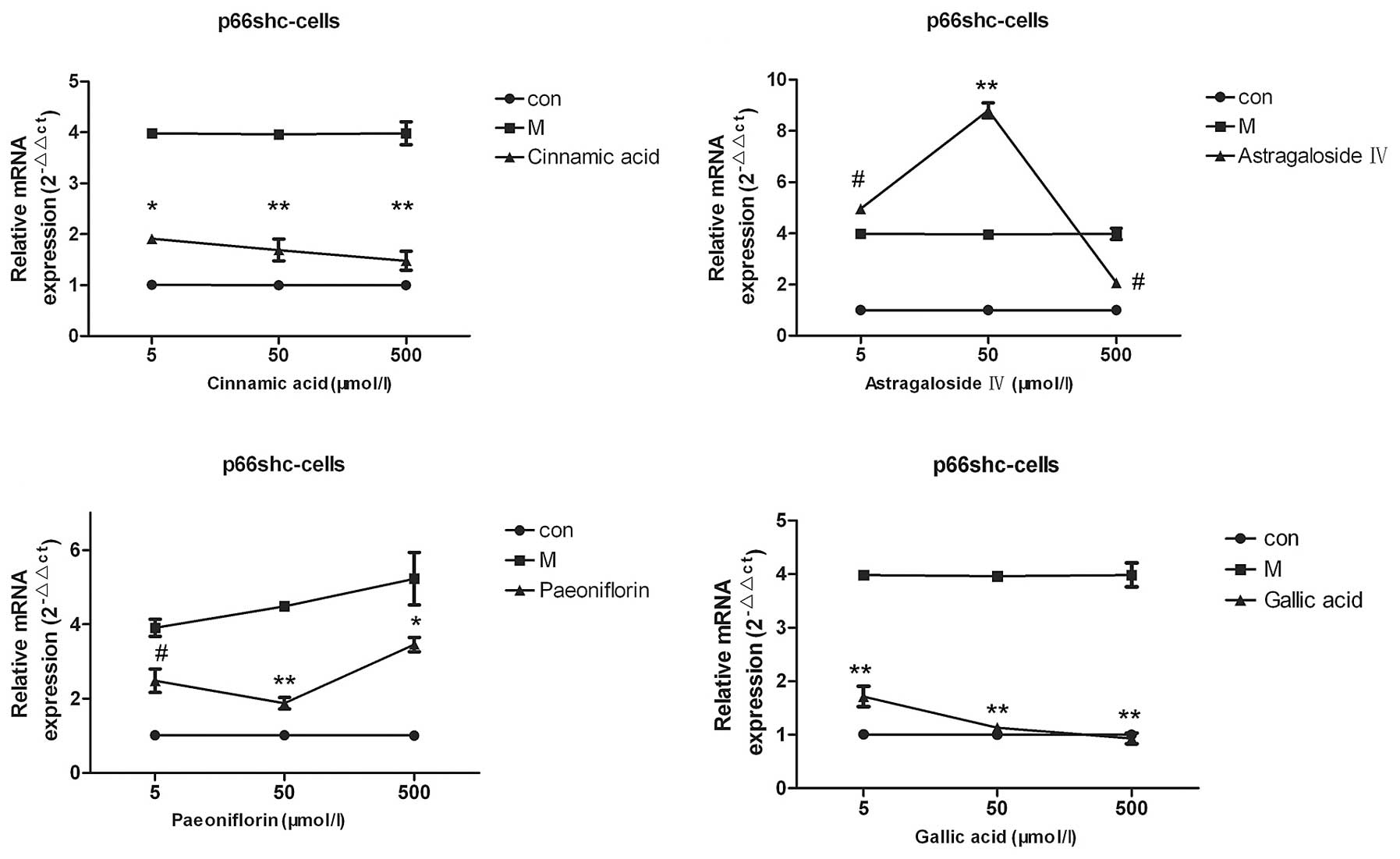
Screening of the small molecule
compounds cinnamic acid, astragaloside IV, paeoniflorin and gallic
acid
The results of the qPCR demonstrated that various
concentrations (5, 50 or 500 µmol/l) of cinnamic acid, paeoniflorin
and gallic acid were able to reduce the p66shc mRNA expression
levels, as compared with the model group (P<0.05; Fig. 4), whereas, only 500 µmol/l
astragaloside IV was able to decrease the p66shc mRNA expression
levels in the cell model (P<0.05). Of the four compounds
screened, cinnamic acid and gallic acid exerted the most pronounced
effects and decreased the p66shc mRNA expression levels to those
that resembled the mRNA expression levels of the cells in the
normal group (Fig. 4).
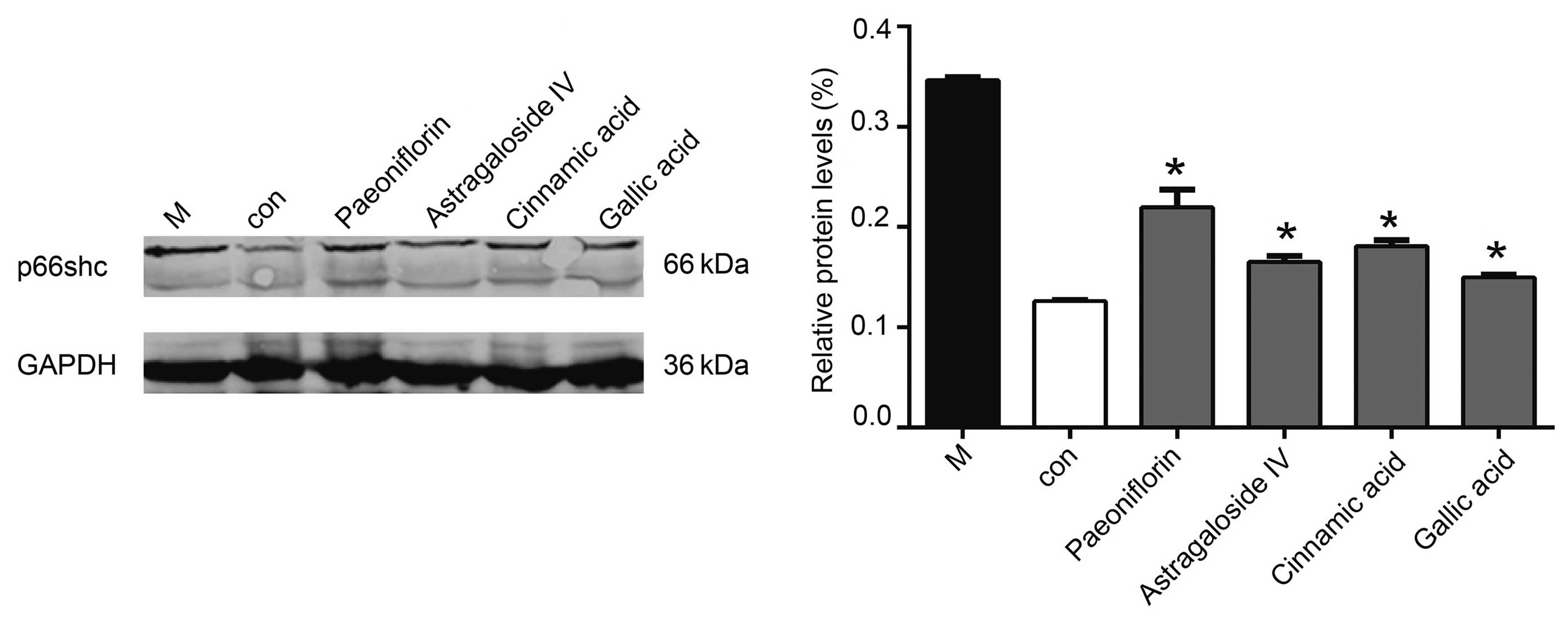
Western blotting demonstrated that 50 µmol/l
cinnamic acid, paeoniflorin or gallic acid reduced the protein
expression levels of p66shc, although the effects of gallic acid
were the most significant (P<0.05; Fig. 5).
As compared with the model group, treatment with 50
µmol/l cinnamic acid and paeoniflorin exerted no significant
effects on the secretion of Aβ1–42 from the SH-SY5Y cells, whereas
treatment with 50 µmol/l astragaloside IV or gallic acid was
associated with decreased Aβ1–42 secretion levels; however, the
effects of gallic acid were more significant (P<0.05; Fig. 6A). As compared with the model group,
treatment of the cells with 50 µmol/l cinnamic acid, astragaloside
IV, paeoniflorin or gallic acid increased the SOD activity and Ach
expression levels in the model cells, with the effects of gallic
acid being particularly significant (P<0.01; Fig. 6B and C).
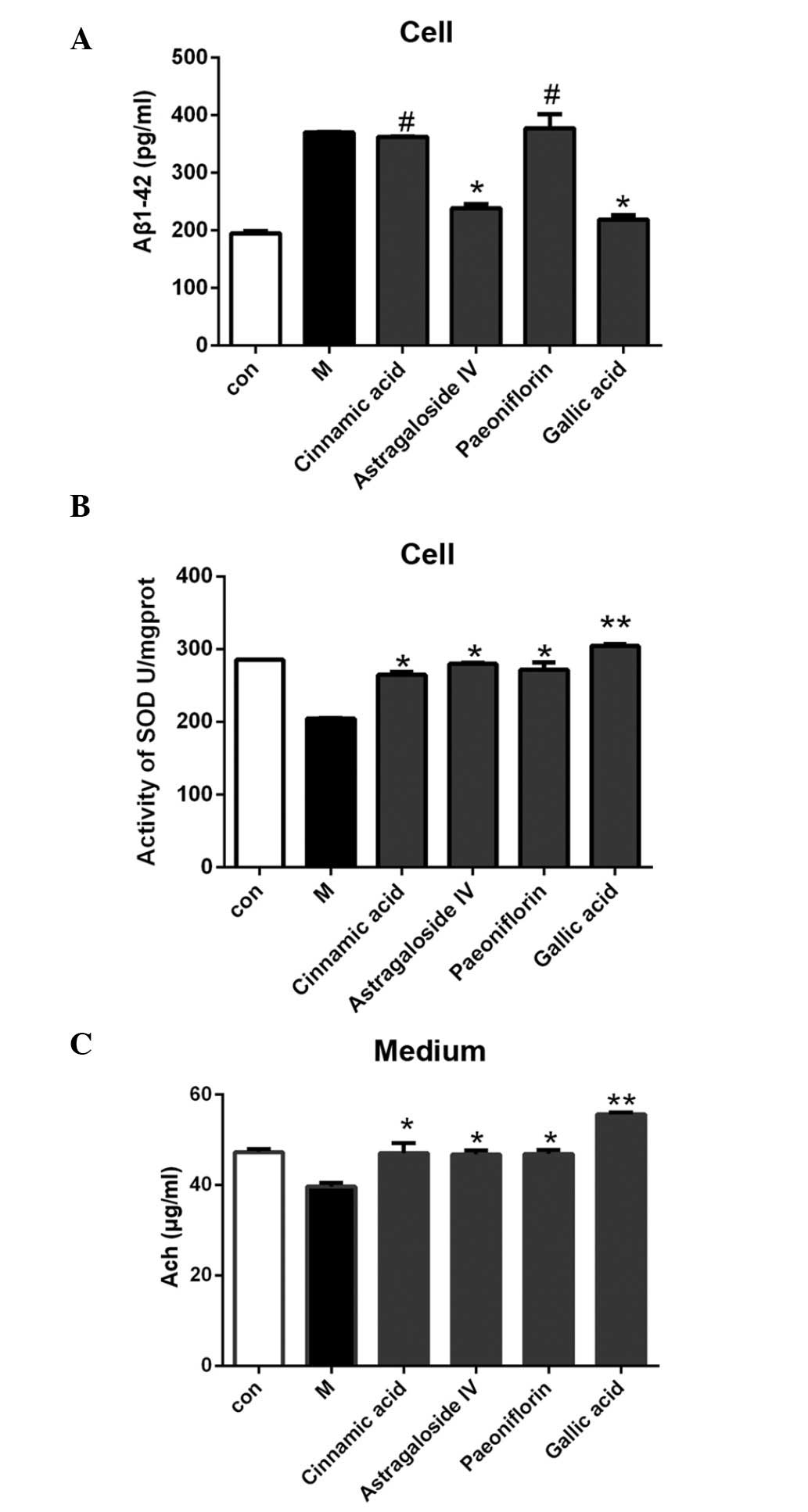 | Figure 6.(A) As compared with the cells in the
model group, Aβ secretion was reduced following treatment with 50
µmol/l astragaloside IV or gallic acid, with the effects of gallic
acid being more significant. (B) The cell model was treated with 50
µmol/l cinnamic acid, astragaloside IV, paeoniflorin or gallic
acid. As compared with the untreated model group, the SOD activity
was significantly elevated following treatment with the small
molecule compounds. (C) As compared with the untreated model group,
the Ach level was elevated after treatment with 50 µmol/l cinnamic
acid, astragaloside IV, paeoniflorin, or gallic acid. The effects
of gallic acid on Aβ secretion, SOD activity, and Ach secretion,
were the most significant of all the small molecule compounds. Data
are presented as the mean ± standard deviation.
#P>0.05 vs. the model group; *P<0.05 vs. the model
group; **P<0.01 vs. the model group. Aβ, β-amyloid; SOD,
superoxide dismutase; Ach, acetylcholine, Con, the control group;
M, the model group. |
Discussion
The pathogenesis of AD is very complex. Currently,
the Aβ hypothesis, in which Aβ destroys synaptic structures and
impairs hippocampal long-term potentiation, resulting in a decline
in learning and memory functions, is widely accepted (17). Extracellular Aβ is associated with
neuronal dysfunction by permeabilising lipid bilayers, reducing
membrane fluidity and inducing the inflammatory cascade and
oxidative stress (1–6).
Previous studies have associated the loss of p66shc
with reduced levels of reactive oxygen species and oxidative stress
responses in the brain, improved spatial learning and memory, and
prevention of ageing-induced behavioral changes (7–13,20,21).
In addition, p66shc has been associated with an increased lifespan,
which is predominantly reflected in the reduction of brain atrophy,
the maintenance of behavioral plasticity and the increase in the
overall level of brain-derived neurotrophic factor (22). Furthermore, Sone et al
(23) demonstrated a positive
correlation between the gene expression levels of p66shc and
ageing, and this correlation was particularly evident in the
brain.
The levels of p66shc have been associated with
oxidation and ageing of the nervous system (10). AD is a neurodegenerative disorder, in
which Aβ formation represents an important mechanism underlying its
pathogenesis; therefore, compounds that are able to reduce the
production of Aβ in neurons and block cell damage may be considered
in the prevention and treatment of patients with AD.
The results of the present study demonstrated that
anisomycin-induced neuronal damage increased Aβ1–42 secretion, and
this was associated with concomitant upregulation of p66shc
expression, an increase in the cellular response to oxidative
stress, and a reduction in neurotransmitter production. Thus
suggesting that anisomycin may induce neuronal damage and increase
cellular deposition of Aβ1–42 via an oxidative stress pathway.
Among the four small molecule compounds examined in
the present study, gallic acid was able to downregulate p66shc
expression in the SH-SY5Y cells, and reduce anisomycin-induced Aβ
deposition, enhance SOD activity and increase Ach secretion. These
results suggested that gallic acid was able to reduce
anisomycin-induced Aβ deposition in the SH-SY5Y cells via the
downregulation of p66shc expression. In addition, cinnamic acid,
astragaloside IV and paeoniflorin were able to suppress the
expression of p66shc, and were able to attenuate anisomycin-induced
Aβ deposition. Astragaloside IV reduced Aβ secretion from cells,
possibly via mechanisms other than downregulation of p66shc.
Although cinnamic acid and paeoniflorin were unable to directly
decrease Aβ secretion, they did reduce the oxidative stress
responses to varying extents and enhanced neurotransmitter
production; thus suggesting that they were able to exhibit minor
neuroprotective effects, although these were less significant than
those detected for gallic acid.
In conclusion, the present study demonstrated that
treatment of SH-SY5Y human neuroblastoma cells with anisomycin was
associated with nerve damage and increased Aβ secretion. In
addition, gallic acid was able to downregulate p66shc expression in
SH-SY5Y cells, which may have reduced anisomycin-induced Aβ
deposition; thus suggesting that gallic acid exerted protective
effects on SH-SY5Y cells, which may be considered a novel
therapeutic strategy in the treatment of patients with AD.
Acknowledgements
The present study was supported by a grant from The
National Natural Science Foundation of China (grant nos. 81373706,
81373619 and 81202811).
References
|
1
|
Götz J, Deters N, Doldissen A, Bokhari L,
Ke Y, Wiesner A, Schonrock N and Ittner LM: A decade of tau
transgenic animal models and beyond. Brain Pathol. 17:91–103. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattson MP: Pathways towards and away
fromA lzheimer's disease. Nature. 430:631–639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minter MR, Taylor JM and Crack PJ: The
contribution of neuro-inflammation to amyloid toxicity in
Alzheimer's disease. J Neurochem Oct. 28:2015.(Epub ahead of
print).
|
|
4
|
Wimo A: Long-term effects of A lzheimer's
disease treatment. Lancet Neurol. doi:
http://dx.doi.org/10.1016/S1474-4422(15)00302-6. PubMed/NCBI
|
|
5
|
Mawuenyega KG, Sigurdson W, Ovod V,
Munsell L, Kasten T, Morris JC, Yarasheski KE and Bateman RJ:
Decreased clearance of CNS beta-amyloid in Alzheimer's disease.
Science. 330:17742010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Migliaccio E, Giorgio M, Mele S, Pelicci
G, Reboldi P, Pandolfi PP, Lanfrancone L and Pelicci PG: The p66shc
adaptor protein controls oxidative stress response and life span in
mammals. Nature. 402:309–313. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan W, Gao L, Zeng W, Hu Y, Wang G, Li M,
Zhou J, Ma X, Tian X and Yao J: Activation of the SIRT1/p66shc
antiapoptosis pathway via carnosic acid-induced inhibition of
miR-34a protects rats against nonalcoholic fatty liver disease.
Cell Death Dis. 6:e18332015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Priami C, De Michele G, Cotelli F,
Cellerino A, Giorgio M, Pelicci PG and Migliaccio E: Modelling the
p53/p66Shc Aging Pathway in the Shortest Living Vertebrate
Nothobranchius Furzeri. Aging Dis. 6:95–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Gao D, Wang H, Li X, Yang J, Yan X,
Liu Z and Ma Z: Negative feedback loop between p66Shc and ZEB1
regulates fibrotic EMT response in lung cancer cells. Cell Death
Dis. 6:e17082015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhat SS, Anand D and Khanday FA: p66Shc as
a switch in bringing about contrasting responses in cell growth,
Implications on cell proliferation and apoptosis. Mol Cancer.
14:762015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perrini S, Tortosa F, Natalicchio A,
Pacelli C, Cignarelli A, Palmieri VO, Caccioppoli C, De Stefano F,
Porro S, Leonardini A, et al: The p66Shc Protein Controls Redox
Signaling and Oxidation-Dependent DNA Damage in Human Liver Cells.
Am J Physiol Gastrointest Liver Physiol: Sep. 3:2015.(Epub ahead of
print).
|
|
12
|
Sampaio SF, Branco AF, Wojtala A,
Vega-Naredo I, Wieckowski MR and Oliveira PJ: p66Shc signaling is
involved in stress responses elicited by anthracycline treatment of
rat cardiomyoblasts. Arch Toxicol Aug. 30:2015.(Epub ahead of
print).
|
|
13
|
Guo X, Wu X, Ren L, Liu G and Li L:
Epigenetic mechanisms of amyloid-β production in anisomycin-treated
SH-SY5Y cells. Neuroscience. 194:272–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pena RR, Pereira-Caixeta AR, Moraes MF and
Pereira GS: Anisomycin administered in the olfactory bulb and
dorsal hippocampus impaired social recognition memory consolidation
in different time-points. Brain Res Bull. 109:151–157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sorg BA, Todd RP, Slaker M and Churchill
L: Anisomycin in the medial prefrontal cortex reduces
reconsolidation of cocaine-associated memories in the rat
self-administration model. Neuropharmacology. 92:25–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dubue JD, McKinney TL, Treit D and Dickson
CT: Intrahippocampal Anisomycin Impairs Spatial Performance on the
Morris Water Maze. J Neurosci. 35:11118–11124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hardy J and Selkoe DJ: The amyloid
hypothesis of Alzheimer's disease: Progress and problems on the
road to therapeutics. Science. 297:353–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou G, Du X, Duan T and Liu T: Application
of a NotI subtraction and methylation specific genome subtractive
hybridization technique in the detection of genomic DNA methylation
differences between hydatidiform moles and villi. Mol Med Rep.
7:77–82. 2013.PubMed/NCBI
|
|
19
|
Chen Q, Qiu C, Huang Y, Jiang L, Huang Q,
Guo L and Liu T: Human amniotic epithelial cell feeder layers
maintain iPS cell pluripotency by inhibiting endogenous DNA
methyltransferase 1. Exp Ther Med. 6:1145–1154. 2013.PubMed/NCBI
|
|
20
|
Berry A, Greco A, Giorgio M, Pelicci PG,
de Kloet R, Alleva E, Minghetti L and Cirulli F: Deletion of the
lifespan determinant p66(Shc) improves performance in a spatial
memory task, decreases levels of oxidative stress markers in the
hippocampus and increases levels of the neurotrophin BDNF in adult
mice. Exp Gerontol. 43:200–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berry A, Capone F, Giorgio M, Pelicci PG,
de Kloet ER, Alleva E, Minghetti L and Cirulli F: Deletion of the
life span determinant p66Shc prevents age-dependent increases in
emotionality and pain sensitivity in mice. Exp Gerontol. 42:37–45.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berry A and Cirulli F: The p66(Shc) gene
paves the way for healthspan: Evolutionary and mechanistic
perspectives. Neurosci Biobehav Rev. 37:790–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sone K, Mori M and Mori N: Selective
upregulation of p66-Shc gene expression in the liver and brain of
aged rats. Arch Gerontol Geriatr. 55:744–748. 2012. View Article : Google Scholar : PubMed/NCBI
|