Introduction
Cannabinoid receptor type 1 (CB1) has been found to
be involved in intrahepatocyte lipid accumulation (1,2). CB1
overexpression in hepatocytes has been hypothesized to contribute
to the formation of hepatic steatosis and inflammation (3–5).
Steatogenic agents, such as ethanol, and a high-fat diet can
upregulate the activity of CB1 via the increased synthesis of
endocannabinoids, 2-arachidonoylglycerol, and anandamide (4). CB1 receptor activation upregulates the
lipogenic transcription factor, sterol regulatory element-binding
protein 1c and its target enzymes, acetyl-CoA carboxylase-1 and
fatty acid synthase. Furthermore, CB1 receptor activation
downregulates carnitine palmitoyltransferase-1, which is the
rate-limiting enzyme associated with fatty acid oxidation.
Therefore, CB1 activation increases fatty acid synthesis and
decreases fatty acid oxidation, which results in a fatty liver
(4). Previously published images of
hepatic CB1 overexpression in human diseases have predominantly
been obtained from patients suffering from serious liver diseases,
such as severe non-alcoholic fatty liver disease found in morbid
obesity (6), stage IV primary
biliary cirrhosis (7) and hepatitis
C (8). However, it remains unclear
whether CB1 overexpression additionally contributes to milder, more
common liver diseases, such as fatty liver disease without or with
mild inflammation.
The primary aim of the present exploratory study was
to determine the extent of CB1 expression in steatotic rat livers.
A secondary aim was to clarify whether steatosis and inflammation
are more extensive in areas of increased CB1 overexpression.
Materials and methods
Animals
A total of 36 male Sprague Dawley rats obtained from
the Karolinska Institute of the Karolinska University Hospital
(Stockholm, Sweden) had free access to regular rodent chow (R36,
Lactamin, Kimstad, Sweden) and water. For 14 (n=12) or 21 days
(n=5), the water for 17 of the rats contained 10% fructose; whereas
the remaining 19 rats received fructose-free water. Duration of
fructose treatment was selected in order to induce hepatic
steatosis and various severities of insulin resistance. Nine rats
additionally received one injection of insulin (1 mU/g actrapid;
Novo Nordisk, Bagsvaerd, Denmark) at 40 min prior to euthanasia.
All institutional (Karolinska Institute) and Swedish national
guidelines for the care and use of laboratory animals were
followed. The rats were sacrificed at 7 weeks of age in the course
of other research. All animal experiments were approved by the
regional Ethics Committee on Animal Research (North Stockholm,
Sweden). Following the experiments, formalin-fixed and
paraffin-embedded liver slices from the rats were obtained. The
Karolinska Institute provided slices from the middle of the right
median lobe for CB1 staining, along with a list of durations of
fructose exposure.
Two male rats, aged 180 days that were homozygous
for leptin receptor gene mutations (9) were bred at the Lund University. The
rats lacked the C-terminal end of the leptin receptor, which
completely inhibited leptin-receptor binding and function. These
rats received Lantmännen R3 breeding chow for rat and mouse
(Lantmännen, Stockholm, Sweden). The rats were hyperleptinemic and
obese. All institutional (Lund University) and Swedish national
guidelines for the care and use of laboratory animals were
followed. The research was approved by the Malmö/Lund Ethics
Committee for Animal Experiment. The rats were euthanized at 180
days of age in the course of other research. Spare formalin-fixed
and paraffin-embedded liver blocks were provided for CB1
staining.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded liver blocks
were sectioned at 4 µm, deparaffinized in two changes of xylene (9
min each) and rehydrated in alcohols (96, 80 and 70% for 1 min
each). Sections were then washed twice in distilled water and
placed in 0.01 M sodium citrate (pH 6.0) in a microwave oven for 9
min for heat-induced epitope retrieval. Following two 5-min washes
in distilled water, specimens were incubated for 10 min with
Peroxidase Blocking Reagent (Dako, Glostrup Denmark) and rinsed
twice with phosphate-buffered saline (PBS), for 5 min each time.
Incubation with Protein Block Reagent was performed for 15 min,
then specimens were incubated with rabbit primary antibodies (CB1;
10006590; Cayman Chemical Company, Ann Arbor, MI, USA) at a
concentration of 1:50 for 1 h at room temperature, and then rinsed
twice with PBS, for 5 min each time. Subsequently, the goat
secondary antibody (Dako EnVision+System-HRP anti-rabbit; K4065;
Dako) was applied and incubated for 30 min at room temperature.
Following rinsing twice with PBS for 5 min each time,
3,3′-diaminobenzidine (Dako) was applied to the samples at the
original dilution, and after the next rinsing (twice for 5 min each
rinse) in distilled water, the slides were counterstained with
hematoxylin. Following washing in tap water for 10 min, the slides
were covered with cover slips. Negative controls were created by
omitting the first antibodies.
Grading and scoring
Steatosis was graded according to the system
proposed by Dixon et al (10). The Dixon system quantifies steatosis,
whereas the present system measured CB1. Furthermore, in order to
determine the extent of inflammatory activity, the Bedossa scoring
system was used (11), where the
activity score (A; from 0–4) was the sum of hepatocyte ballooning
(0–2) and lobular inflammation (0–2). A0 indicates no activity. The
immunoreactivity of CB1 was evaluated using two parameters: i)
Intensity of staining; and ii) number of stained cells. Briefly,
score 1 was assigned to weak staining, 2 to moderate staining and 3
to strong. To report the number of CB1-stained cells, a system
based on the aforementioned Dixon system was used, as follows:
Score 1, 0–5% CB1-stained cells; score 2, 5–25% CB1-stained cells;
score 3, 25–75% CB1-stained cells; and score 4, >75% CB1-stained
cells. The total CB1 score was calculated by adding the two scores
together, and therefore ranged between 1 and 7 points. Two
pathologists independently evaluated all histological slides using
a BX51 light microscope (Olympus Corporation, Tokyo, Japan). Images
were captured using a DP10 digital camera (Olympus Corporation) at
magnifications of ×100 and ×200.
Results
All cases exhibited CB1 expression,
which coexisted with steatosis
Positive staining for CB1 was observed in a large
proportion of the hepatocytes in each case. The total score of
positive staining (i.e. the score for intensity of staining plus
the score for the number of stained cells) is shown in Table I. Two out of the seven cases that
scored 6 were from the rats lacking normal leptin receptors.
 | Table I.Scoring of the liver tissue
samples. |
Table I.
Scoring of the liver tissue
samples.
|
| Livers (n) |
|---|
|
|
|
|---|
| Score | CB1 | Dixon |
|---|
| 1 | 0 | 5 |
| 2 | 3 | 4 |
| 3 | 8 | 15 |
| 4 | 11 | 14 |
| 5 | 9 | – |
| 6 | 7 | – |
| 7 | 0 | – |
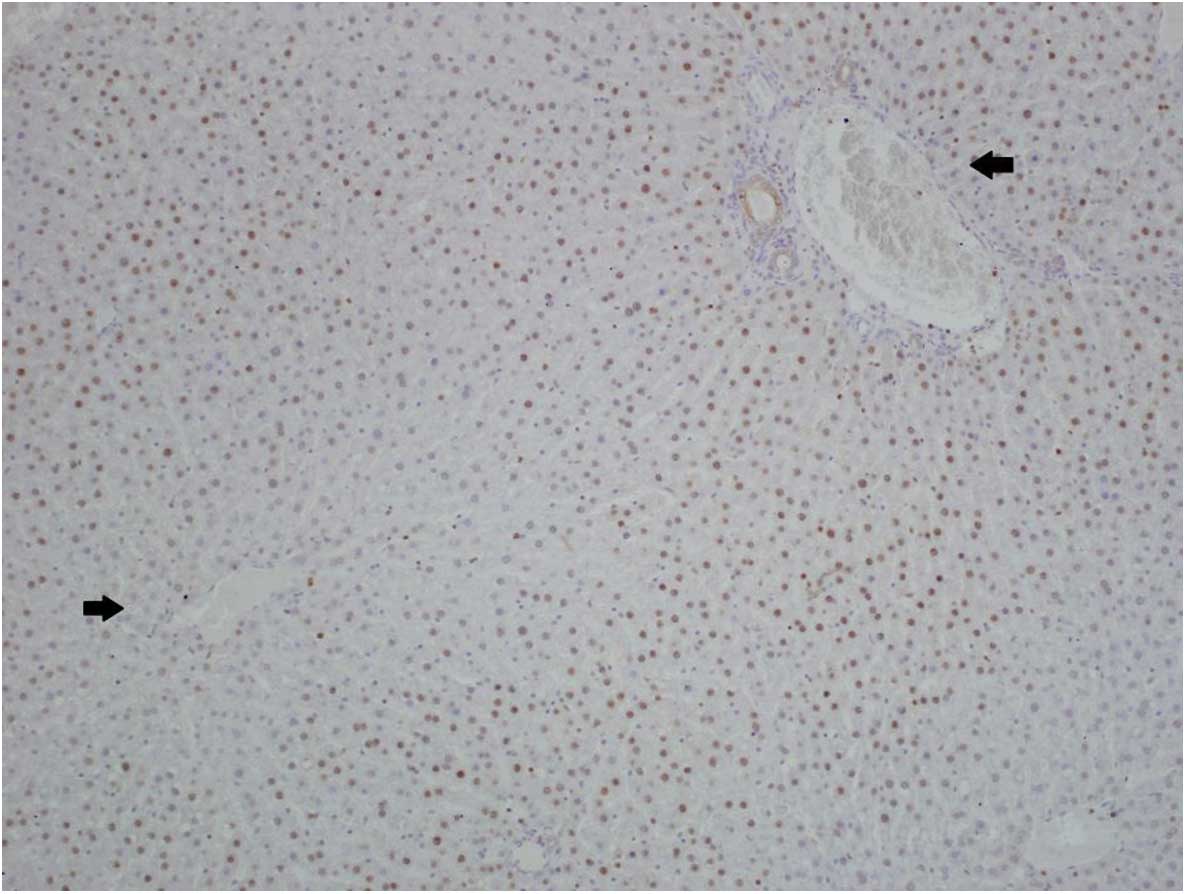
The expression of CB1 was more marked in hepatocytes
located next to portal triads and significantly reduced in
hepatocytes near to the central veins (Fig. 1). Similar results were obtained for
steatosis, which was less marked in hepatocytes near to the central
veins. Therefore, increased CB1 expression and steatosis coexisted
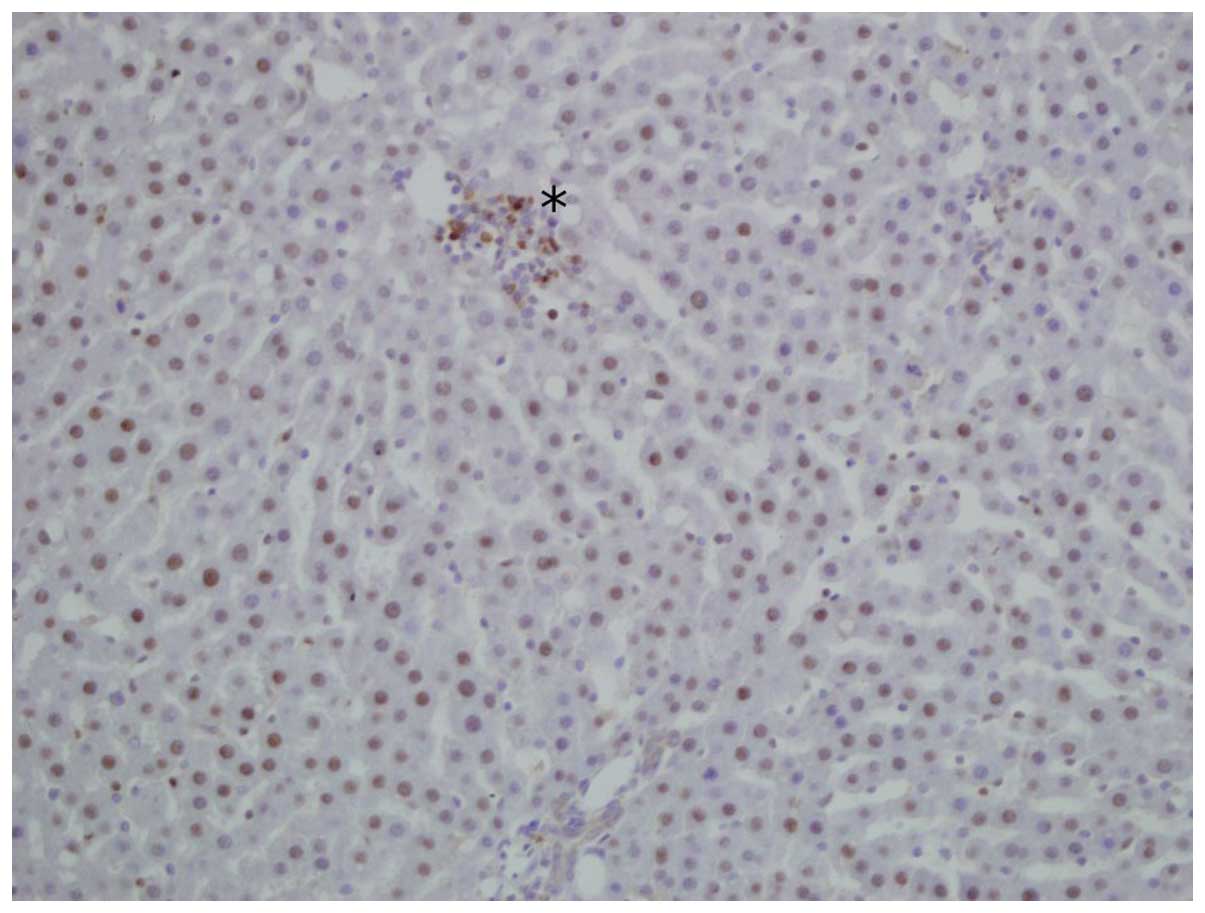
to a considerable extent. In addition, immunohistochemical staining
for CB1 receptor was also performed in certain smaller groups of
intralobular lymphocytic inflammatory infiltrations in the liver
samples (Fig. 2). Inflammatory
activity represented by lymphocytic infiltrations was slightly more
common in samples that exhibited increased CB1 expression; however,
the inflammatory activity did not exceed A0 grade of the Bedossa
scoring system.
The liver samples from obese rats lacking normal
leptin receptors scored a total CB1 score of 6 and exhibited
macrovesicular grade 4 steatosis, whereas the livers from Sprague
Dawley rats exhibited a reduced CB1 score and/or less severe
steatosis.
Details of steatosis
The stage of steatosis was determined based on the
Dixon system (10). Respective Dixon
scores are presented in Table I. In
20 cases the steatosis was microvesicular, whereas in 18 cases the
steatosis was macrovesicular. The two rats lacking normal leptin
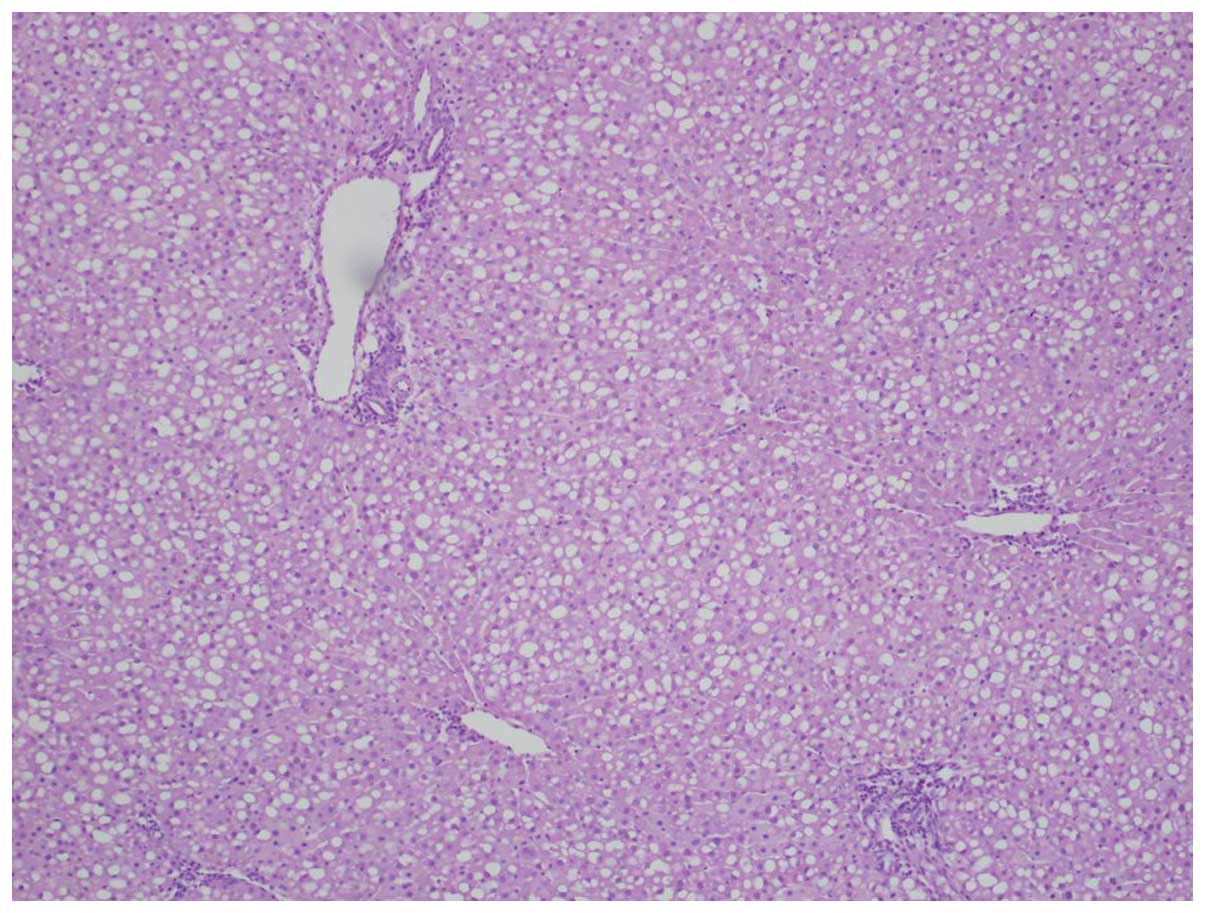
receptors exhibited stage 4 macrovesicular steatosis.
Hematoxylin-eosin staining revealed stage 4 hepatic steatosis with
a diffuse pattern (Fig. 3).
Discussion
Previously published images of CB1 expression have
predominantly been captured from cases with severe liver diseases,
including severe non-alcoholic fatty liver disease found in morbid
obesity (6), stage IV primary
biliary cirrhosis (7) and hepatitis
C (8). Liu et al (6) have previously captured
immunohistochemistry images of human livers stained with CB1
antibodies. In these figures, the nuances of the hepatocytes in the
liver with no obvious pathology appear identical to the negative
control, which had not been stained with CB1. Therefore, the
immunohistochemistry images captured in the present study and those
attained by Liu et al (6)
suggested that the visible CB1 expression levels exhibited in the
present study constitute overexpression. The present study used
similar CB1 antibodies to those that generated the aforementioned
images (6–8). The present results indicated that CB1
was overexpressed in the hepatocytes of rats with steatosis with
mild or without any inflammatory activity in two strains of rats
(Figs. 1 and 2). In these images, steatosis was most
marked in areas exhibiting the overexpression of CB1. This is
consistent with previous studies, which have reported that CB1
contributes to the formation of steatosis (4,12). In
addition, lymphocyte infiltrations were more commonly observed in
areas where CB1 was highly overexpressed, which is consistent with
studies that have suggested that CB1 contributes to inflammation
(12) and plays a role in
inflammatory cells (13). In
particular, a CB1 antagonist, rimonabant, has been demonstrated to
suppress the lipopolysaccharide-induced production of the
pro-inflammatory interleukins (IL), IL-6 and IL-8 in human
macrophages (13).
The livers extracted from the 2 rats lacking normal
leptin receptors had a total CB1 score of 6, and macrovesicular
grade 4 steatosis. By contrast, the livers from the 36 Sprague
Dawley rats exhibited a reduced CB1 score and/or less severe
steatosis. These results may have been due to the increased age of
the rats lacking normal leptin receptors, which allowed more time
for disease progression. High CB1 activity has previously been
associated with obesity (14) and
hepatic steatosis.
The present results suggested that hepatic CB1
contributes to steatosis in rats, even prior to its progression to
steatohepatitis. Although the present results are preliminary and
require further confirmation, it is to be expected that a hepatic
CB1 antagonist that is actively transported into hepatocytes may
exert a protective effect against steatosis and inflammatory
complications. Previous studies have suggested that a hepatic CB1
antagonist may be designed that would be actively transported into
hepatocytes (12,15). For example, a glucokinase activator
has been optimized for active liver uptake, leading to a
>50-fold increase in free concentration in the liver compared
with that in the pancreas (15).
Furthermore, a hepatoselective CB1 antagonist may be capable of
ameliorating steatosis, insulin resistance, dyslipidemia and
inflammatory liver disease (12).
The present results suggest that a hepatoselective CB1 antagonist
could ameliorate steatosis and steatohepatitis in rodents, even
prior to the occurrence of serious complications. Centrally acting
CB1 antagonists are known to produce notable psychiatric side
effects in certain patients (16);
however a hepatoselective CB1 antagonist may have the potential to
be effective with negligible psychiatric side effects (12).
The present study investigated tissue from spare,
archived liver blocks, which were obtained from 38 rats that had
been euthanized during the course of previous research at the
Karolinska Institute of the Karolinska University Hospital and Lund
University in order to keep the number of animals used for research
to a minimum. Therefore, among the limitations of this study is the
lack of individual data for the food and fructose intake levels of
the rats per day, in addition to the final body weight of the rats.
However, these variables do not appear to be necessary for the
present investigation. In the livers of 7-week-old Sprague-Dawley
rats in a previous study (17), the
triglyceride content did not differ significantly between males and
females, which supports the generalization of the present findings
to female rats. In addition, to the best of our knowledge, no
previous results have suggested that the hepatic molecular pathways
associated with steatosis differ markedly between males and
females. Another limitation of the present study is that the
descriptive statics are not supplemented with inferential
statistics. The scoring system used assigned identical scores to
tissues with steatosis ranging from 26 to 75%; however, these
tissues may not have had identical disease severity. In future
studies, actual percentages, rather than scores, should be recorded
as a basis for calculating correlations between CB1 upregulation,
steatosis and inflammation
On the basis of the present histological findings
and the results of previous studies on CB1 overexpression in more
serious inflammatory liver diseases, we have come to the conclusion
that it is likely that CB1 overexpression contributes to increased
steatosis and complications across several stages of the disease,
including the early stages.
Acknowledgements
The authors thank Ms Linda Faxius from the Diabetes
and Celiac Unit (Faculty of Medicine, Lund University) for her
assistance in obtaining tissue from rats lacking normal leptin
receptors.
References
|
1
|
De Gottardi A, Spahr L,
Ravier-Dall'Antonia F and Hadengue A: Cannabinoid receptor 1 and 2
agonists increase lipid accumulation in hepatocytes. Liver Int.
30:1482–1489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pai WY, Hsu CC, Lai CY, Chang TZ, Tsai YL
and Her GM: Cannabinoid receptor 1 promotes hepatic lipid
accumulation and lipotoxicity through the induction of SREBP-1c
expression in zebrafish. Transgenic Res. 22:823–838. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Auguet T, Berlanga A, Guiu-Jurado E, Terra
X, Martinez S, Aguilar C, Filiu E, Alibalic A, Sabench F, Hernández
M, et al: Endocannabinoid receptors gene expression in morbidly
obese women with nonalcoholic fatty liver disease. Biomed Res Int.
2014:5025422014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purohit V, Rapaka R and Shurtleff D: Role
of cannabinoids in the development of fatty liver (Steatosis). AAPS
J. 12:233–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osei-Hyiaman D, DePetrillo M, Pacher P,
Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L,
Wang L and Kunos G: Endocannabinoid activation at hepatic CB1
receptors stimulates fatty acid synthesis and contributes to
diet-induced obesity. J Clin Invest. 115:1298–1305. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Zhou L, Xiong K, Godlewski G,
Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, et al:
Hepatic cannabinoid receptor-1 mediates diet-induced insulin
resistance via inhibition of insulin signaling and clearance in
mice. Gastroenterology. 142:1218–1228.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Floreani A, Lazzari R, Macchi V,
Porzionato A, Variola A, Colavito D, Leon A, Guido M, Baldo V, De
Caro R and Bergasa NV: Hepatic expression of endocannabinoid
receptors and their novel polymorphisms in primary biliary
cirrhosis. J Gastroenterol. 45:68–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Poorten D, Shahidi M, Tay E, Sesha
J, Tran K, McLeod D, Milliken JS, Ho V, Hebbard LW, Douglas MW and
George J: Hepatitis C virus induces the cannabinoid receptor 1.
PLoS One. 5:e128412010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moralejo DH, Hansen CT, Treuting P,
Hessner MJ, Fuller JM, Van Yserloo B, Jensen R, Osborne W, Kwitek
AE and Lernmark A: Differential effects of leptin receptor mutation
on male and female BBDR Gimap5-/Gimap5-spontaneously diabetic rats.
Physiol Genomics. 41:9–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixon JB, Bhathal PS, Hughes NR and
O'Brien PE: Nonalcoholic fatty liver disease: Improvement in liver
histological analysis with weight loss. Hepatology. 39:1647–1654.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bedossa P, Poitou C, Veyrie N, Bouillot
JL, Basdevant A, Paradis V, Tordjman J and Clement K:
Histopathological algorithm and scoring system for evaluation of
liver lesions in morbidly obese patients. Hepatology. 56:1751–1759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper ME and Regnell SE: The hepatic
cannabinoid 1 receptor as a modulator of hepatic energy state and
food intake. Br J Clin Pharmacol. 77:21–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaplan BL: The role of CB1 in immune
modulation by cannabinoids. Pharmacol Ther. 137:365–374. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matias I, Gonthier MP, Orlando P,
Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L,
Festy F, Pasquali R, et al: Regulation, function, and dysregulation
of endocannabinoids in models of adipose and beta-pancreatic cells
and in obesity and hyperglycemia. J Clin Endocrinol Metab.
91:3171–3180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pfefferkorn JA, Guzman-Perez A, Litchfield
J, Aiello R, Treadway JL, Pettersen J, Minich ML, Filipski KJ,
Jones CS, Tu M, et al: Discovery of
(S)-6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)
propanamido) nicotinic acid as a hepatoselective glucokinase
activator clinical candidate for treating type 2 diabetes mellitus.
J Med Chem. 55:1318–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christensen R, Kristensen PK, Bartels EM,
Bliddal H and Astrup A: Efficacy and safety of the weight-loss drug
rimonabant: A meta-analysis of randomised trials. Lancet.
370:1706–1713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gustavsson C, Yassin K, Wahlström E,
Cheung L, Lindberg J, Brismar K, Ostenson CG, Norstedt G and
Tollet-Egnell P: Sex-different hepatic glycogen content and glucose
output in rats. BMC Biochem. 11:382010. View Article : Google Scholar : PubMed/NCBI
|