Introduction
Inflammation is an orchestrated biological process,
induced by tissue injury or microbial infection, which protects the
body from these inflammatory stimuli. However, persistent or
excessive inflammation is associated with a variety of pathological
conditions, including rheumatoid arthritis, bacterial sepsis and
skin inflammation (1,2). Macrophages play a key role in the host
defense against noxious substances and are involved in numerous
inflammatory diseases (3). The
activation of macrophages by inflammatory stimuli can generate
reactive oxygen species, such as H2O2 and
superoxide, and induce the expression of various genes such as
interleukin (IL)-6 and tumor necrosis factor (TNF)-α, in addition
to other inflammatory mediators, including nitric oxide (NO) and
prostaglandin E2 (PGE2), which are synthesized by inducible nitric
oxide synthase (iNOS) and cyclooxygenase (COX)-2, respectively.
Inflammatory cytokines and mediators contribute to the pathogenesis
of numerous inflammation-associated human diseases (4). Lipopolysaccharide (LPS) from
gram-negative bacteria induces inflammation and is frequently used
to stimulate macrophages in order to study inflammation and the
mechanisms of action of potential anti-inflammatory agents.
The anti-inflammatory actions of various
phytochemicals have been found to be mediated through suppression
of the NF-κB pathway (5). NF-κB is a
key regulator of a various genes involved in immune and
inflammatory responses (6). In
resting cells, NF-κB is complexed with the inhibitor of κB (IκB)
protein in the cytoplasm, and is thereby inactivated. When cells
receive pathological stimuli, IκB kinase phosphorylates IκB,
causing it to break away from NF-κB. The uncomplexed NF-κB then
translocates to the nucleus, where it binds to DNA and activates
the transcription of various genes including iNOS, COX-2, IL-6 and
TNF-α (7). Therefore, NF-κB is
regarded as a target molecule for anti-inflammatory drug
development (8).
In addition to activating NF-κB, LPS also activates
mitogen-activated protein kinases (MAPKs) (9). There are three major subgroups of MAPK,
namely extracellular signal-regulated kinase (ERK), c-Jun
N-terminal protein kinase (JNK) and p38. These kinases play key
roles in the regulation of numerous cellular functions, including
cell survival, apoptosis and cellular responses to inflammation
(10).
Hedyotis diffusa Willd (HDW), which is a herb of the
Rubiaceae family, has a long history of use in Chinese medicine,
and is widely distributed in northeast Asia. According to the
theories of traditional Chinese medicine (TCM), it has
heat-clearing, detoxification, blood circulation promoting and
blood stasis eliminating effects (11,12).
Pharmacological studies have shown that it contains compounds with
anticancer, anti-inflammatory, antibacterial and immunomodulatory
activities, which include flavonoids, anthraquinones, hemiterpenes,
polyphenols, organic acids and polysaccharides (13–16).
The anti-inflammatory effects of total flavonoids of
HDW (TFHDW) have been investigated in various inflammatory models,
including ulcerative colitis induced by dextran sulfate sodium in
mice (17,18), ear edema induced by dimethylbenzene
in mice, granuloma pouch induced by turpentine in rats, and paw
edema caused by egg white in rats (19). However, few studies on its
anti-inflammatory activity in vitro and its mechanisms have
been carried out. In the present study, the cell-based
anti-inflammatory activities and the mechanisms of action of TFHDW
in LPS-stimulated RAW 264.7 macrophages were investigated. In order
to elucidate the mechanisms underlying the anti-inflammatory
actions, the effect of TFHDW on the expression of iNOS, TNF-α, IL-6
and IL-1β at the mRNA and protein levels, as well as on NF-κB and
MAPK signaling pathways were also studied.
Materials and methods
Materials and reagents
Fetal bovine serum (FBS), RPMI-1640, penicillin,
streptomycin, 0.05% (w/v) trypsin-ethylenediamine tetraacetic acid
(EDTA) and phosphate-buffered saline (PBS) were HyClone products
(GE Healthcare, Logan, UT, USA). Cytokine (IL-6, TNF-α and IL-1β)
enzyme-linked immunosorbent (ELISA) kits were purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). TRIzol reagent and
SuperScript II reverse transcriptase were Invitrogen products
(Thermo Fisher Scientific, Waltham, MA, USA). Anti-phosphorylated
IκBα (anti-p-IκBα; #2859), anti-IκBα (#4812), anti-NF-κB p65
(#6956), anti-p-NF-κB p65 (#3033)and anti-β-actin monoclonal mouse
or rabbit (#4970)antibodies and anto-rabbit IgG (#7074) and
anti-mouse IgG (#7076) horseradish peroxidase (HRP)-conjugated
secondary antibodies were from Cell Signaling Technology (Danvers,
MA, USA). Bio-Plex phosphoprotein assay kits were purchased from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA). All other
chemicals, unless otherwise stated, were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Preparation of TFHDW from Hedyotis
diffusa Willd
Dried plant materials of Hedyotis diffusa Willd.
were purchased from Guo Yi Tang Chinese Herbal Medicine Store
(Fujian, China). The original herb was identified as Hedyotis
diffusa Willd by Dr Wei Xu at the Department of Pharmacology,
Fujian University of Traditional Chinese Medicine (Fuzhou, China).
The material was coarsely ground prior to extraction. A total of
300 g of the material was extracted three times with 80% ethanol
for 3 h at 50°C. The fluid was filtered through a filter with a
1-mm pore-size. The filtrate was then evaporated, and the crude
extract was isolated using a column containing AB-8 macroporous
adsorption resin (CangZhou Bon Chemical Co., Ltd., Hebei, China)
with the application of 80% aqueous ethanol to elute the
flavonoids. The ethanol solvent was then evaporated using a rotary
evaporator (Model RE-2000; Shanghai Yarong Biochemical Instrument
Factory, Shanghai, China).
Cell culture and treatment
Cells of the RAW 264.7 mouse monocyte-macrophage
cell line (American Type Culture Collection, Manassas, VA, USA)
were maintained in RPMI-1640 supplemented with FBS (10%), 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were incubated at 37°C
in a humidified atmosphere of 95% air and 5% CO2. Cells
were plated in 96-, 24- and 6-well plates at densities of
8×104, 1×105 and 5×105 cells/well.
When cell treatments were conducted, the cells were incubated in
serum-free medium for 4 h, then treated with LPS (1 µg/ml) and/or
TFHDW for 24 h (for ELISA), or for 12 h [for reverse
transcription-polymerase chain reaction (RT-PCR)] or for 20 min
(for western blotting) for the detection of protein or mRNA
expression.
Cytotoxicity assay
RAW 264.7 cells were grown in 96-well plates at a
density of 8×104 cells/ml. TFHDW was added at various
concentrations (0, 25, 50, 100, 200, 400 and 800 µg/ml). A methyl
thiazolyl tetrazolium assay (MTT) assay was used to measure the
viability of the cells. Briefly, after 24 h incubation with or
without TFHDW, MTT solution (0.05 mg/ml) was added and the cells
were incubated for another 4 h at 37°C. Then, the supernatant was
removed and 100 µl dimethylsulfoxide was added to dissolve the
formazan. The absorbance of the cells was measured using a
microplate reader (ELx800; BioTek, Winooski, VT, USA) at wavelength
of 570 nm. The control group, which consisted of untreated cells,
was considered to comprise 100% viable cells. Results are expressed
as a percentage of viable cells compared with the control
group.
Determination of NO production
The release of NO by iNOS is one of the major
factors contributing to the inflammatory process (20). The production of nitrite, a
metabolite of NO, was assessed by the Griess reaction. Cells were
plated in 96-well plates (8×104 cells/ml) and treated
with LPS (1 µg/ml) in the presence or absence of TFHDW (50, 100 and
200 µg/ml). After incubation for 24 h, suspended media were
collected for measurement of the nitrite concentrations using the
Griess reaction. This involved taking a 50-µl aliquot of the
culture supernatant, mixing it with an equal volume of Griess
reagent [0.1% N-(1-naphthyl)-ethylenediamine, 1% sulfanilamide in
5% phosphoric acid] and incubating the mixture at room temperature
(RT) for 10 min. The absorbance at 540 nm was measured using a
microplate absorbance reader. The concentration of nitrite was
determined from a sodium nitrite standard curve.
Measurement of inflammatory
cytokines
RAW 264.7 cells were plated in a 24-well cell
culture plate (1×105 cells/ml) and incubated with TFHDW
(50, 100 and 200 µg/ml) in the presence or absence of LPS (1 µg/ml)
for 24 h. A 1-ml volume of culture-medium supernatant was then
collected for measurement of the levels of IL-6, TNF-α and IL-1β
using the relevant ELISA kit according to the manufacturer's
instructions.
RT-PCR
Total RNA was extracted from the cells using TRIzol
reagent following the manufacturer's protocol. The purity and
integrity of the RNA were assessed using a NanoDrop
spectrophotometer (ND-2000C; Thermo Fisher Scientific).
Subsequently, first-strand cDNA synthesis was performed with 2 µg
total RNA using SuperScript II reverse transcriptase kit
(Fermentas; Thermo Fisher Scientific) according to the
manufacturer's protocol. The obtained cDNA was used to determine
the mRNA levels of TNF-α, IL-6, IL-1β and iNOS using a DreamTaq
Green PCR Master Mix (2X) PCR kit (Fermentas; Thermo Fisher
Scientific). The primers used were as follows: TNF-α forward,
5′-CTCAAGGACAACAGCCAGTTC-3′ and reverse,
5′-GGCACTAAGGGCTCAGTCAG-3′; IL-6 forward,
5′-GGATACCACCCACAACAGACC-3′ and reverse,
5′-AATCAGAATTGCCATTGCAC-3′; IL-1β forward,
5′-ATCACTCATTGTGGCTGTGG-3′ and reverse, 5′-GTCGTTGCTTGGTTCTCCT-3′;
iNOS forward, 5′-CAGATCGAGCCCTGGAAGAC-3′ and reverse,
5′-CTGGTCCATGCAGACAACCT-3′; and GADPH forward,
5′-CACTCACGGCAAATTCAACGGCA-3′ and reverse,
5′-GACTCCACGACATACTCAGCAC-3′. The PCR cycling reaction was
performed using an S1000 thermocycler (Bio-Rad Laboratories, Inc.).
Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was used as an
internal control.
Western blot analysis
Total cells were harvested after treatment, washed
twice with ice-cold PBS, and gently lysed in
radioimmunoprecipitation assay buffer containing phosSTOP
phosphatase inhibitor cocktail and protease inhibitor cocktail
(Roche Diagnostics, Mannheim, Germany). Lysates were centrifuged
for 15 min at 12,000 × g to obtain a supernatant for further
analysis. The protein concentration of the lysate was measured
using a bicinchoninic acid (BCA) quantification assay (Pierce,
Rockford, IL, USA). Proteins (50 µg) were separated by 10% sodium
dodecylsulfate-polyacrylamide gel electrophoresis and transferred
to polyvinylidene difluoride membranes (0.45-µm pore size
IPVH00010; Millipore, Billerica, MA, USA). The membranes were
incubated with primary antibody overnight at 4°C. The primary
antibodies were monoclonal antibodies targeting IκBα, p-IκBα, NF-κB
p65, p-NF-κB p65 and β-actin (1:1,000) diluted in immunoblot buffer
(TBS containing 0.05% Tween-20 and 5% non-fat dry milk). Following
washing with TBS and Tween 20 three times, membranes were incubated
with the secondary HRP-conjugated anti-mouse (or rabbit) IgG
antibody (1:1,000) for 1 h at RT. After washing, the blots were
detected with Clarity ECL Western Blotting Substrate (Bio-Rad
Laboratories, Inc.) for 1 min using a camera with the ChemiDoc
XRS+ System (Bio-Rad Laboratories, Inc.). The pixel
intensities of the immunoreactive bands were quantified using the
percentage adjusted volume feature of Image Lab software (Bio-Rad
Laboratories, Inc.). β-actin served as an internal control.
Bio-Plex phosphoprotein assay
A bead-based multiplex assay for phosphoproteins
(Bio-Plex Phosphoprotein assay) was used to detect p-ERK1/2, p-JNK
and p-p38. Cells were lysed using a lysis kit (Bio-Rad
Laboratories, Inc.) and were then centrifuged at 15,000 × g for 15
min. Protein concentrations were determined by BCA protein assay.
Then, 25 µl protein extract and 25 µl testing assay buffer were
transferred into a 96-well filter plate coated with antibodies
against p-ERK1/2, p-JNK and p-p38 and the plate was incubated
overnight on a platform shaker at room temperature. Following a
series of washes to remove the unbound proteins, a mixture of
biotinylated detection antibodies, each specific for a different
epitope, was added to the reaction. Streptavidin-phycoerythrin was
then added to bind to the biotinylated detection antibodies. Data
acquisition and analysis was conducted using the Bio-Plex 200
suspension array system (Bio-Rad Laboratories, Inc.). The total
proteins for ERK1/2, JNK and p38 were also quantified using the
Bio-Plex total protein assay kit (Bio-Rad Laboratories, Inc.). The
phosphorylation level was expressed as the ratio of phosphoprotein
to total protein.
Statistical analysis
Data are expressed as mean ± standard deviation.
One-way analysis of variance was used when comparing the data
obtained under different experimental conditions. In vitro
experiments were conducted in triplicates; representative results
are shown. A P-value of <0.05 was considered to indicate a
statistically significant result.
Results
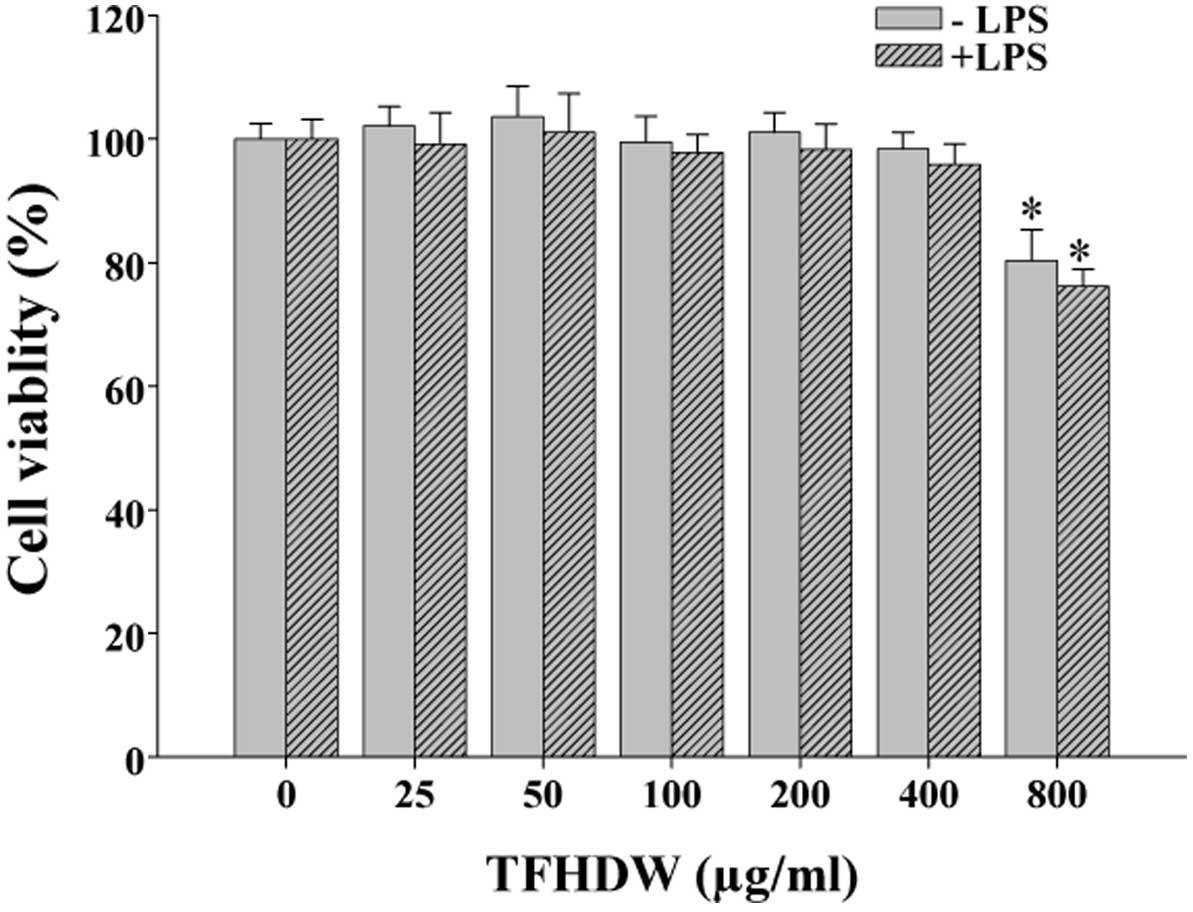
TFHDW did not exhibit cytotoxicity
against RAW 264.7 cells
RAW 264.7 cells were treated with various
concentrations of TFHDW for 24 h, and the viability and
cytotoxicity were determined by MTT assay. TFHDW did not exhibit
cytotoxicity to RAW 264.7 cells in the absence and presence of LPS
at concentrations of 50, 100 and 200 µg/ml, and these
concentrations were used in the following experiments. However,
when the concentrations reached 800 µg/ml, TFHDW appeared to
inhibit cell viability (Fig. 1).
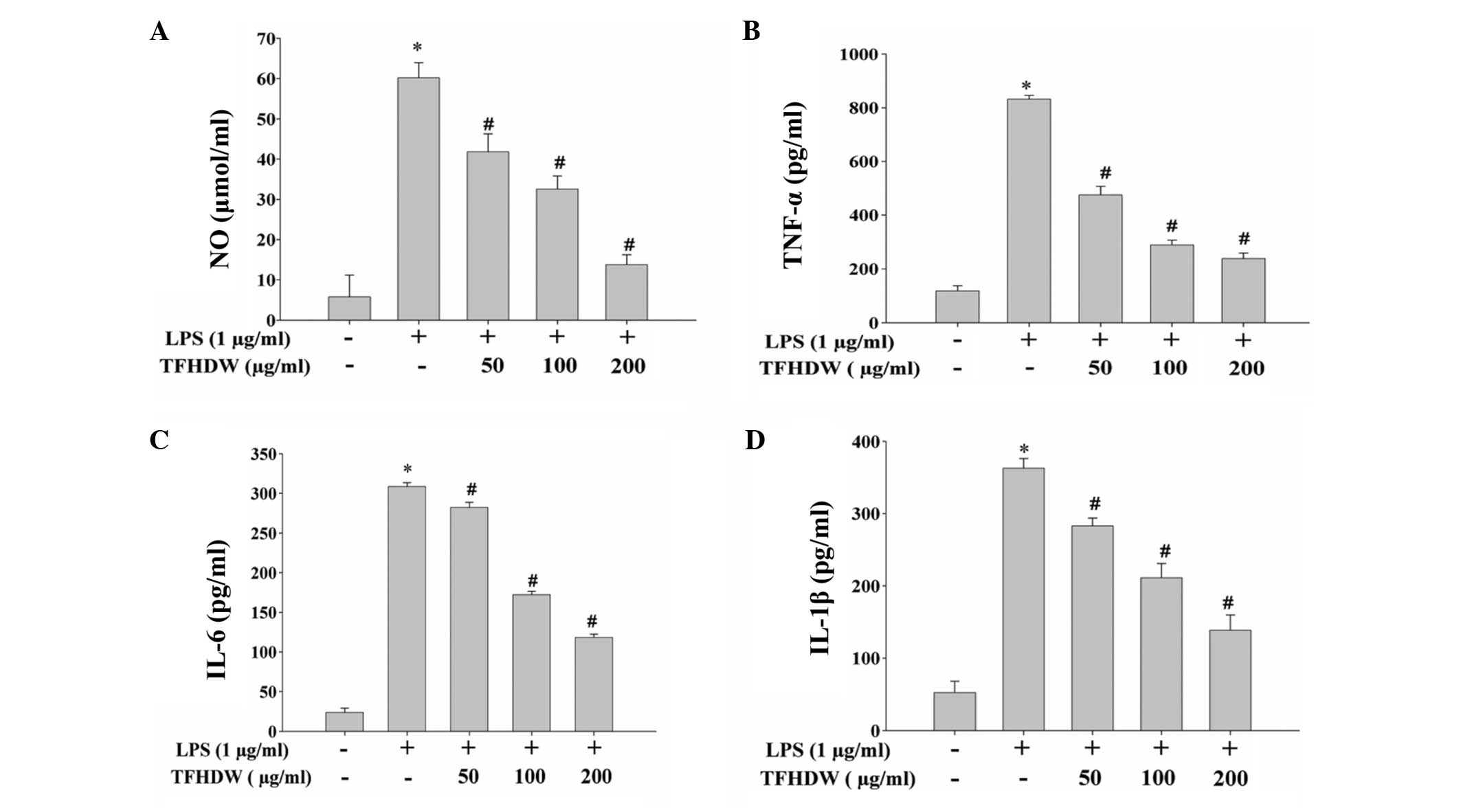
TFHDW inhibits the LPS-induced
inflammatory response in RAW 264.7 cells
The effect of TFHDW on LPS-induced inflammation in
RAW 264.7 cells was evaluated by measuring the production of NO and
pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β). As shown in
Fig. 2, stimulation with LPS for 24
h significantly induced the release of NO, TNF-α, IL-6 and IL-1β,
indicating that an inflammatory response was induced in the RAW
264.7 cells. However, the LPS-induced release of inflammatory
mediators was significantly inhibited by TFHDW treatment in a
concentration-dependent manner. To further verify these
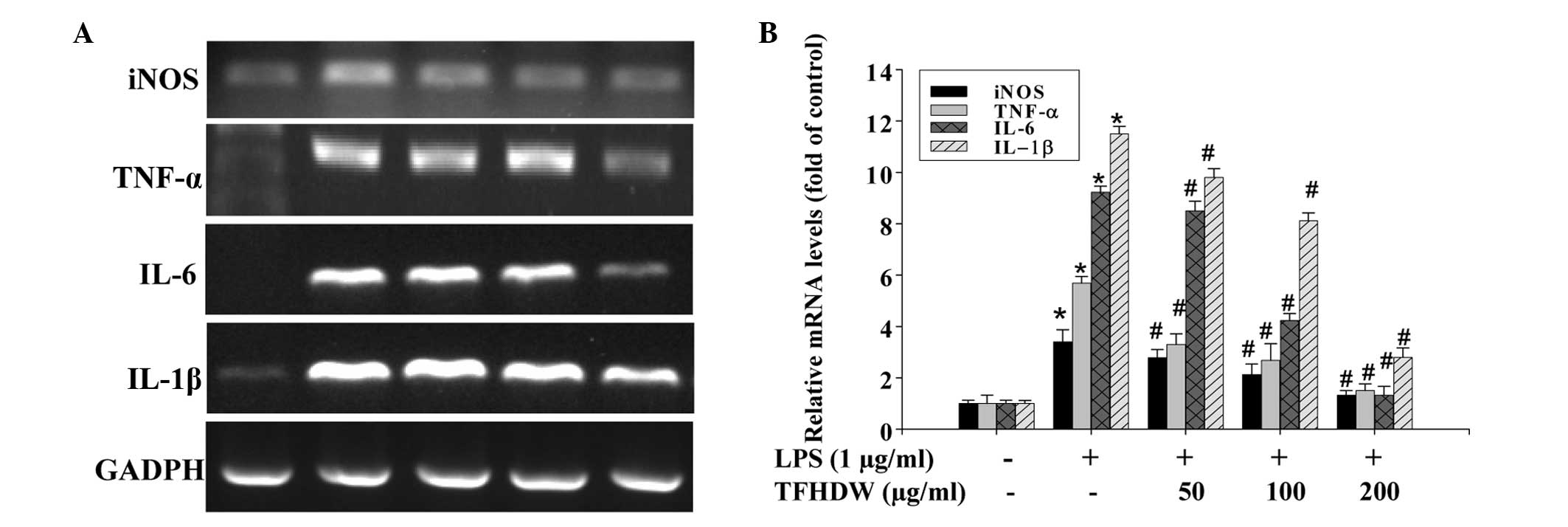
observations, the effect of TFHDW on the mRNA expression of these
pro-inflammatory factors was determined. As shown in Fig. 3, stimulation with LPS significantly
and concentration-dependently increased the mRNA expression levels
of iNOS, TNF-α, IL-6 and IL-1β in RAW 264.7 cells and these
increases were markedly suppressed by TFHDW treatment.
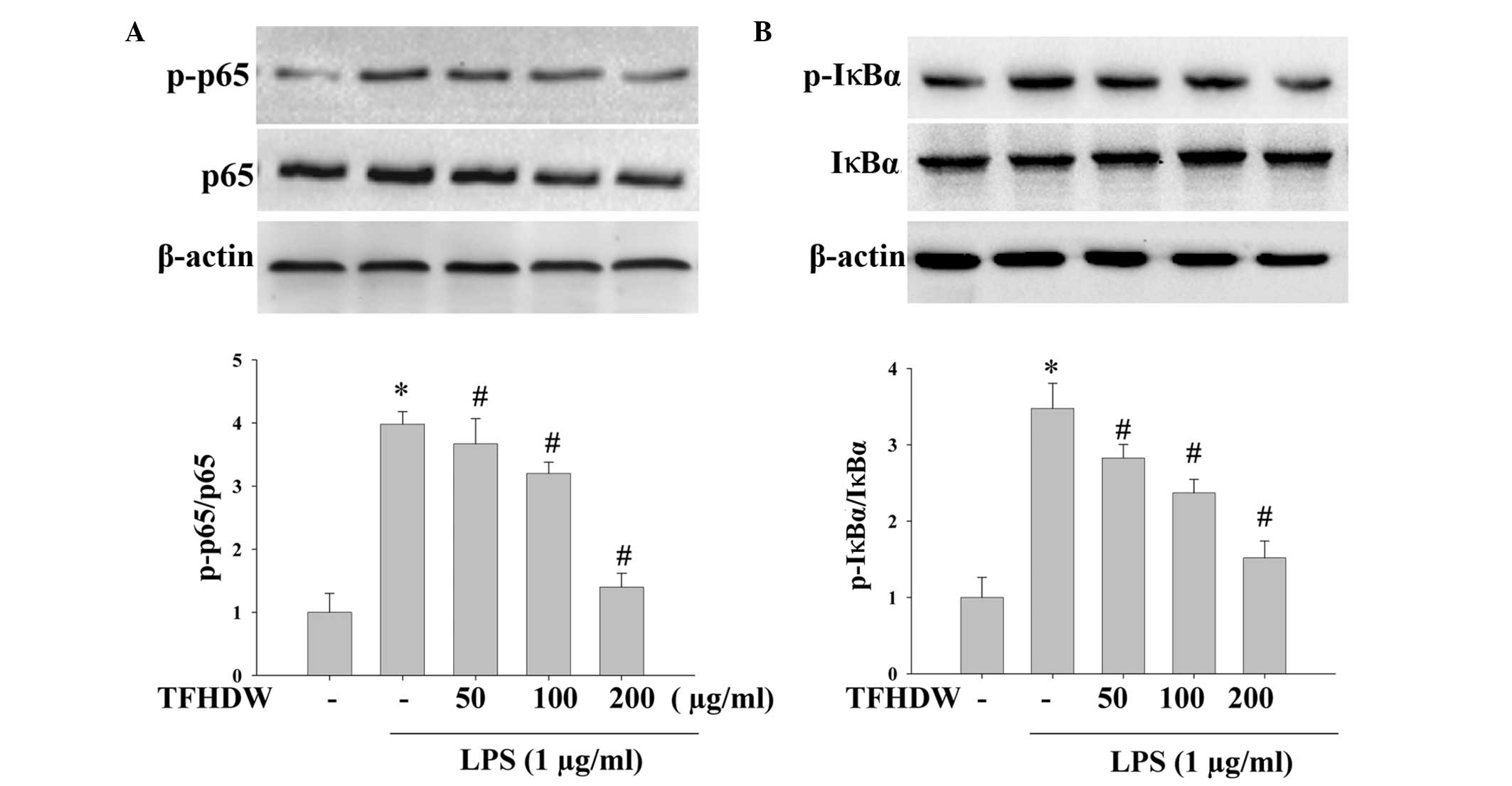
TFHDW prevents LPS-induced activation
of the NF-κB pathway in RAW 264.7 cells
NF-κB is an important upstream transcription factor
inducing the expression of iNOS, TNF-α, IL-6 and IL-1β mRNA
following stimulation by LPS. As p65 is the functional subunit of
NF-κB activated by LPS in macrophages, the phosphorylation of p65
was analyzed by western blot analysis. As shown in Fig. 4, treatment with LPS significantly
increased the phosphorylation of p65 in the RAW 264.7 cells;
However, pretreatment with TFHDW notably inhibited this excessive
phosphorylation. During the p65 phosphorylation process,
phosphorylation of IκBα is essential to release NF-κB from the
NF-κB/IκBα complex. Therefore, the effect of TFHDW on the
LPS-induced phosphorylation of IκBα was also investigated. The
results showed that TFHDW strongly suppressed the phosphorylation
of IκBα, suggesting that TFHDW inhibited NF-κB activation and the
phosphorylation of p65 by reducing the phosphorylation of IκBα
(Fig. 4).
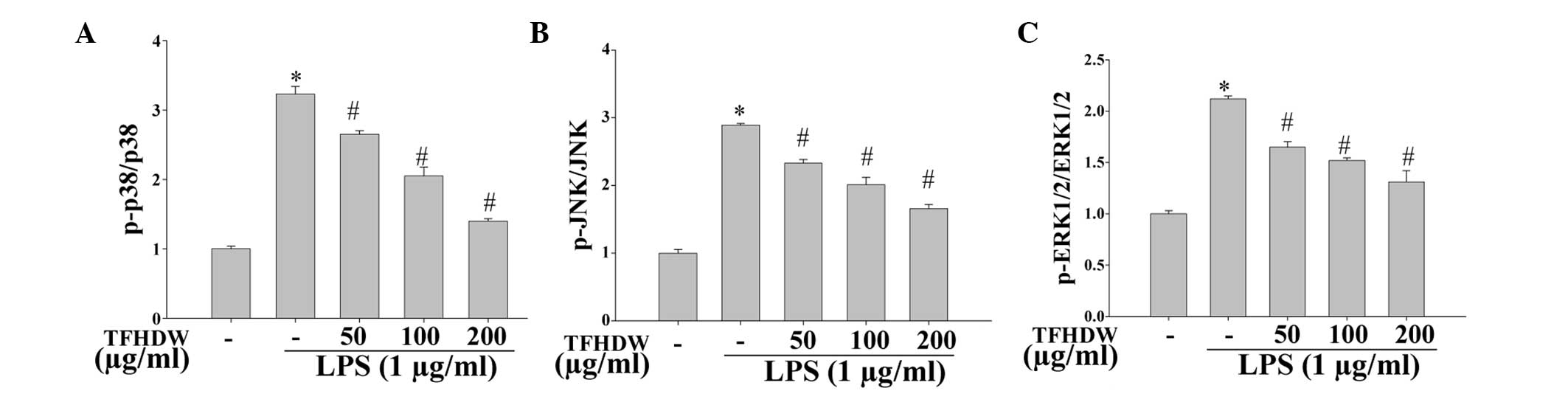
TFHDW suppresses the phosphorylation
of p38, JNK and ERK 1/2
In order to investigate whether the
anti-inflammatory effects of TFHDW are mediated via MAPK signaling
pathways, the phosphorylation of the MAPK signaling molecules p38,
JNK and ERK 1/2 was analyzed using a Bio-Plex phosphoprotein assay.
The results suggest that LPS significantly induced the
phosphorylation of p38, JNK and ERK 1/2; however, the
phosphorylation levels of the MAPK isoforms were markedly decreased
in the cells treated with TFHDW and LPS compared with the cells
treated with LPS alone. These results indicate that TFHDW
effectively blocks MAPK signaling pathways in activated macrophages
(Fig. 5).
Discussion
Inflammation is a biological protective response to
tissue injury or microbial invasion capable of causing cell injury,
under which pro-inflammatory mediators are released (21). Inflammatory factors are important
elements in the chronic inflammation associated with diseases such
as arteriosclerosis, obesity, diabetes, neurodegenerative diseases
and cancer. Steroidal and non-steroidal anti-inflammatory drugs are
currently used to treat acute inflammation. However, these drugs
are not entirely successful in curing chronic inflammatory
disorders, and often have side effects. Therefore, the
identification of new, safer and more effective anti-inflammatory
compounds is necessary (22). TCM,
which plays an important role in primary health care in China,
involves the use of extracts of various plants for the treatment of
pathological disturbances, including acute and chronic
inflammation. Flavonoids, which are an active constituent of TCM
have been found to have notable biological properties, including
anticancer, antimicrobial, antiviral, anti-inflammatory,
immunomodulatory and antithrombotic activities (23–25).
TFHDW, the flavonoid compounds of the traditional Chinese herb HDW,
has been demonstrated to be effective for the treatment of
inflammation in vivo (17–19). The
purpose of the present study was to further investigate the
anti-inflammatory effects of TFHDW on pro-inflammatory mediators
(NO, TNF-α, IL-6, IL-1β) and pro-inflammatory enzyme (iNOS)
expression in LPS-activated RAW 264.7 cells and to explore the
mechanism. It was found that TFHDW inhibited LPS-induced iNOS,
TNF-α, IL-6 and IL-1β expression at the protein and transcription
levels, confirming that TFHDW exhibits an anti-inflammatory
effect.
The NF-κB signaling pathway is an important
LPS-activated pathway. NF-κB is present as a complex with IκB
located in the cytoplasm in unstimulated cells. When stimulated,
IκB is phosphorylated, ubiquitinated and rapidly degraded, which
releases the p65 subunit of NF-κB from IκB, resulting in p65
translocation into the nucleus where it regulates gene
transcription (26,27). To explore the mechanism of NF-κB
inactivation by TFHDW, the effects of the extract on the
LPS-induced phosphorylation of p65 and IκB were examined by western
blotting. The levels of phosphorylation were increased upon
exposure to LPS, and treatment with TFHDW reduced the
phosphorylation of p65 and IκB without affecting the total cellular
levels of these proteins.
Inhibition of the MAPK pathway is also known to
disrupt the synthesis of proinflammatory mediators (28). Several studies have shown that MAPKs
are involved in the activation of NF-κB (28–30). The
effects of TFHDW on the LPS-induced phosphorylation of p38, JNK and
ERK1/2 were examined in the present study in order to further
elucidate its anti-inflammatory mechanism. TFHDW inhibited the
phosphorylation of p38, JNK and ERK1/2 in LPS-stimulated cells in a
concentration-dependent manner. The attenuation of MAPK activation
may be a mechanism by which TFHDW reduces cytokine production. The
results of the current study suggest that the anti-inflammatory
activity of TFHDW is mediated by the inhibition of iNOS, TNF-α,
IL-6 and IL-1β expression via the downregulation of the NF-κB and
MAPK signaling pathways.
In conclusion, in the present study, it was
demonstrated that TFHDW negatively modulates the production of NO
and cytokines (TNF-α, IL-6 and IL-1β) in LPS-treated RAW 264.7
cells in a concentration-dependent manner. It was also found that
TFHDW blocks the activation of NF-κB and MAPK signaling pathways.
These data suggest that TFHDW has notable anti-inflammatory
activity and is worthy of further development as a herbal remedy
for the treatment of various inflammatory diseases.
Acknowledgements
This study was supported as Project No. 81173203 by
the National Natural Science Foundation of China.
Glossary
Abbreviations
Abbreviations:
|
HDW
|
Hedyotis diffusa Willd
|
|
TFHDW
|
total flavones of HDW
|
|
LPS
|
lipopolysaccharide
|
|
NO
|
nitric oxide
|
|
iNOS
|
inducible NO synthase
|
|
IL-1β
|
interleukin-1β
|
|
TNF-α
|
tumor necrosis factor-α
|
|
NF-κB
|
nuclear factor-κB
|
|
IκBα
|
inhibitor of κBα
|
|
MAPK
|
mitogen-activated protein kinase
|
|
JNK
|
c-Jun N-terminal protein kinase
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
References
|
1
|
Dinarello CA: Proinflammatory and
anti-inflammatory cytokines as mediators in the pathogenesis of
septic shock. Chest. 112(Suppl 6): S321–S329. 1997. View Article : Google Scholar
|
|
2
|
Palladino MA, Bahjat FR, Theodorakis EA
and Moldawe LL: Anti-TNF-alpha therapies: The next generation. Natl
Rev Drug Discov. 2:736–746. 2003. View
Article : Google Scholar
|
|
3
|
Pierce GF: Macrophages: Important
physiologic and pathologic sources of polypeptide growth factors.
Am J Respir Cell Mol Biol. 2:233–234. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ,
Ha KS, Han JA, Yim SV, Chang K, Kwon YG, et al: Astaxanthin
inhibits nitric oxide production and inflammatory gene expression
by suppressing I (kappa)B kinase dependent NF-kappaB activation.
Mol Cells. 16:97–105. 2003.PubMed/NCBI
|
|
5
|
Kundu JK and Surh YJ: Breaking the relay
in deregulated cellular signal transduction as a rationale for
chemo prevention with anti-inflammatory phytochemicals. Mutat Res.
591:123–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie QW, Kashiwabara Y and Nathan C: Role
of transcription factor NF-kappaB/Rel in induction of nitric oxide
synthase. J Biol Chem. 269:4705–4708. 1994.PubMed/NCBI
|
|
7
|
Rice NR and Ernst MK: In vivo control of
NF-kappaB activation by I kappaB alpha. EMBO J. 12:4685–4695.
1993.PubMed/NCBI
|
|
8
|
Makarov SS: NF-kappaB as a therapeutic
target in chronic inflammation: Recent advances. Mol Med Today.
6:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearson G, Robinson F, Gibson Beers T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu YG and Song LR: Zhong Hua Ben Cao.
Shanghai: Shanghai Science and Technology Press. 1530–1533.
1998.(In Chinese).
|
|
12
|
Cai QY, Lin JM, Wei L, Zhang L, Wang L,
Zhan Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis
diffusa willd inhibits colorectal cancer growth in vivo via
inhibition of STAT3 signaling pathway. Int J Mol Sci. 13:6117–6128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YY and Luo JB: Analysis of the
chemical constituents of Hedyotis diffusa. Nan Fang Yi Ke Da
Xue Xue Bao. 28:127–128. 2008.(In Chinese). PubMed/NCBI
|
|
14
|
Lee HZ, Bau DT, Kuo CL, Tsai RY, Chen YC
and Chang YH: Clarification of the phenotypic characteristics and
anti-tumor activity of Hedyotis diffusa. Am J Chin Med.
39:201–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang W, Li Y and Jiang J: Chemical
constituents from Hedyotis diffusa. Zhongguo Zhong Yao Za
Zhi. 34:712–714. 2009.(In Chinese). PubMed/NCBI
|
|
16
|
Wang YL, Zhang Y, Fang M, Li QJ, Jiang Q
and Ming L: Immunomodulatory effects of total flavonoids of
Oldenlandia diffusa Willd. Zhongguo Yao Li Xue Tong Bao.
21:444–447. 2005.(In Chinese).
|
|
17
|
Luo SY, Zhong ZG and Zhou L: Experimental
study of the total flavonids of Oldenlandia diffusa on
ulcerative colitis in the rats. Zhongguo Yi Yuan Yao Xue Za Zhi.
31:437–440. 2011.(In Chinese).
|
|
18
|
Luo SY, Le Z, Lv XH and Zhong ZG: Study on
effect of total flavonids of Oldenlandia diffusa on
ulcerative colitis and its immunological mechanism. Zhongguo Zhong
Yao Za Zhi. 39:896–900. 2014.(In Chinese). PubMed/NCBI
|
|
19
|
Wang YL, Zhang Y, Fang M, Li QJ, Jiang Q
and Ming L: Anti-inflammatory and antibacterial effects of total
flavones of Oldenlandia diffusa Willd. Zhongguo Yao Li Xue
Tong Bao. 21:348–350. 2005.(In Chinese).
|
|
20
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
22
|
Yoon JH and Baek SJ: Molecular targets of
dietary polyphenols with anti-inflammatory properties. Yonsei Med
J. 46:585–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robak J and Gryglewski RJ: Bioactivity of
flavonoids. Pol J Pharmacol. 48:555–564. 1996.PubMed/NCBI
|
|
24
|
Russo A, Acquaviva R, Campisi A, Sorrenti
V, Di Giacomo C, Virgata G, Barcellona ML and Vanella A:
Bioflavonoids as antiradicals, antioxidants and DNA cleavage
protectors. Cell Biol Toxicol. 16:91–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de las Heras B and Hortelano S: Molecular
basis of the antiinflammatory effects of terpenoids. Inflamm
Allergy Drug Targets. 8:28–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Israf DA, Khaizurin TA, Syahida A, Lajis
NH and Khozirah S: Cardamonin inhibits COX and iNOS expression via
inhibition of p65NF-kappaB nuclear translocation and Ikappa-B
phosphorylation in RAW 264.7 macrophage cells. Mol Immunol.
44:673–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K,
Kang SS, Zou LB and Kim YS: Anti-inflammatory activity of
4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2
expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK
inactivation. Eur J Pharmacol. 586:340–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HG, Yoon DH, Lee WH, Han SK, Shrestha
B, Kim CH, Lim MH, Chang W, Lim S, Choi S, et al: Phellinus
linteus inhibits inflammatory mediators by suppressing
redox-based NF-kappaB and MAPKs activation in
lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol.
114:307–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung WK, Choi I, Lee DY, Yea SS, Choi YH,
Kim MM, Park SG, Seo SK, Lee SW, Lee CM, et al: Caffeic acid
phenethyl ester protects mice from lethal endotoxin shock and
inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible
nitric oxide synthase expression in RAW 264.7 macrophages via the
p38/ERK and NF-kappaB pathways. Int J Biochem Cell Biol.
40:2572–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|