Introduction
Hypogonadism is a highly prevalent disease in
middle-aged men and is associated with an increased risk of
numerous chronic diseases, including metabolic syndrome,
osteoporosis and obesity, which significantly affect quality of
life (1). Male hypogonadism can
chiefly be divided into two forms; these are fetal-onset male
hypogonadism and late-onset hypogonadism (LOH). Fetal-onset male
hypogonadism is associated with disorders of sexual development or
pubertal development (2). Associated
with advanced age, LOH is a clinical and biochemical syndrome with
characteristic low serum testosterone levels, amongst other
symptoms (3). Previous studies have
indicated that LOH has a significant effect upon quality of life,
and affects the function of multiple organ systems (4,5).
Testosterone replacement treatment (TRT) was first
approved in 1972 by the U.S. Food and Drug Administration as a
treatment for the symptoms of male hypogonadism; currently, TRT is
prescribed for men diagnosed with androgen deficiency to alleviate
symptoms and improve quality of life (5). Previous studies that have assessed the
impact of TRT on bone mineral density, body weight, bone mass,
total lean body mass, metabolic syndrome and Aging Male Symptoms
(AMS) over the past decade have been highly inconsistent (6–21); thus,
it remains unclear whether testosterone therapy is beneficial with
regard to these features in men with hypogonadism. Furthermore,
controversy remains over whether TRT is safe for long-term
treatment due to the concern that higher serum testosterone
presents an increased risk for prostate cancer, which has been a
widely accepted hypothesis for ~70 years (22). In the present study, a meta-analysis
was performed to evaluate the efficacy and safety of TRT in men
with hypogonadism, which may resolve a number of the current
controversies in the use of this drug.
Materials and methods
Eligibility criteria
Inclusion criteria were established prior to a
literature search, following the guidelines from the Quality of
Reporting of Meta-Analyses conference (23). Only placebo-controlled, randomized
controlled trials (RCTs) of men with testosterone deficiency that
compared TRT-treated with placebo-treated patients were included.
All included studies were required to provide treatment of the
subjects for at least 6 months. Studies examining TRT therapy for
men with cancer, benign prostatic hyperplasia (BPH) and diabetes
were excluded, in addition to studies of men with a severe organic
disease, a mental disease diagnosed by a psychiatrist (for
instance, major depression currently being treated with
antidepressant medication), Parkinson disease or heart failure.
Search strategy
A literature search was performed in December 2014
using the databases of Medline (www.ncbi.nlm.nih.gov/pubmed), Embase (www.elsevier.com/solutions/embase-biomedical-research),
Elsevier (www.sciencedirect.com) and the Cochrane Library
(www.cochranelibrary.com). The Medline
and Embase searches included only a free-text protocol using the
term ‘testosterone’ within the ‘Title’ and ‘Abstract’ fields of the
records. Furthermore, the search strategy employed the following
limitations: Human subjects, English language publications, RCT
publication type and publication time between January 2004 and
December 2014. Searches of the Cochrane Library used the same
free-text protocol using the terms ‘testosterone’ and ‘hypogonadal’
within the ‘Title’ and ‘Abstract’ fields of the records, applying
no limits. All studies published between January 2004 and December
2014 were considered. A total of 1,435 records were initially
collated in this study, with 272 records retrieved from the
Elsevier database and 1,158 records from the Medline database and
Embase. A total of 5 records were retrieved from the Cochrane
Library. The records were independently reviewed by 3 authors in
order to identify the studies comparing testosterone and control
treatments. Furthermore, other, relevant studies cited in the
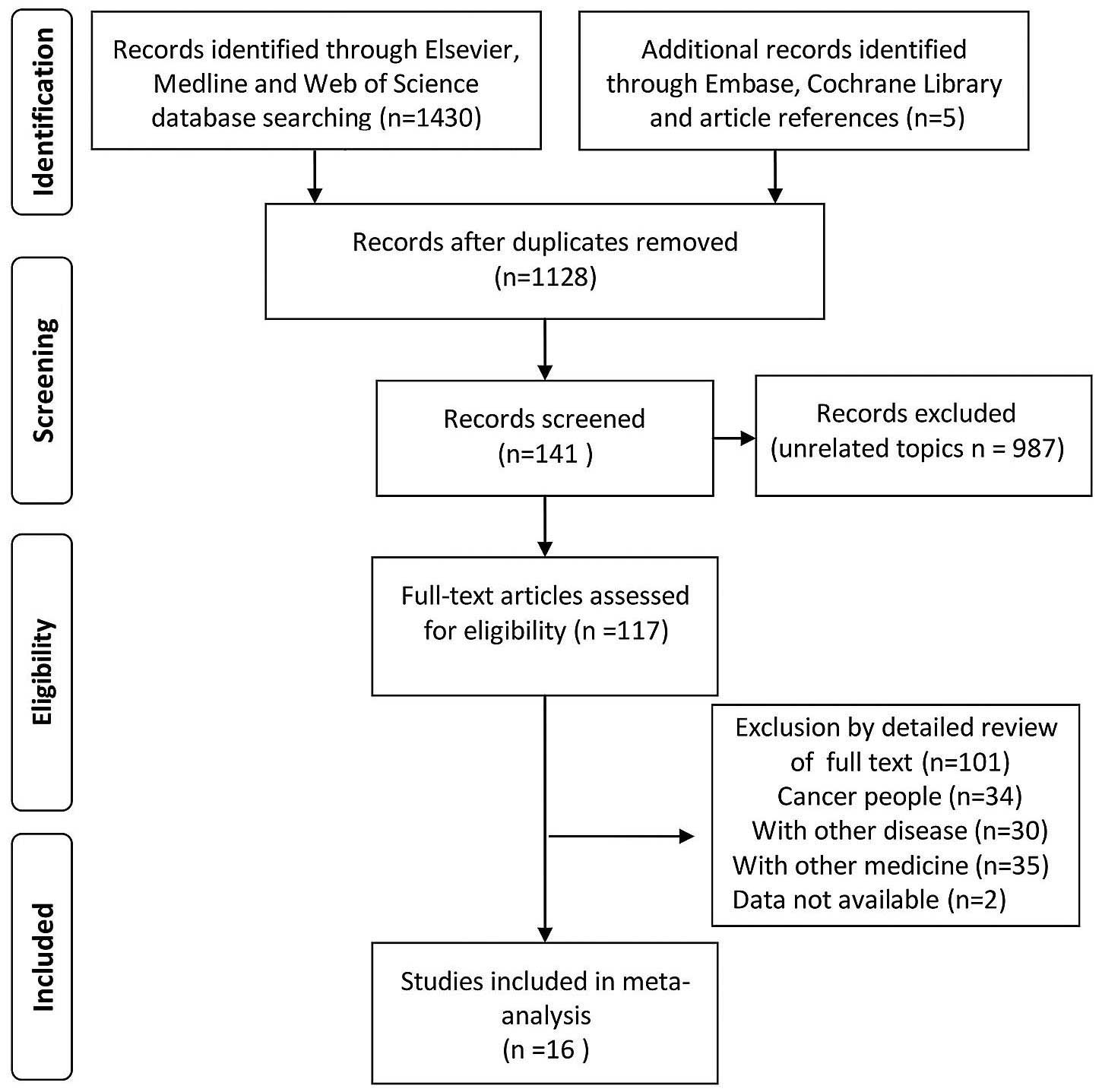
reference lists of the selected studies (Fig. 1) were also evaluated.
Study selection
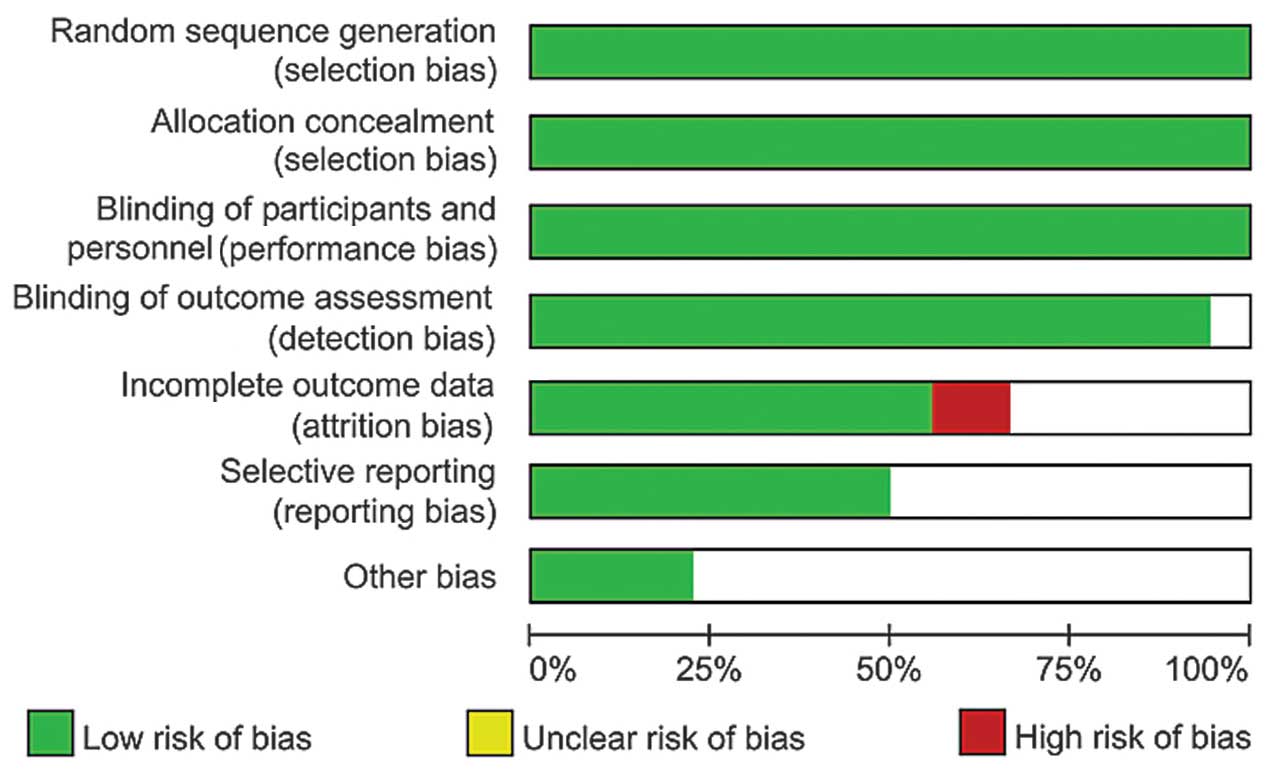
The quality of the randomized trials was assessed
based on methods as follows: Method of randomization, method of
blinding, allocation concealment, evidence of selective reporting
and incomplete outcome data. Studies that were deemed of
high-quality by consensus among study authors (Fig. 2) were included.
Data extraction
The first outcomes of interest were associated with
the efficacy of TRT, including the AMS score, body weight (in kg),
body mass index (BMI), bone mineral density (in g/cm2),
total lean body mass (in kg), total fat mass (in kg) and total
cholesterol (in mg/dl). AMS score was determined based on the
responses to the AMS questionnaire (www.aging-males-symptoms-scale.info/documents/question.pdf),
which investigated 17 different symptoms. The second outcomes of
interest were associated with the safety of TRT, including the
prostate-specific antigen (PSA) level (in ng/ml), International
Prostate Symptom Scores (IPSS), prostate volume (in ml) and the
nature of adverse events (mild to moderate and serious). Data
extraction was independently conducted by 2 investigators, and a
third reviewer would make a judgment when disagreement arose
regarding eligibility, as described previously (24).
Statistical analysis
Statistical analyses were independently performed by
2 authors who were not involved in data extraction. Q-test was used
to measure inter-study heterogeneity. I2 metric was used
to quantify heterogeneity, which is independent of the number of
studies included in the cumulative analysis. The I2
values were 0–100%, with higher values denoting a greater degree of
heterogeneity. I2<25% reflects a small level of
inconsistency, and I2>50% reflects significant
inconsistency. Data were pooled using a fixed-effects model. In
studies demonstrating heterogeneity, a sensitivity analysis was
conducted in order to establish the cause of heterogeneity.
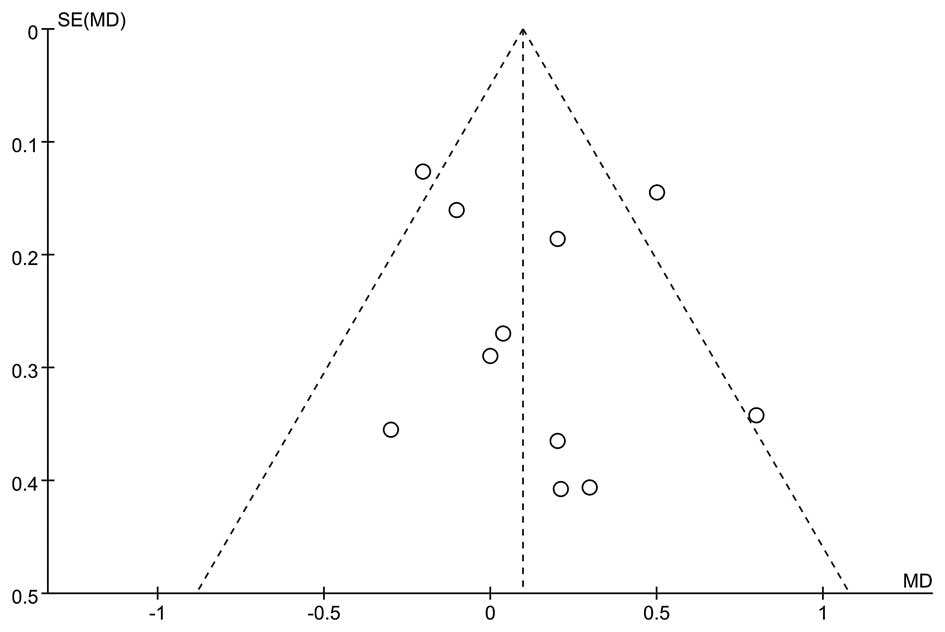
Potential publication bias was identified using a funnel plot,
which would suggest a publication bias through asymmetry of the
plot. All analyses were performed using RevMan version 5.3
(Cochrane, London, UK). All P-values were calculated using
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Subgroup and sensitivity analysis
To investigate the causes of heterogeneity, subgroup
analyses were made according to the method of administration of
testosterone (injection, oral, transdermal) and duration of
treatment (6 months vs. >6 months).
Results
Identification of data
A total of 1,435 references were identified in the
initial database search. As shown in Table I, through an abstract and full text
review, 16 RCTs that met the study criteria were identified. A
total of 1,921 patients were randomized across the 16 studies
(Table I). All 16 RCTs were
double-blinded. A total of 7 RCTs provided treatment for 6 months,
with the remainder providing treatment for 1 year. A funnel plot
revealed no evidence of publication bias (Fig. 3).
 | Table I.Study and patient
characteristics. |
Table I.
Study and patient
characteristics.
|
|
| Sample size, n |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author, year
(ref.) | Country | Ex | C | Inclusion
criteria | Exclusion of PSA
levels | Duration of
intervention | Dosage and
administration method | Main outcomes |
|---|
| Amory et al,
2004 (8) | USA | 24 | 24 | TT<350 ng/dl,
Y>65 | >4.0 ng/ml | 36 months | 200 mg TE IM, every
2 weeks | Bone mineral
density, prostate volume, PSA |
| Cavallini et
al, 2004 (9) | Italy | 40 | 45 | FT<6 pg/ml
Y>60, with symptoms of androgen decline | >4.0 ng/ml | 6 months | TU 160 mg/day,
PO | PSA, prostate
volume |
| Page et al,
2005 (10) | USA | 24 | 24 | TT<350 ng/dl,
Y>65 | >4.0 ng/ml | 36 months | 200 mg TE IM, every
2 weeks | Weight, total
cholesterol |
| Marks et al,
2006 (11) | USA | 21 | 19 | TT< 300 ng/dl,
Y=44–78 with symptoms | 10.0 ng/ml | 6 months | 150 mg TE IM, every
2 weeks | IPSS, prostate
volume, PSA |
| Vaughan et
al, 2007 (12) | USA | 24 | 23 | TT<350 ng/dl,
Y>65 | >4.0 ng/ml | 3 years | 200 mg TE IM, every
2 weeks | PSA, prostate
volume |
| Emmelot-Vonk et
al, 2008 (7) | Netherlands | 113 | 110 | TT<13.7 nmol/l
Y=60–80 | Y=60–69, >4.5
µg/l Y>70, >6.5 µg/l | 6 months | 160 mg/day TU,
PO | BMI, body weight,
total cholesterol, total lean body mass, total fat mass, IPSS, BMD,
adverse event, PSA, prostate volume |
| Aversa et
al, 2010 (13) | Italy | 40 | 10 | TT<3.0 ng/ml,
with metabolic syndrome, Y=45–65 | Age-adjusted
elevated PSA | 24 months 12
weeks | 1,000 mg TU IM,
every | BMI, total
cholesterol |
| Aversa et
al, 2010 (14) | Italy | 32 | 10 | TT<3.20 ng/ml,
with metabolic syndrome, Y=50–65 | Age-adjusted
elevated PSA | 12 months | 1,000 mg TU IM,
every 12 weeks | BMI, total
cholesterol, AMS score, IPSS, prostate volume |
| Idan et al,
2010 (15) | USA | 55 | 55 | Healthy,
Y>50 | >4.0 g/l | 24 months | 70 mg DHT daily,
transdermal | BMI, total
cholesterol, prostate volume, IPSS, PSA, mild to moderate adverse
event, total lean body mass, BMD |
| Kalinchenko et
al, 2010 (16) | Russia | 105 | 65 | TT<12.0 nM, with
metabolic syndrome, Y=35–70 | >4.0 g/l | 30 weeks | 1,000 mg TU IM,
every every 12 weeks | BMI, body weight,
total cholesterol, IPSS, prostate volume, PSA |
| Srinivas-Shankar
et al, 2010 (17) | New Zealand | 130 | 132 | TT<345 ng/dl,
presence of frailty, Y>65 | >4.0 g/l | 6 months | 50 mg/day
testosterone gel, transdermal | AMS score, total
lean body mass, total fat mass, total cholesterol, IPSS, PSA, mild
to moderate adverse event, serious adverse event |
| Kaufman et
al, 2011 (18) | USA | 234 | 40 | TT<300 ng/dl,
Y=18.80 | >2.5 g/l | 6 months | 1.62% testosterone
gel, serum total testosterone | Mild.to.moderate
adverse event, serious adverse event |
| Shigehara et
al, 2011 (19) | Japan | 23 | 23 | TT<11.8 pg/ml,
with BPH | >2.0 g/l | 12 months | 250 mg TE IM, every
4 weeks | AMS score, IPSS,
PSA |
| Behre et al,
2012 (6) | Germany | 183 | 179 | TT<15 nmol/l,
Y=50.80 with symptoms of testosterone deficiency | >4.0 g/l | 6 months | Hydroalcoholic 1%
testosterone gel (50 mg) daily, transdermal | AMS score, BMD,
PSA, total lean body mass, total fat mass, bone mass, body weight
serious adverse event |
| Frederiksen et
al, 2012 (20) | Denmark | 20 | 18 | TT<7.3 nmol/l,
Y=60.78 | >3.0 g/l | 6 months | Hydroalcoholic 1%
testosterone gel (50 mg) daily, transdermal | BMI, body weight,
total cholesterol, total lean body mass, total fat mass, PSA |
| Ho et al,
2012 (21) | Malaysia | 60 | 60 | TT>12 nmol/l,
Y>40 | >4.0 g/l | 12 months | 1,000 mg TU IM,
every 12 weeks | AMS score, serious
adverse event, mild to moderate adverse event |
Efficacy
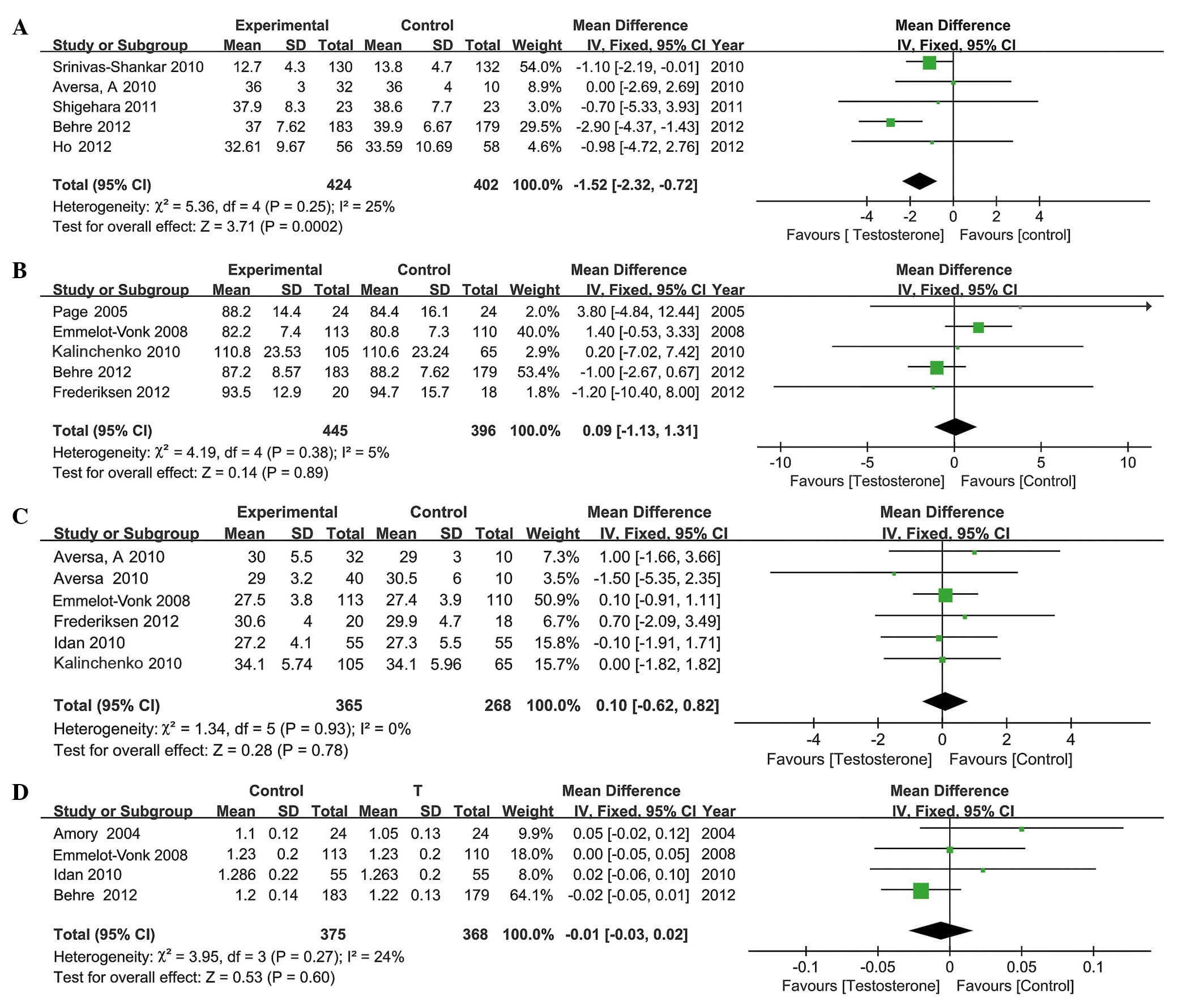
AMS score
A total of 5 RCTs, involving 826 participants (424
in the testosterone group and 402 in the control group), included
AMS scores. The present data revealed that the AMS scores improved
significantly following TRT. The mean decrease in AMS score was
1.52 (95% CI, 0.72 to 2.32; P=0.0002; Fig. 4A) following TRT. Additional analyses
indicated that AMS was significantly improved in short-term studies
with transdermal administration (within the same studies), but not
in long-term studies with administration via injection. The average
decrease in AMS score was 1.74 (95% CI, 0.86 to 2.61; P=0.0001) in
short-term and transdermal administration studies, and 0.40 (95%
CI, −2.37 to 1.57; P=0.69) in long-term and injection studies.
Body weight
A total of 5 RCTs, involving 841 participants (445
in the testosterone group and 396 in the control group), included
body weight data. No significant difference was identified in body
weight between the control and the TRT group; the mean difference
between these groups was 0.09 (95% CI, −1.13 to 1.31; P=0.89;
Fig. 4B). Subgroup analyses
indicated that there was no significant change to body weight
during the short-term (MD, 0; 95% CI, −1.25 to 1.26; P=0.99) or the
long-term (MD, 1.68; 95% CI, −3.86 to 7.22; P=0.55) studies
following TRT. Subgroup analyses also indicated that there were no
significant changes to body weight associated with administration
via injection (MD, 1.68; 95% CI, −3.86 to 7.22; P=0.55),
transdermal administration (MD, −1.01, 95% CI, −2.65 to 0.64;
P=0.23) or oral administration (MD, 1.4; 95% CI, −0.53 to 3.33;
P=0.38).
BMI
BMI measurements were included in 6 RCTs, involving
a total of 633 participants (365 in the testosterone group and 268
in the control group). No significant decrease was reported in BMI
following TRT; the mean difference between the two groups was 0.10
(95% CI, −0.62 to 0.82; P=0.78; Fig.
4C). The results of the subgroup analyses revealed a mean
increase of 0.17 (95% CI, −0.78 to 1.12; P=0.73) during short-term
studies and 0.01 (95% CI, −1.1 to 1.12; P=0.98) during long-term
studies. Subgroup analyses also indicated that there were no
significant differences in BMI following administration by
injection (MD, 0.08; 95% CI, −1.32 to 1.48; P=0.91), transdermal
administration (MD, 0.14; 95% CI, −1.38 to 1.66; P=0.86) or oral
administration (MD, 0.10; 95% CI, −0.62 to 0.82; P=0.85).
Bone mineral density
A total of 4 RCTs, involving 743 participants (375
in the testosterone group and 368 in the control group), included
the bone mineral density (BMD) of patients. TRT did not increase
BMD in men with hypogonadism compared with control patients. The
mean difference between the two groups was −0.01 (95% CI, −0.03 to
0.02; P=0.60; Fig. 4D). Additional
analysis of patients' responses to TRT administered with differing
duration and administration methods revealed no statistically
significant differences associated with duration of treatment
(short-term: MD, −0.01; 95% CI, −0.04 to 0.01; P=0.31; long-term:
MD, 0.05; 95% CI, −0.02 to 0.12; P=0.17), or with different
administration methods (injection: MD, 0.05; 95% CI, −0.02 to 0.12;
P=0.17; transdermal: MD, −0.02; 95% CI, −0.04 to 0.01; P=0.26; and
oral: MD, 0.0; 95% CI, −0.05 to 0.05; P=0.60) of TRT treatment.
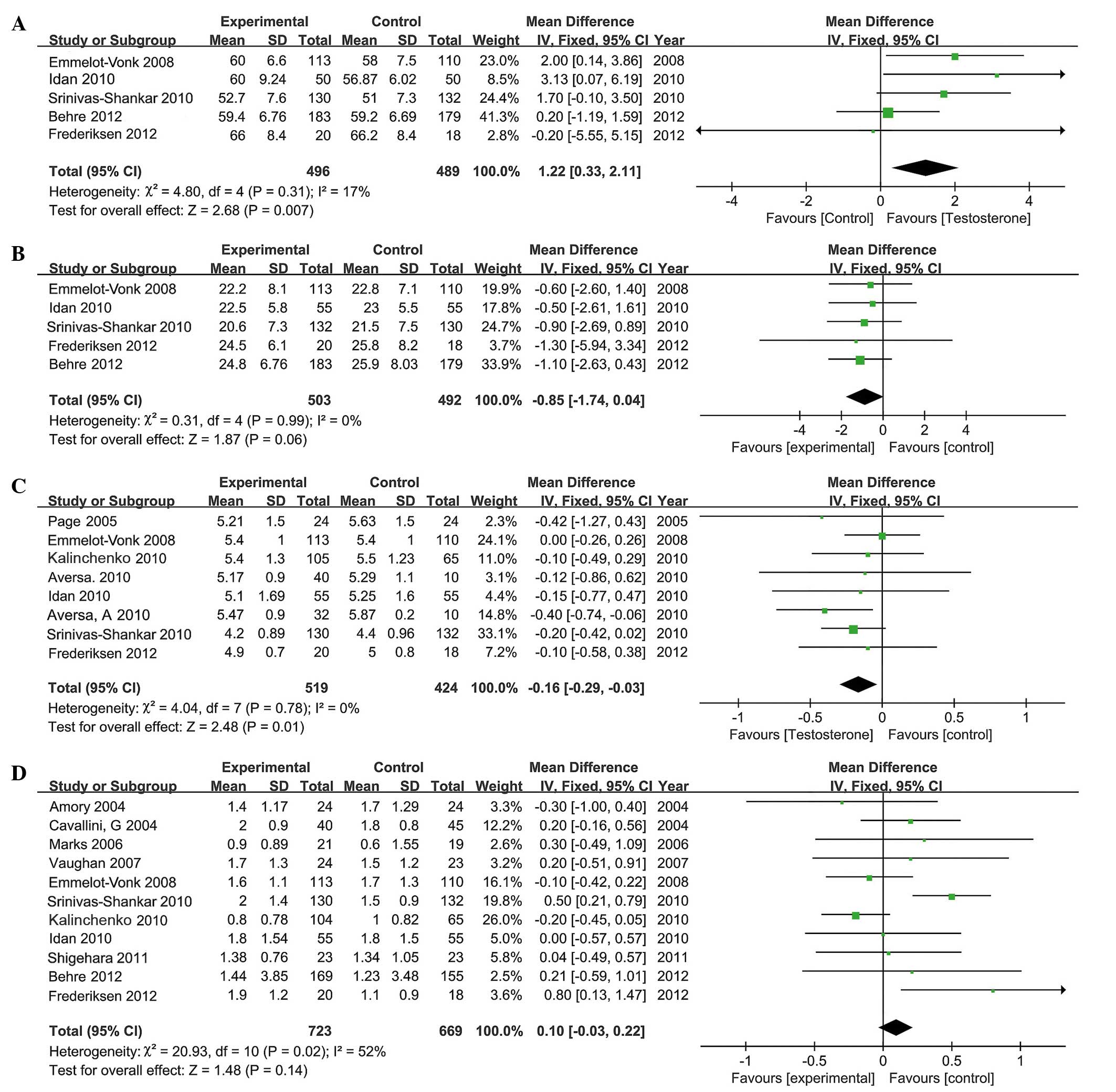
Total lean body mass
A total of 5 RCTs, involving 985 participants (496
in the testosterone group and 489 in the control group), reported
upon total lean body mass. Lean body mass was significantly
increased following TRT; the mean difference between the two groups
was 1.22 (95% CI, 0.33 to 2.11; P=0.007; Fig. 5A). Additional analysis of patients'
response to TRT administered for different durations and via
different methods indicated that TRT significantly increased lean
body mass during short-term (MD, 1.04; 95% CI, 0.11 to 1.97;
P=0.03) and long-term (MD, 3.13; 95% CI, 0.07 to 6.19; P=0.04)
treatment. However, no significant difference was identified in
total lean body mass amongst the patients administered TRT via
injection (MD, 1.7; 95% CI, −0.1 to 3.5; P=0.06) and transdermally
(MD, 0.65; 95% CI, −0.58 to 1.88; P=0.30). The included studies did
not present any data on oral administration of TRT.
Total fat mass
A total of 5 RCTs, involving 995 participants (503
in the testosterone group and 492 in the control group), reported
upon the total fat mass of patients. No statistical difference was
identified between the two groups (MD, −0.85; 95% CI, −1.74 to
0.04; P=0.06; Fig. 5B). Subgroup
analysis revealed no significant changes in the total fat mass
between patients administered TRT transdermally (MD, −0.91; 95% CI,
−1.91 to 0.08; P=0.07) and via injection (MD, −0.6; 95% CI, −2.6 to
1.4; P=0.56).
Total cholesterol
A total of 8 RCTs, involving 943 participants (519
in the testosterone group and 424 in the control group), included
the total cholesterol levels of patients. TRT decreased total
cholesterol in the testosterone group compared with the control
patients. The mean difference between the two groups was −0.16 (95%
CI, −0.29 to −0.03; P=0.01; Fig.
5C). Subgroup analysis revealed that total cholesterol levels
significantly decreased in the long-term group (MD, −0.23; 95% CI,
−0.39 to −0.07; P=0.005). However, no significant change in total
cholesterol was noted in the short-term group (MD, −0.11; 95% CI,
−0.27 to 0.05; P=0.16). Subgroup analysis also demonstrated that
administration by injection (MD, −0.27; 95% CI, −0.5 to −0.04;
P=0.02) reduced cholesterol to a greater degree than
transdermally-administered (MD, −0.18; 95% CI, −0.37 to 0.01;
P=0.07) and orally-administered (MD, 0; 95% CI, −0.26 to 0.26;
P=1.00) TRT.
Safety
PSA levels
A total of 11 RCTs, involving 1,392 participants
(723 in the testosterone group and 669 in the control group),
reported the PSA levels of patients. The present analysis revealed
no significant increase in PSA levels following TRT (MD, 0.10; 95%
CI, −0.03 to 0.22; P=0.14; Fig. 5D).
Subgroup analysis revealed that PSA levels did not increase
following long-term TRT (MD, −0.12; 95% CI, −0.32 to 0.07; P=0.21).
However, following short term TRT, the PSA levels increased
significantly (MD, 0.26; 95% CI, 0.09–0.43; P=0.002) Furthermore,
transdermal administration (MD, 0.43; 95% CI, 0.2 to 0.66;
P=0.0002) increased PSA levels more than the injection (MD, −0.11;
95% CI, −0.31 to 0.09; P=0.27) or the oral administration (MD,
0.03; 95% CI, −0.21 to 0.27; P=0.81) methods in men with
hypogonadism. Following short term TRT, the prostate volume
increased significantly (MD, 2.62; 95% CI, 1.42–3.81; P=0.001).
Furthermore, the oral administration (MD, 2.61; 95% CI, 1.41–3.8;
P=0.0001) resulted in higher increase in prostate volume compared
with the injection (MD, −0.5; 95% CI, −2.27 to 1.26; P=0.58) or the
transdermal administration (MD, −0.9; 95% CI, −8.17 to 6.37;
P=0.81) methods in men with hypogonadism.
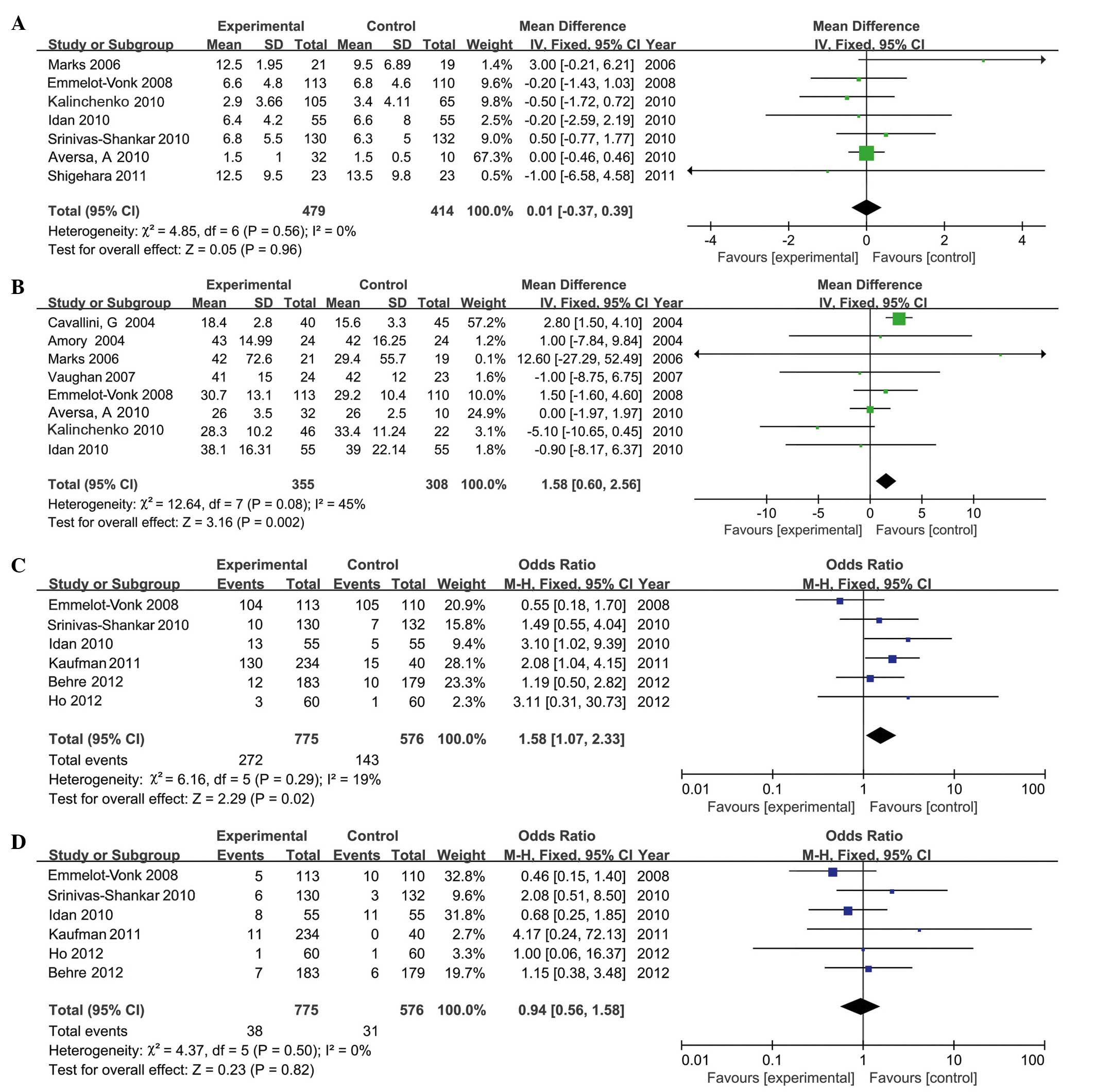
IPSS
A total of 7 RCTs, involving 893 participants (479
in the testosterone group and 414 in the control group), provided
IPSS. The present analysis indicated that, compared with the
control group, TRT did not increase IPSS significantly in the
experimental group (MD, 0.01; 95% CI, −0.37 to 0.39; P=0.96;
Fig. 6A). Subgroup analysis
indicated that there were no significant differences in IPSS in
long-term or short-term treatment. Similarly, no significant
differences in IPSS were identified within the injection (MD,
−0.01; 95% CI, −0.44 to 0.42; P=0.95), transdermal (MD, 0.35; 95%
CI, −0.78 to 1.47; P=0.55) or oral (MD, −0.20; 95% CI, −1.43 to
1.03; P=0.96) administration groups.
Prostate volume
A total of 8 RCTs, involving 663 participants (355
in the testosterone group and 308 in the control group), included
the results of prostate volume. The data revealed that the average
increase in prostate volume was 1.58 (95% CI, 0.6 to 2.56; P=0.002;
Fig. 6B) following TRT. However, no
significant difference in prostate volume was reported following
long-term TRT (MD, −0.55; 95% CI, −2.27 to 1.17; P=0.96).
Mild to moderate adverse events
A total of 6 RCTs, involving 1,351 participants (775
in the testosterone group and 576 in the control group), included
details of mild to moderate adverse events (Table II). Analysis demonstrated that the
frequency of mild to moderate adverse events in the testosterone
group was higher than in the control group (MD, 1.58; 95% CI, 1.07
to 2.33; P=0.02; Fig. 6C),
particularly in patients undergoing long-term treatment (MD, 3.10;
95% CI, 1.14 to 8.41; P=0.03). However, no significant differences
were reported in the number of mild to moderate adverse events in
the short-term studies (MD, 1.38; 95% CI, 0.90 to 2.11; P=0.15).
Subgroup analyses demonstrated that these adverse events occurred
more frequently in the transdermal administration group compared
with the other administration groups.
 | Table II.Adverse events. |
Table II.
Adverse events.
|
| Discontinued
participants, n | Mild to moderate
adverse events, n/total n | Serious adverse
events n/total n |
|---|
|
|
|
|
|
|---|
| First author, year
(ref.) | Experimental | Control | Experimental | Control | Experimental | Control |
|---|
| Idan et al,
2010 (15) | 19 | 14 | 13/55 | 5/55 | 8/55 | 11/55 |
| Ho et al,
2012 (21) | 4 | 2 | 3/60 | 1/60 | 1/60 | 1/60 |
| Kaufman et
al, 2011 (18) | 66 | 12 | 134/234 | 15/40 | 5/234 | 1/40 |
| Emmelot-Vonk et
al, 2008 (7) | 16 | 14 | 104/113 | 105/110 | 5/113 | 10/110 |
| Srinivas-Shankar
et al, 2010 (17) | 15 | 16 | 10/130 | 7/132 | 6/130 | 3/132 |
| Behre et al,
2012 (6) | 15 | 24 | 12/183 | 10/179 | 7/183 | 6/179 |
Serious adverse events
Serious adverse events reported in the included
studies were: Cancer, mortality, pulmonary embolism, myocardial
infarction, heart failure, constrictive pericarditis, elective
surgery for intervertebral disc, inguinal hernia repair and deep
vein thrombosis.
A total of 6 RCTs, involving 1,351 participants (775
in the testosterone group and 576 in the control group), reported
upon the number of serious adverse events. TRT did not increase the
number of serious adverse events (MD, 0.85; 95% CI, 0.50 to 1.44;
P=0.55; Fig. 6D). Similarly, no
significant differences were identified upon comparing alternative
duration or methods of treatment.
Discussion
Numerous clinical trials have examined the efficacy
and safety of TRT for men with testosterone deficiency based on
serum levels (6–21); however, paradoxical results have
prevented conclusions from being made. A meta-analysis was
therefore conducted of 16 RCTs on aging men with primary or
secondary hypogonadism to assess the efficacy and safety of TRT.
The present analysis indicated that the patients' quality of life
improved markedly following TRT, as indicated by decreased AMS
scores, which supports the results of previous studies (6,25).
However, conflicting data have been reported in studies regarding
the efficacy of TRT treatment upon BMD (26). Mittan et al (27) reported that using TRT during the
post-operative period for patients undergoing hypogonadal pituitary
tumor surgery may have beneficial effects on the BMD of the spine,
but that no significant changes occur in the femoral neck or total
femur BMD. The current analysis revealed that TRT had no beneficial
effects on BMD in men with hypogonadism without tumors, in contrast
with the results of previous studies (28–30).
There are three possible reasons for this discrepancy. First, the
participants of studies included in the present analysis were
selected on the basis of their androgen status, as opposed to their
health status or symptoms. The majority of participants in the
current study were healthy and had no pre-existing health problems
that may have prejudiced results (7). Second, in aging men with low
testosterone levels, the effect of TRT on BMD appears to be
associated with baseline testosterone levels; testosterone
treatment increased BMD only in men whose baseline levels were
below the reference range (31).
Third, the majority of studies included in the present
meta-analysis involved <2 years of TRT, while conventional
osteoporosis studies typically extend over a 3-year period. BMD
following TRT therefore requires additional study.
The present analysis revealed that, compared with
the placebo group, TRT significantly increased lean body mass,
which was in accordance with previous studies (25,32).
Other previous studies (26,27,32)
reported that the weight and fat mass of patients with prostate
cancer significantly increased and that their lean mass
significantly decreased during androgen deprivation therapy.
However, in the present analysis, no improvement was identified in
body weight and BMI following TRT. Another previous study
demonstrated that body weight and BMI improved significantly in men
with hypogonadism also exhibiting type 2 diabetes following TRT
(28), suggesting that TRT was more
efficacious in improving body weight and BMI in patients with type
2 diabetes.
Clinical trials have examined whether exogenous
testosterone administration can decrease serum concentrations of
high-density lipoprotein cholesterol (HDL-C) (25). As low HDL-C correlates with an
increased risk of cardiovascular disease (CVD), this decrease may
contribute to the presumed adverse cardiovascular effects of
testosterone. By contrast, a previous study demonstrated that low
circulating androgen levels in men are associated with increased
risk of CVD and mortality (8), which
challenges the assumption that testosterone adversely impacts
cardiovascular health in men. However, as there were not enough
data to analyze the CVD risk following TRT, additional
investigative trials are required to determine whether TRT
correlates with altered CVD risk.
Prostate growth is dependent on the presence of
testosterone; higher serum testosterone is associated with a larger
prostate volume and higher prevalence of BPH. Antiandrogens and
orchidectomy decrease the prostate volume in patients with BPH
(29,33). Previous evidence has indicated that
androgens affect prostate volume and the development of prostate
cancer (31). The present analysis
revealed that prostate volume increased in the testosterone-treated
group compared with the placebo group. However, no statistical
differences were identified in PSA levels and IPSS in the
testosterone-treated group compared with those in the placebo
group. Raynaud et al (34)
reported that long-term TRT was not associated with significant
changes to PSA concentration and PSA velocity or any significant
prostate risks. However, Khera et al (32) reported that patients with a baseline
total testosterone level of <250 ng/dl were more likely to
demonstrate increased PSA levels following TRT than those with a
baseline total testosterone level of >250 ng/dl, which supports
the prostate saturation hypothesis. In the present meta-analysis,
the total testosterone of participants was <300 ng/dl, and PSA
levels and IPSS did not increase following TRT. However, it cannot
be concluded that PSA levels and IPSS did not increase following
TRT in patients with a baseline total testosterone level of <50
ng/dl; patients who are severely hypogonadal may experience
increased PSA following TRT. The current meta-analysis revealed
that prostate volume increased significantly following TRT. The PSA
concentration typically demonstrated a greater association with TRT
than total prostate volume. The PSA concentration did not increase
whilst prostate volume increased significantly; this may be as cell
proliferation of the peripheral prostate affected by testosterone
is more efficient than the synthesis and secretion of PSA per cell
in prostate epithelial cells. This hypothesis conflicts with a
previous study reporting that PSA secretion is more rapidly altered
by exogenous androgens than prostate growth is (35). The molecular mechanism behind this
phenomenon requires additional study.
A novel and unexpected result of the present study
was that mild to moderate adverse events associated with TRT
occurred at a greater frequency in TRT patients than in the control
group, particularly in patients receiving long-term TRT; this was
also true of the transdermal administration group. However, the
frequency of serious adverse events did not significantly differ
between the testosterone and control groups (Table II). A previous meta-analysis
(36) demonstrated that TRT was
associated with a significantly higher risk of detection of
prostate events and adverse events. However, the frequency of
cardiovascular events, sleep apnea and mortality did not
significantly increase in the present study.
The 16 RCTs (6–21)
included in the current meta-analysis were all double-blind, and
the quality of the individual studies in the meta-analysis was high
(Fig. 6). The results of these
analyses may, therefore, be of great importance from a scientific
and a clinical standpoint. However, there are a number of
limitations to the present analysis, as follows: i) All 16 RCTs
included rigorous periodic monitoring of patients and excluded
patients at PSA levels of >4 ng/ml, which may be why the
meta-analysis failed to detect an increased likelihood of prostate
cancer amongst patients receiving TRT; ii) differences in
testosterone dosage used and baseline PSA levels were reported,
which may explain heterogeneity associated with a number of
outcomes; and iii) the adverse events of TRT were analyzed, but not
enough data were available to assess specific types of adverse
events (for instance, gastrointestinal disorders, psychiatric
disorders, infections and infestations, muscle and connective
tissue disorders or nervous system disorders) that occur following
TRT. Any of the aforementioned factors may affect the results of
the meta-analysis.
In conclusion, the present meta-analysis indicated
that TRT improved the quality of life, increased lean body mass and
significantly decreased total cholesterol. In addition, this
treatment is well-tolerated and safe for men with hypogonadism who
are exhibiting PSA levels of <4 ng/ml.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31100702/C100307)
and by the Specialized Research Fund for the Doctoral Program of
Higher Education in China (grant no. 20110072120054).
References
|
1
|
Cardarelli R, Singh M, Meyer J, Balyakina
E, Perez O and King M: The association of free testosterone levels
in men and lifestyle factors and chronic disease status: A North
Texas Healthy Heart Study. J Prim Care Community Health. 5:173–179.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferreira SR: Cushing and Arquivos
Brasileiros de Endocrinologia e Metabologia Award 2006. Arq Bras
Endocrinol Metabol. 51:11812007.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui Y, Zong H, Yan H and Zhang Y: The
effect of testosterone replacement therapy on prostate cancer: A
systematic review and meta-analysis. Prostate Cancer Prostatic Dis.
17:132–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bassil N: Late-onset hypogonadism. Med
Clin North Am. 95:507–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donatucci C, Cui Z, Fang Y and Muram D:
Long-term treatment patterns of testosterone replacement
medications. J Sex Med. 11:2092–2099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Behre HM, Tammela TL, Arver S, Tolrá JR,
Bonifacio V, Lamche M, Kelly J, Hiemeyer F, Giltay EJ and Gooren
LJ: European Testogel Study Team: A randomized, double-blind,
placebo-controlled trial of testosterone gel on body composition
and health-related quality-of-life in men with hypogonadal to
low-normal levels of serum testosterone and symptoms of androgen
deficiency over 6 months with 12 months open-label follow-up. Aging
Male. 15:198–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emmelot-Vonk MH, Verhaar HJ, Pour Nakhai
HR, Aleman A, Lock TM, Bosch JL, Grobbee DE and van der Schouw YT:
Effect of testosterone supplementation on functional mobility,
cognition, and other parameters in older men: A randomized
controlled trial. JAMA. 299:39–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amory JK, Watts NB, Easley KA, Sutton PR,
Anawalt BD, Matsumoto AM, Bremner WJ and Tenover JL: Exogenous
testosterone or testosterone with finasteride increases bone
mineral density in older men with low serum testosterone. J Clin
Endocrinol Metab. 89:503–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cavallini G, Caracciolo S, Vitali G,
Modenini F and Biagiotti G: Carnitine versus androgen
administration in the treatment of sexual dysfunction, depressed
mood, and fatigue associated with male aging. Urology. 63:641–646.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Page ST, Amory JK, Bowman FD, Anawalt BD,
Matsumoto AM, Bremner WJ and Tenover JL: Exogenous testosterone (T)
alone or with finasteride increases physical performance, grip
strength, and lean body mass in older men with low serum T. J Clin
Endocrinol Metab. 90:1502–1510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marks LS, Mazer NA, Mostaghel E, Hess DL,
Dorey FJ, Epstein JI, Veltri RW, Makarov DV, Partin AW, Bostwick
DG, et al: Effect of testosterone replacement therapy on prostate
tissue in men with late-onset hypogonadism: A randomized controlled
trial. JAMA. 296:2351–2361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaughan C, Goldstein FC and Tenover JL:
Exogenous testosterone alone or with finasteride does not improve
measurements of cognition in healthy older men with low serum
testosterone. J Androl. 28:875–882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aversa A, Bruzziches R, Francomano D,
Rosano G, Isidori AM, Lenzi A and Spera G: Effects of testosterone
undecanoate on cardiovascular risk factors and atherosclerosis in
middle-aged men with late-onset hypogonadism and metabolic
syndrome: Results from a 24-month, randomized, double-blind,
placebo-controlled study. J Sex Med. 7:3495–3503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aversa A, Bruzziches R, Francomano D,
Spera G and Lenzi A: Efficacy and safety of two different
testosterone undecanoate formulations in hypogonadal men with
metabolic syndrome. J Endocrinol Invest. 33:776–783. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Idan A, Griffiths KA, Harwood DT, Seibel
MJ, Turner L, Conway AJ and Handelsman DJ: Long-term effects of
dihydrotestosterone treatment on prostate growth in healthy,
middle-aged men without prostate disease: A randomized,
placebo-controlled trial. Ann Intern Med. 153:621–632. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalinchenko SY, Tishova YA, Mskhalaya GJ,
Gooren LJ, Giltay EJ and Saad F: Effects of testosterone
supplementation on markers of the metabolic syndrome and
inflammation in hypogonadal men with the metabolic syndrome: The
double-blinded placebo-controlled Moscow study. Clin Endocrinol
(Oxf). 73:602–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Srinivas-Shankar U, Roberts SA, Connolly
MJ, O'Connell MD, Adams JE, Oldham JA and Wu FC: Effects of
testosterone on muscle strength, physical function, body
composition, and quality of life in intermediate-frail and frail
elderly men: A randomized, double-blind, placebo-controlled study.
J Clin Endocrinol Metab. 95:639–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaufman JM, Miller MG, Garwin JL,
Fitzpatrick S, McWhirter C and Brennan JJ: Efficacy and safety
study of 1.62% testosterone gel for the treatment of hypogonadal
men. J Sex Med. 8:2079–2089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shigehara K, Sugimoto K, Konaka H, Iijima
M, Fukushima M, Maeda Y, Mizokami A, Koh E, Origasa H, Iwamoto T
and Namiki M: Androgen replacement therapy contributes to improving
lower urinary tract symptoms in patients with hypogonadism and
benign prostate hypertrophy: A randomised controlled study. Aging
Male. 14:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frederiksen L, Højlund K, Hougaard DM,
Brixen K and Andersen M: Testosterone therapy increased muscle mass
and lipid oxidation in aging men. Age (Dordr). 34:145–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho CC, Tong SF, Low WY, Ng CJ, Khoo EM,
Lee VK, Zainuddin ZM and Tan HM: A randomized, double-blind,
placebo-controlled trial on the effect of long-acting testosterone
treatment as assessed by the Aging Male Symptoms scale. BJU Int.
110:260–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgentaler A: Testosterone and prostate
cancer: An historical perspective on a modern myth. Eur Urol.
50:935–939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moher D, Cook DJ, Eastwood S, Olkin I,
Rennie D and Stroup F: Improving the Quality of Reports of
Meta-Analyses of Randomised Controlled Trials: The QUOROM
Statement. Onkologie. 23:597–602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oxman AD and Guyatt GH: The science of
reviewing research. Ann N Y Acad Sci. 703:125–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhasin S and Buckwalter JG: Testosterone
supplementation in older men: A rational idea whose time has not
yet come. J Androl. 22:718–731. 2001.PubMed/NCBI
|
|
26
|
Smith MR: Changes in fat and lean body
mass during androgen-deprivation therapy for prostate cancer.
Urology. 63:742–745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mittan D, Lee S, Miller E, Perez RC,
Basler JW and Bruder JM: Bone loss following hypogonadism in men
with prostate cancer treated with GnRH analogs. J Clin Endocrinol
Metab. 87:3656–3661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hackett G, Cole N, Bhartia M, Kennedy D,
Raju J and Wilkinson P: BLAST Study Group: Testosterone replacement
therapy improves metabolic parameters in hypogonadal men with type
2 diabetes but not in men with coexisting depression: The BLAST
study. J Sex Med. 11:840–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roehrborn CG, Siami P, Barkin J, Damião R,
Major-Walker K, Nandy I, Morrill BB, Gagnier RP and Montorsi F:
CombAT Study Group: The effects of combination therapy with
dutasteride and tamsulosin on clinical outcomes in men with
symptomatic benign prostatic hyperplasia: 4-year results from the
CombAT study. Eur Urol. 57:123–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaplan SA: Re: The effects of combination
therapy with dutasteride and tamsulosin on clinical outcomes in men
with symptomatic benign prostatic hyperplasia: 4-year results from
the CombAT study. J Urol. 185:1384–1385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsing AW, Reichardt JK and Stanczyk FZ:
Hormones and prostate cancer: Current perspectives and future
directions. Prostate. 52:213–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khera M, Bhattacharya RK, Blick G, Kushner
H, Nguyen D and Miner MM: Changes in prostate specific antigen in
hypogonadal men after 12 months of testosterone replacement
therapy: Support for the prostate saturation theory. J Urol.
186:1005–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao CH, Li HY, Chung SD, Chiang HS and Yu
HJ: Significant association between serum dihydrotestosterone level
and prostate volume among Taiwanese men aged 40–79 years. Aging
Male. 15:28–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raynaud JP, Gardette J, Rollet J and
Legros JJ: Prostate-specific antigen (PSA) concentrations in
hypogonadal men during 6 years of transdermal testosterone
treatment. BJU Int. 111:880–890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozata M, Bulur M, Beyhan Z, Sengül A,
Saglam M, Turan M, Corakci A and Gundogan Ali M: Effects of
gonadotropin and testosterone treatments on prostate volume and
serum prostate specific antigen levels in male hypogonadism. Endocr
J. 44:719–724. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Calof OM, Singh AB, Lee ML, Kenny AM,
Urban RJ, Tenover JL and Bhasin S: Adverse events associated with
testosterone replacement in middle-aged and older men: A
meta-analysis of randomized, placebo-controlled trials. J Gerontol
A Biol Sci Med Sci. 60:1451–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|