Introduction
The wound healing potency of an aqueous human
placental extract (HPE) has been clinically established in previous
studies (1,2). HPE has numerous applications in the
treatment of chronic soft tissue ulcers and in the stimulation of
endogenous growth factors, while it possesses anti-inflammatory and
antimicrobial properties (1,2). HPE efficiently activates adenosine A2A
receptors and is not degraded in vivo, meaning it is more
efficient than other types of therapy, including vacuum sealing
drainage, local application of growth factor, and traditional
Chinese medicine (3). However, the
use of appropriate vehicles to maintain the biological activity of
HPE during the delivery process is crucial to the success of the
treatment (4,5).
Short poly-N-acetyl glucosamine (sNAG) nanofibers
have been found to activate the integrin receptor on the surface of
platelets, promote the formation of fibrous protein complexes and
cell proliferation and migration, and stimulate the release of a
variety of growth factors (6,7). The
activated integrin receptor was also found to increase the
expression of vascular endothelial growth factor (VEGF) and
accelerate the formation of new blood vessels through a
transcriptional factor-dependent signaling pathway (8). Furthermore, sNAG nanofibers may also
accelerate the expression of α-defensins and defensin β-1 in
endothelial and keratin cells (9).
Therefore, self-assembled sNAG nanofiber gel encapsulating aqueous
HPE can serve as an ideal matrix material and tool for the
treatment of chronic soft tissue ulcers (9,10).
Layer-by-layer (LbL) self-assembly is a widely used
technique of alternating the adsorption of materials onto a surface
using complementary interactions, one layer of material at a time,
in order to create nanometer-thin films (11,12). Due
to its versatility and simplicity, the LbL self-assembly technique
has been under intensive investigation for a wide range of
purposes, including bone tissue engineering for vaccine delivery
and the creation of biological interfaces (13,14). The
development of stimuli-responsive LbL-assembled nanoparticles has
advanced in recent years (15).
In the present study, the aqueous HPE was
encapsulated in a novel nanocomposite gel fiber (16,17)
prepared by the ideal formulation of IKVAV, RGD, RAD16 and FGL-PA
through LbL self-assembly (17,18,19,20), and
the morphology, particle size, drug loading, drug release efficacy,
encapsulation rate and structure validation were examined. IKVAV is
an amphiphilic molecule, also known as
isoleucine-lysine-valine-alanin-valine. The sequence of IKVAV is
C16H31O-NH-AAAGGGEIKVAV-COOH, and this was
prepared by the solid phase synthesis method (21). RAD16 is a self-assembled peptide
composed of alternating negative and positive amino acids
(ACN-RADARADARADARADA-CONH2). In addition, the FGL-PA
sequence was
C22H43O-NH-AAAGGGEVYVVAENQQGKSKA-COOH, and
the RGD sequence was C16
H31O-NHAAAAGGGS(PO4)-RGD-COOH. The study
aimed to investigate whether this novel type of composite
nanocapsules may offer a promising delivery system for HPE.
Materials and methods
Materials
IKVAV (relative molecular mass of 1,351.6 with 98%
purity; batch number, 20140520; Shanghai Bootech Bioscience &
Technology Co., Ltd., Shanghai, China), RAD16 (relative molecular
mass of 1207.34 with 98% purity; Shanghai Bootech Bioscience &
Technology Co., Ltd.), FGL-PA (relative molecular mass of 2,485.92
with 98% purity; Shanghai Bootech Bioscience & Technology Co.,
Ltd.), and RGD (relative molecular mass of 1,207.34 with 99%
purity; Shanghai Bootech Bioscience & Technology Co., Ltd.)
were used in the present study.
Preparation of vehicle nanofiber
gel
For the preparation of the vehicle nanofiber gel, 10
mg IKVAV, 10 mg RAD16, 10 mg FGL-PA and 10 mg RGD were added to a
mixture of 0.1 M NaOH (400 µl) and twice-distilled water (400 µl).
The mixture was then placed in 37°C for 30 min and stirred
vigorously until a clear liquid was obtained. The pH value was
detected using a pH meter (pH 9.4) and 0.1 M HCl was used to adjust
the pH to 8.5–9.0. Distilled water was used to adjust the peptide
concentration to 1 mg/ml. Next, 0.1 ml Dulbecco's modified Eagle's
medium/nutrient mixture F12 (DMEM-F12; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added to 0.1 ml peptide to
trigger self-assembly.
A greater nanofiber gel viscosity resulted in better
performance of the gel. Based on the preliminary results, IKVAV,
FGL-PA and RGD were used for the formula screening (22). The appropriate proportion and
concentration of IKVAV, FGL-PA and RGD were screened to prepare the
optimum nanofibers gel for the appropriate times as follows: i)
IKVAV:RGD = 2:1 (4.5 sec); ii) IKVAV:RGD = 1:1 (4 sec); iii)
IKVAV:RGD = 1:2 (5 sec); iv) IKVAV:FGL-PA = 2:1 (5.5 sec); v)
IKVAV:FGL-PA = 1:1 (6 sec); vi) IKVAV:FGL-PA = 1:2 (7 sec); vii)
RGD:FGL-PA = 2:1 (8 sec); viii) RGD:FGL-PA = 1:1 (8.2 sec); ix)
RGD:FGL-PA = 1:2 (8.5 sec); x) IKVAV:RGD:FGL-PA = 1:1:1 (11 sec);
xi) IKVAV:RGD:FGL-PA = 3:2:1 (9 sec); xii) IKVAV:RGD:FGL-PA = 1:2:3
(10 sec).
Preparation of nanofiber
gel-encapsulated HPE
HPE was obtained from a 28 year-old patient
recruited from the Union Hospital of the Tongi Medical College
(Wuhan, China) on February 2014. According to the appropriate
proportion and concentration, IKVAV, FGL-PA and RGD were added into
the HPE, and then mixed with an equal volume DMEM-F12 to trigger
self-assembly at room temperature. The solution was then diluted 0,
50 and 100 times with distilled water and observed at
magnifications of x30,000, x39,000, x65,000, x93,000 and x135,000
using a Tecnai-10 transmission electron microscope (Philips,
Amsterdam, Netherlands).
Transmission electron microscopy
The morphology of the HPE nanofiber gels was
analyzed using a Tecnai-10 transmission electron microscope
(Philips). Briefly, following dilution (1:100) in water, the sample
was negatively-stained with 1% (weight/volume) phosphotungstic acid
(Shanghai Muhong Industrial Co., Ltd., Shanghai, China) for 5 min
at 25°C. Subsequently, the sample was placed on copper film grids
(Wuhan Boster Biological Technology, Ltd., Wuhan, China) and
observed using transmission electron microscopy after drying for 10
min at 25°C (23).
Particle size determination
After diluting 100-fold with distilled water, the
mean particle size of the HPE nanofiber gel was determined using
Zetasizer 3000 HS (Malvern Instruments Ltd., Malvern, UK). Three
parallel measurements were performed for each sample as previously
described (24).
Encapsulation efficiency and drug
loading testing of HPE nanofiber gel
The encapsulation efficiency and drug loading was
analyzed by dissolving ~5 mg HPE nanofiber gel in either 1.2 ml
distilled water or absolute ethyl alcohol. Next, the mixture was
centrifuged at 8,000 × g for 10 min and the precipitation was
resuspended in 1.2 ml distilled water. The supernatant and
precipitation were detected using an ultraviolet (UV) detector
(Alpha 1500; Shanghai Lab-Spectrum Instruments Co., Ltd., Shanghai,
China) at 280 nm. The formula used to measure the encapsulation
efficiency was as follows: Encapsulation efficiency =
(concentration of supernatant + concentration of precipitation in
demulsified samples - concentration of precipitation in
non-demulsified samples)/total concentration of demulsified
samples. The formula used to measure drug loading was as follows:
Drug loading = actual drug concentration / (drug-loading particle +
drug concentration). Three parallel measurements were performed for
each sample.
Slow-releasing potential detection of
HPE nanofiber gel
The slow-releasing potential of the HPE nanofiber
gel was evaluated using a dialysis method. Briefly, dialysis bags
(molecular weight cut-off, 1,000 Da) containing 0.1 ml HPE
nanofiber gel were immersed in a thermostatic gas bath containing
20 ml release medium (phosphate-buffered saline, pH 7.2) at 37°C.
At predefined intervals (0.25, 0.5, 1, 1.5, 2, 4, 6, 9, 12, 24, 48,
72, and 96 h), 1 ml aliquots of phosphate-buffered saline (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) were withdrawn
and replaced with the same volume of release medium. The HPE
release behavior of the nanofiber gel was determined using a UV
spectrophotometer (Agilent 8453; Agilent Technologies, Santa
Barbara, CA, USA) at 280 nm. The measured concentrations were
calculated according to the standard curve, followed by calculation
of the cumulative release rate (22).
Crystal structure validation of HPE
nanofiber gel
The HPE stock solution and nanofiber gel were
diluted 100 times with distilled water. The solution was stirred
for 10 min at 20 × g. The crystal structure of the HPE stock
solution and HPE nanofiber gel was then evaluated using an X-ray
diffractometer (Siemens D5000; Siemens AG, Munich, Germany). The
condition of the diffraction was as follows: λ = 1.5064 A°;
voltage, 20 kV; electric current, 20 mA; 2θ scanning in the range
of 10° to 80°; scanning frequency, 0.02° Q/S.
Statistical analysis
Statistical analysis was performed using the SPSS
version 16 software (SPSS, Inc., Chicago, IL, USA). The statistical
significance of differences was determined using one-way analysis
of variance. All statistical tests were two-sided, and P<0.05
was considered to indicate a statistically significant difference.
Results are presented as the mean ± standard deviation.
Results
Preparation and characterization of
HPE encapsulated in nanofiber gels
The IKVAV, RAD16, FGL-PA and RGD peptides were
synthesized and used to form nanofiber skeletons using the
self-assembly method via electrostatic and hydrophobic
interactions. After a preliminary screening, IKVAV, RGD and FGL-PA
were able to form nanofiber skeletons with greater viscosity.
Twelve different combinations of IKVAV, RGD and FGL-PA were used
for the formula screening. The results indicated that the optimal
proportion of the nanofiber gel was IKVAV:RGD:FGL-PA = 1:1:1 and
the optimal concentration was 0.6 mg/ml, as determined by observing
the liquidity, diffusion time and viscosity.
Subsequently, the HPE was encapsulated into the
nanofibers. The morphology of the HPE nanofibers was observed using
transmission electron microscopy. The results revealed that the HPE
nanofibers had a fibrous appearance under different dilutions. With
the increase of the dilution, the nanofiber gel appeared to be more
homogeneously dispersed. At a dilution of 100, the nanofiber gel
was found to be directly observed (Fig.
1).
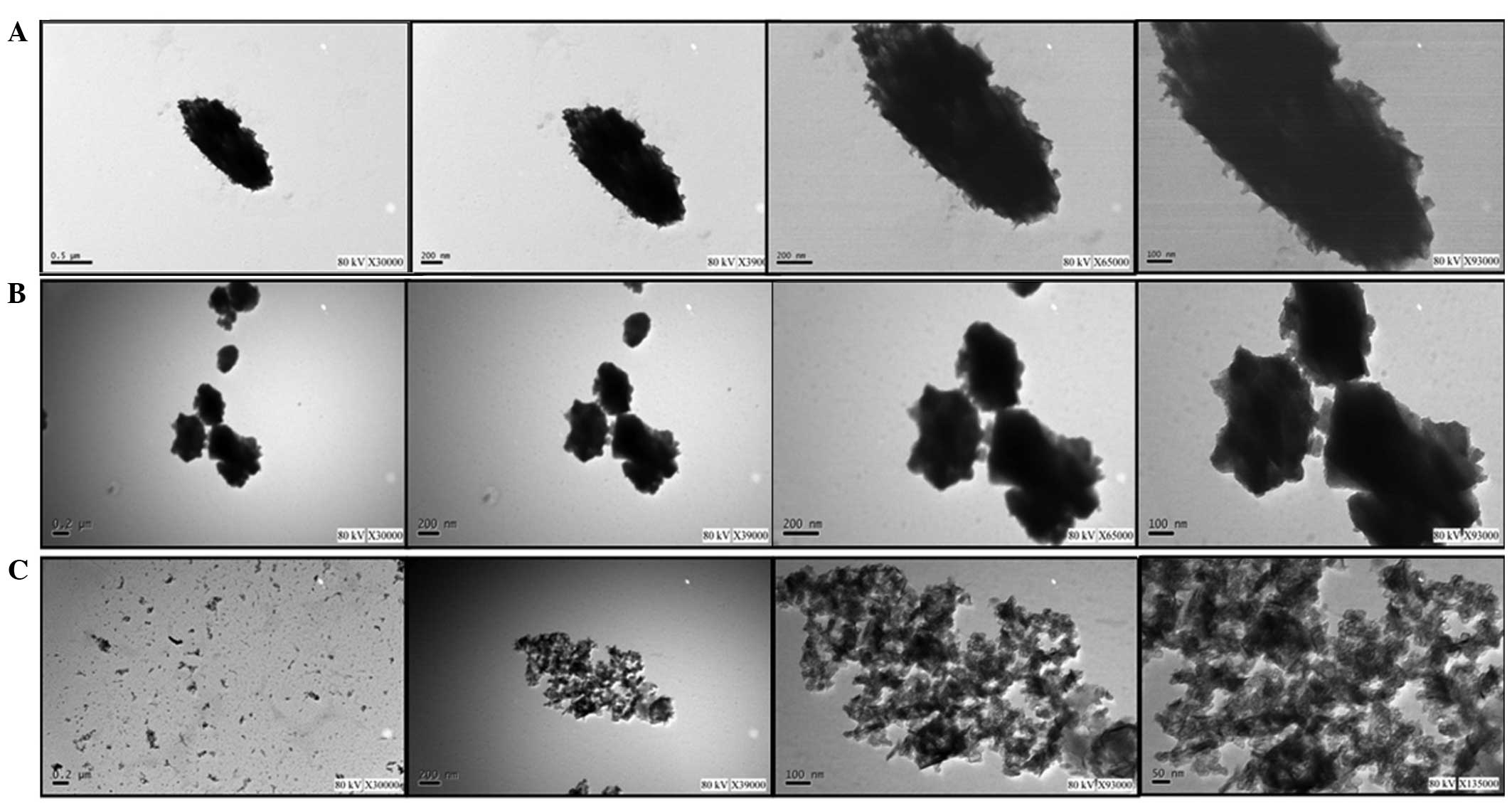 | Figure 1.Morphology of human placental extract
encapsulated in nanofibers, as observed using transmission electron
microscopy. Different dilutions were used, including dilution by
(A) 0, (B) 50 and (C) 100 times. The four images in each part of
the figure were captured at different magnifications. In (A) and
(B), the magnification is x30,000, x39,000, x65,000 and x93,000
(from left to right), while in (C) the magnifications are x30,000,
x39,000, x93,000 and x135,000 (from left to right). |
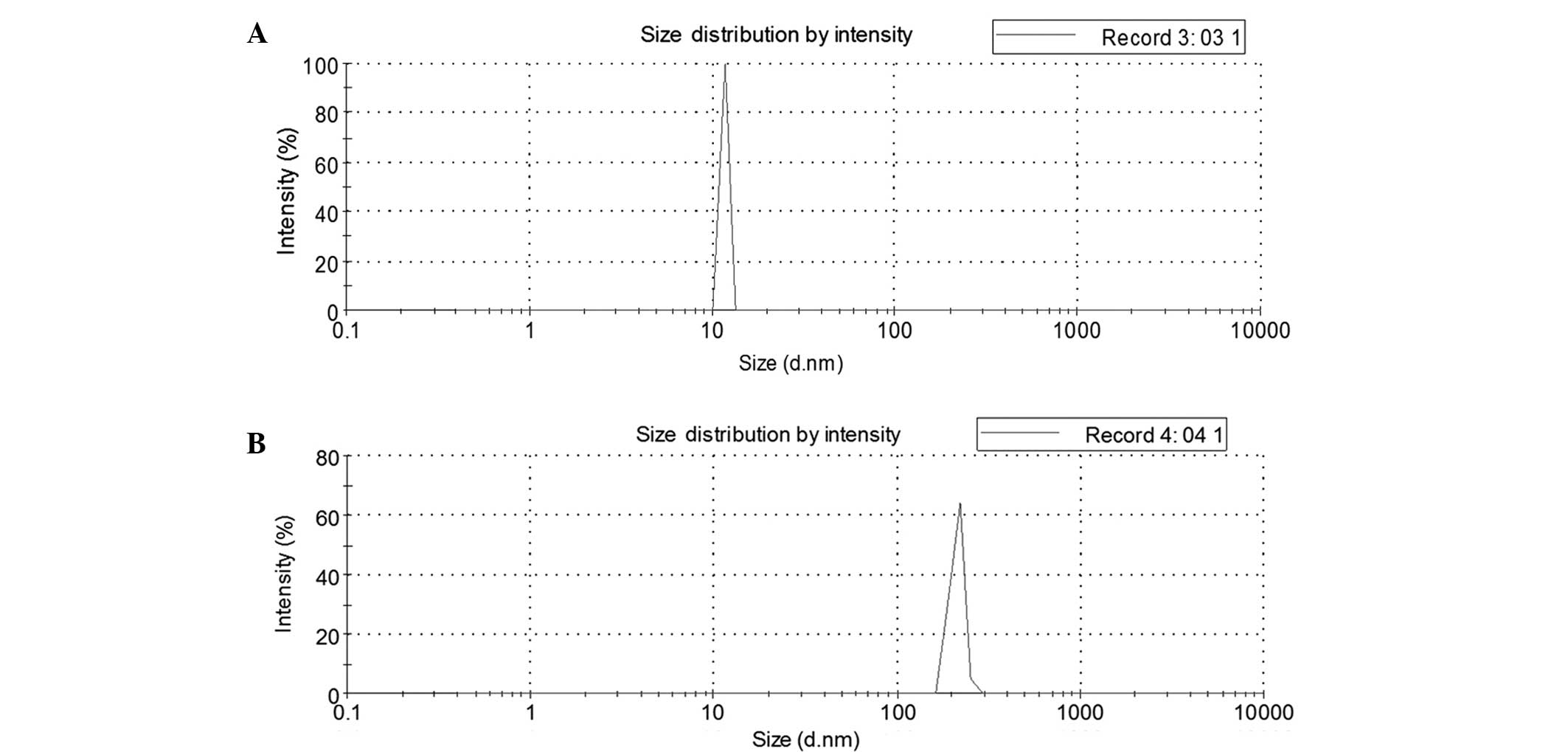
As shown in Fig. 2,
the mean particle widths of the vehicle and of the HPE nanofiber
gels were ~11.7 and 212 nm, respectively.
Encapsulation efficiency and in vitro
release of HPE
The results of UV spectrophotometric analysis
revealed that the encapsulation efficiency of all three HPE samples
(dilution performed in triplicate) was >90% (96.8, 98.3 and
94.8%), with a mean encapsulation efficiency of 96.6±1.75%. The
drug loading of the three samples (dilution performed in
triplicate) was found to be 5.2, 5.13 and 5.11 mg/g, with a mean
drug loading of 5.15±0.04 mg/g (data not shown).
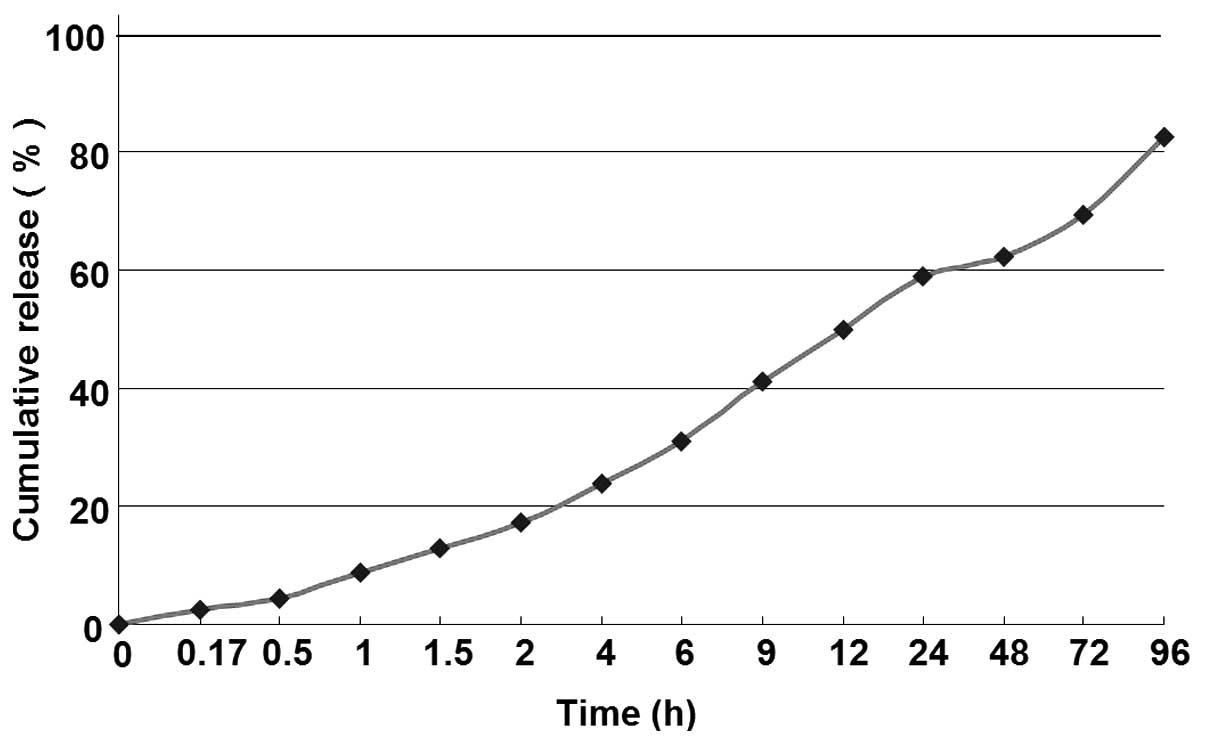
The in vitro release of HPE nanofiber gel was
examined using a dialysis method. The drug release profile was
performed under simulated physiologic conditions (pH 7.2). As shown
in Fig. 3, the HPE nanofiber gel
showed a sustained drug release profile, with a total HPE release
from the nanofibers of 2.35–82.58%. The nanofiber gel exhibited a
slow-releasing profile (>4 days).
Crystal structure validation of HPE
nanofiber gel
The results indicated that the peak number and time
of HPE nanofiber gel was similar to that of the HPE stock solution.
The preliminary results confirmed that the structure of HPE
encapsulated in the nanofiber gel was unvaried compared with the
HPE structure.
Discussion
The absolute or relative reduction of exogenous
growth factor and its receptor is one of the main reasons why the
treatment of chronic soft tissue ulcer is challenging (25,26). The
topical application of active growth factors can promote the
healing of chronic ulcer (27);
however, our previous study revealed that the exogenous growth
factors were easily degraded and diluted due to their short
half-life (28). Signaling pathways
that are able to awaken the body's self-healing mechanisms are
therefore required to promote the sustained release of endogenous
growth factors. Recent studies have shown that adenosine A2A
receptor activation may play an important role in the protection
and promotion of wound healing in ischemia, hypoxia, inflammation,
trauma and numerous other pathological processes (29,30). The
adenosine A2A receptor activation can inhibit the generation of
reactive oxygen species and promote the release of cytokines, such
as tumor necrosis factor-α, accelerate osteoblast, fibroblast and
fat precursor cell proliferation, as well as the release of VEGF,
angiogenin and glutamine transferase II, and thus promoting wound
healing (31,32). Therefore, selective activation of
adenosine A2A receptors awakens the body's self-healing mechanisms
(33).
Numerous studies have demonstrated the biological
actions of HPE in various diseases, and HPE has been used in wound
healing in several countries for years as a folk remedy (34,35).
Another study suggested that HPE exerts an anti-inflammatory
function that suppresses chemical mediators, and that these effects
may be associated with innate immune functions (36). Furthermore, previous studies
indicated that oligomeric DNA nucleotides, such as
polydeoxyribonucleotide (PDRN), are linear DNA nucleotide polymers
from HPE with a length range of 50–2,000 bp (37,38).
PDRN can efficiently activate adenosine A2A receptors, without
being degraded into small fragments, thus preventing the activation
of other adenosine receptors (39,40).
PRDN treatment for chronic soft tissue ulcer has been found to
significantly increase wound granulation, thus promoting wound
healing (41). However, the method
used to promote the PDRN late release will directly affect its
therapeutic effect on the chronic soft tissue ulcer.
To date, limited information is available regarding
the dosage of HPE required for wound healing. In the present study,
aqueous HPE was encapsulated in a novel nanofiber gel prepared by
the appropriate formulation of IKVAV, RGD, RAD16 and FGL-PA through
LbL assembly. Subsequently, the morphology, particle size, drug
loading efficacy, encapsulation rate, release efficacy and
structure validation were examined. The results showed that the
particle size of the HPE nanofiber gel was 212 nm, which was
included in the 1–1,000 nanometer materials. Following the
detection of the encapsulation efficiency and drug loading, the HPE
nanofiber gel was found to have high drug loading (5.15 mg/g) and
high encapsulation efficiency (96.6%). Furthermore, the HPE
nanofiber gel exhibited a slow-releasing profile (>4 days).
Investigation of the crystal diffraction structure confirmed that
the structure of HPE that was encapsulated in the nanofiber gel did
not undergo any apparent changes. Therefore, these novel composite
nanocapsules may offer a promising delivery system for HPE.
Acknowledgements
The present study was supported by the National
Natural Science Fund of China (grant no. 81271968).
References
|
1
|
De D, Chakraborty PD and Bhattacharyya D:
Regulation of trypsin activity by peptide fraction of an aqueous
extract of human placenta used as wound healer. J Cell Physiol.
226:2033–2040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De D, Chakraborty PD and Bhattacharyya D:
Analysis of free and bound NADPH in aqueous extract of human
placenta used as wound healer. J Chromatogr B Analyt Technol Biomed
Life Sci. 877:2435–2442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Li-jie and Wang Zhi-ping: An analysis
for 50 examples of curative effect about ulcer powder for external
use curing skin soft tissue injury. China Practical Medicine.
3:13–14. 2008.
|
|
4
|
Nath S and Bhattacharyya D: Cell adhesion
by aqueous extract of human placenta used as wound healer. Indian J
Exp Biol. 45:732–738. 2007.PubMed/NCBI
|
|
5
|
Chakraborty PD and Bhattacharyya D:
Isolation of fibronectin type III like peptide from human placental
extract used as wound healer. J Chromatogr B Analyt Technol Biomed
Life Sci. 818:67–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beeson CC, Beeson GC, Buff H, Eldridge J,
Zhang A, Seth A, Demcheva M, Vournakis JN and Muise-Helmericks RC:
Integrin-dependent Akt1 activation regulates PGC-1 expression and
fatty acid oxidation. J Vasc Res. 49:89–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muise-Helmericks RC, Demcheva M, Vournakis
JN and Seth A: Poly-N-acetyl glucosamine fibers activate bone
regeneration in a rabbit femur injury model. J Trauma. 71(2 Suppl
1): S194–S196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Erba P, Adini A, Demcheva M, Valeri CR and
Orgill DP: Poly-N-acetyl glucosamine fibers are synergistic with
vacuum-assisted closure in augmenting the healing response of
diabetic mice. J Trauma. 71(2 Suppl 1): S187–S193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lindner HB, Zhang A, Eldridge J, Demcheva
M, Tsichlis P, Seth A, Vournakis J and Muise-Helmericks RC:
Anti-bacterial effects of poly-N-acetyl-glucosamine nanofibers in
cutaneous wound healing: Requirement for Akt1. PloS One.
6:e189962011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scherer SS, Pietramaggiori G, Matthews J,
Perry S, Assmann A, Carothers A, Demcheva M, Muise-Helmericks RC,
Seth A, Vournakis JN, et al: Poly-N-acetyl glucosamine nanofibers:
A new bioactive material to enhance diabetic wound healing by cell
migration and angiogenesis. Ann Surg. 250:322–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang F, Yeh CK, Wen J and Sun Y:
N-trimethylchitosan/alginate layer-by-layer self-assembly coatings
act as ‘fungal repellents’ to prevent biofilm formation on
healthcare materials. Adv Healthc Mater. 4:469–475. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv H, Chen Z, Yang X, Cen L, Zhang X and
Gao P: Layer-by-layer self-assembly of minocycline-loaded
chitosan/alginate multilayer on titanium substrates to inhibit
biofilm formation. J Dent. 42:1464–1472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee H, Hong D, Choi JY, Kim JY, Lee SH,
Kim HM, Yang SH and Choi IS: Layer-by-layer-based silica
encapsulation of individual yeast with thickness control. Chem
Asian J. 10:129–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi Y, Choi S, Jeong HY, Liu M, Kim BS
and Kim G: Highly efficient layer-by-layer-assisted infiltration
for high-performance and cost-effective fabrication of
nanoelectrodes. ACS Appl Mater Interfaces. 6:17352–17357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Z, Yao Y, Han L and Che S: Control
of chiral nanostructures by self-assembly of designed amphiphilic
peptides and silica biomineralization. Chemistry. 20:17068–17076.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shah RN, Shah NA, Del Rosario Lim MM,
Hsieh C, Nuber G and Stupp SI: Supramolecular design of
self-assembling nanofibers for cartilage regeneration. Proc Natl
Acad Sci USA. 107:3293–3298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun X, Xu Y, Wang C and Li Q:
Self-assembly of RGD peptide nanofiber hydrogels and its
biocompatibility studies combined with adipose derived stem cells.
Acta Universitatis Medicinalis Anhui. 46:1253–1256. 2011.
|
|
18
|
Sehgal RR and Banerjee R:
IKVAV-functionalized self-assembling peptide hydrogel for improved
neural stem cell transplantation. Nanomedicine (Lond). 8:521–522.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Z, Zheng Q, Wu Y and Wu B:
Biocompatibility of FGL peptide self-assembly nano-fibers with
neural stem cells in vitro. Chinese Journal of Reconstructive
surgery. 22:1369–1372. 2008.(In Chinese).
|
|
20
|
Martínez-Ramos C, Arnal-Pastor M,
Vallés-Lluch A and Pradas MM: Peptide gel in a scaffold as a
composite matrix for endothelial cells. J Biomed Mater Res A.
103:3293–3302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao C, Hedrick M, Pareek G, Renzulli J,
Haleblian G and Webster TJ: Nanostructured
polyurethane-poly-lactic-co-glycolic acid scaffolds increase
bladder tissue regeneration: An in vivo study. Int J
Nanomedicine. 8:3285–3296. 2013.PubMed/NCBI
|
|
22
|
Gu X, Wang J, Wang Y, Wang Y, Gao H and Wu
G: Layer-by-layer assembled polyaspartamide nanocapsules for
pH-responsive protein delivery. Colloids Surf B Biointerfaces.
108:205–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parwe SP, Chaudhari PN, Mohite KK, Selukar
BS, Nande SS and Garnaik B: Synthesis of ciprofloxacin-conjugated
poly (L-lactic acid) polymer for nanofiber fabrication and
antibacterial evaluation. Int J Nanomedicine. 9:1463–1477.
2014.PubMed/NCBI
|
|
24
|
Qi R, Guo R, Zheng F, Liu H, Yu J and Shi
X: Controlled release and antibacterial activity of
antibiotic-loaded electrospun halloysite/poly (lactic-co-glycolic
acid) composite nanofibers. Colloids Surf B Biointerfaces.
110:148–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coull AF, Atherton I, Taylor A and
Watterson AE: Prevalence of skin problems and leg ulceration in a
sample of young injecting drug users. Harm Reduct J. 11:222014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okrainec K, Booth GL, Hollands S and Bell
CM: Impact of language barriers on complications and mortality
among immigrants with diabetes: A population-based cohort study.
Diabetes Care. 38:189–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levy A, Kopplin K and Gefen A: An
air-cell-based cushion for pressure ulcer protection remarkably
reduces tissue stresses in the seated buttocks with respect to
foams: Finite element studies. J Tissue Viability. 23:13–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu G, Wang J, Yang S, Xu W, Ye S and Xia
T: Effect of a porous tantalum rod on early and intermediate stages
of necrosis of the femoral head. Biomed Mater. 5:0650032010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keränen H, Gutiérrez-de-Terán H and Aqvist
J: Structural and energetic effects of A2A adenosine receptor
mutations on agonist and antagonist binding. PloS One.
9:e1084922014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim DG and Bynoe MS: A2A adenosine
receptor regulates the human blood-brain barrier permeability. Mol
Neurobiol. 52:664–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Metsola J, Maksimow M, Ojaniemi M, Metsola
H, Marttila-Ichihara F, Vuolteenaho R, Yegutkin GG, Salmi M,
Hallman M and Jalkanen S: Postnatal development and LPS
responsiveness of pulmonary adenosine receptor expression and of
adenosine-metabolizing enzymes in mice. Pediatr Res. 76:515–521.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Beggiato S, Antonelli T, Tomasini MC,
Borelli AC, Agnati LF, Tanganelli S, Fuxe K and Ferraro L:
Adenosine A2A-D2 receptor-receptor interactions in putative
heteromers in the regulation of the striato-pallidal GABA pathway:
Possible relevance for Parkinson's disease and its treatment. Curr
Protein Pept Sci. 15:673–680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuxe K, Borroto-Escuela D, Fisone G,
Agnati LF and Tanganelli S: Understanding the role of
heteroreceptor complexes in the central nervous system. Curr
Protein Pept Sci. 15:6472014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mitsui Y, Bagchi M, Marone PA, Moriyama H
and Bagchi D: Safety and toxicological evaluation of a novel,
fermented, peptide-enriched, hydrolyzed swine placenta extract
powder. Toxicol Mech Methods. 25:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bogacz B, Bartkowiak-Wieczrek J,
Mikołajczak PŁ, Rakowska-Mrozikiewicz B, Grześkowiak E, Wolski H,
Czerny B and Mrozikiewicz PM: The influence of soybean extract on
the expression level of selected drug transporters, transcription
factors and cytochrome P450 genes encoding phase I
drug-metabolizing enzymes. Ginekol Pol. 85:348–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mrozikiewicz PM, Bogacz A, Czerny B,
Karasiewicz M, Kujawski R, Mikolajczak PL, Seremak-Mrozikiewicz A,
Grzeskowiak E and Bobkiewicz-Kozlowska T: The influence of a
standardized soybean extract (Glycine max) on the expression level
of cytochrome P450 genes in vivo. Ginekol Pol. 81:516–520.
2010.PubMed/NCBI
|
|
37
|
Bitto A, Polito F, Irrera N, D'Ascola A,
Avenoso A, Nastasi G, Campo GM, Micali A, Bagnato G, Minutoli L,
Marini H, et al: Polydeoxyribonucleotide reduces cytokine
production and the severity of collagen-induced arthritis by
stimulation of adenosine A(2A) receptor. Arthritis
Rheum. 63:3364–3371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Minutoli L, Arena S, Bonvissuto G, Bitto
A, Polito F, Irrera N, Arena F, Fragalà E, Romeo C, Nicotina PA, et
al: Activation of adenosine A2A receptors by
polydeoxyribonucleotide increases vascular endothelial growth
factor and protects against testicular damage induced by
experimental varicocele in rats. Fertil Steril. 95:1510–1513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Squadrito F, Bitto A, Altavilla D,
Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino
C, Minutoli L, et al: The effect of PDRN, an adenosine receptor A2A
agonist, on the healing of chronic diabetic foot ulcers: Results of
a clinical trial. J Clin Endocrinol Metab. 99:E746–E753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bitto A, Oteri G, Pisano M, Polito F,
Irrera N, Minutoli L, Squadrito F and Altavilla D: Adenosine
receptor stimulation by polynucleotides (PDRN) reduces inflammation
in experimental periodontitis. J Clin Periodontol. 40:26–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Minutoli L, Antonuccio P, Squadrito F,
Bitto A, Nicotina PA, Fazzari C, Polito F, Marini H, Bonvissuto G,
Arena S, Morgia G, et al: Effects of polydeoxyribonucleotide on the
histological damage and the altered spermatogenesis induced by
testicular ischaemia and reperfusion in rats. Int J Androl.
35:133–144. 2012. View Article : Google Scholar : PubMed/NCBI
|