Introduction
Cerebral ischemia occurs as a result of insufficient
blood flow to the brain (1,2), which leads to limited oxygen supply, or
cerebral hypoxia, and may eventually result in the death of brain
tissue, cerebral infarction or ischemic stroke (3). It is a leading cause of death and the
predominant cause of adult disability (4). At present, there is no specific therapy
for improving functional recovery.
Mesenchymal stem cells (MSCs) are a class of adult
progenitor cells capable of differentiating into several cell types
(5–8). Several previous studies have indicated
that bone marrow derived MSCs may serve as a source of cells for
cell transplantation therapy following cerebral ischemia and have
been successfully used for the treatment of experimental stroke
(9–11). In the aforementioned studies, bone
marrow derived MSCs selectively targeted injured brain tissue and
promoted functional recovery via various cell delivery routes.
Although the transplantation of bone marrow derived MSCs has been
revealed to provide therapeutic benefit to cerebral ischemia, the
underlying mechanisms have yet to be elucidated.
The adult mammalian central nervous system (CNS)
possesses a poor capability to regenerate axons following injury.
The failure of CNS axons to regenerate after damage has significant
consequences for brain ischemia (12). Several factors contributing to this
regenerative failure include the intrinsic state of the injured
neuron, the formation of the inhibitory glial scar and the presence
of inhibitory myelin debris. A previous study demonstrated that
myelin-associated neurite growth inhibitors had a crucial role in
preventing CNS regeneration after brain injury (13). Three inhibitors of regeneration have
been identified in myelin thus far, including: Nogo-A,
oligodendrocyte myelin glycoprotein (OMgp) and myelin-associated
glycoprotein (MAG). These molecules are membrane proteins of myelin
and expressed predominately by oligodendrocytes in the adult
mammalian CNS (12,14–17).
It has been previously reported that numerous brain
neurons in ischemia penumbia undergo apoptosis following either
global or focal ischemic insults (18). Apoptosis is a unique, gene-directed
form of cell death, and is characterized by nuclear condensation
and cytoplasmic fragmentation that contributes to physiological and
pathological processes (19). The
Caspases, in particular Caspase-3, perform a key role in the
execution of apoptosis (20). B-cell
lymphoma 2 (Bcl-2) is the crucial member of the Bcl-2 family of
regulator proteins that regulate apoptosis, and is able to inhibit
apoptosis without affecting cellular proliferation (21,22).
In the present study, the intraluminal model of
transient middle cerebral artery occlusion (MCAO) was established
in rats to investigate the effects of the transplantation of bone
marrow derived MSCs on CNS functional recovery. It was determined
in the present study that the transplantation of bone marrow
derived MSCs promoted the functional recovery of the CNS following
brain ischemia via the inhibition of the expression of Nogo-A, OMgp
and MAG, as well as neuronal apoptosis.
Materials and methods
Rats
A total of 90 healthy adult male Sprague-Dawley (SD)
rats weighing 220–280 g, and 15 healthy adult male SD rats weighing
80–120 g, were obtained from the Experimental Animal Center of the
Second Affiliated Hospital of Harbin Medical University (Harbin,
China). The rats were maintained in a room at 23±1°C under a 12-h
light/dark cycle with ad libitum access to food and water.
All experiments were performed with approval from the Ethics
Committee of Harbin Medical University.
Rat MCAO model
Adult male SD rats (n=90; weight, 220–280 g) were
used to establish the intraluminal model of transient MCAO. A
transient (2-h) MCAO was induced using a modified version of the
method described by Longa et al (23). Briefly, rats were anesthetized by
intraperitoneal injection with 10% chloral hydrate (0.35 ml/100 g;
Sigma-Aldrich, St. Louis, MO, USA) and placed in the supine
position. The left common carotid artery (CCA), external carotid
artery and internal carotid artery (ICA) were exposed and isolated
through a carotid midline incision (2.0–2.5 cm). A surgical nylon
suture 0.234 mm in diameter and wax-coated tip was advanced from
the CCA into the lumen of the ICA until it blocked the origin of
the middle cerebral artery. From the bifurcation of the CCA, 18–20
mm of the nylon suture was in the lumen and 1 cm was left outside.
After 2 h occlusion, the suture was withdrawn to permit reperfusion
until resistance was felt. Neurological examinations, as described
by Longa et al (23), were
performed subsequent to the onset of occlusion. The neurological
results were scored using a 5-point scale as follows: A score of 0
indicated no neurological deficit; 1, (failure to extend left
forepaw fully) indicated a mild focal neurological deficit; 2,
(circling to the left) indicated a moderate focal neurological
deficit and 3, (falling to the left) indicated a severe focal
deficit. Rats with a score of 4 did not walk unaided and had a
decreased level of consciousness. A total of 90 rats with a score
of 1–3 were considered as suitable models of MCAO and were used in
further experiments.
Bone marrow-derived MSCs culture
MSCs were harvested from 15 SD rats (weight, 80–120
g). Primary MSCs were isolated from the bone marrow of the tibias
and femurs of the aforementioned rats by their adherence to plastic
in vitro culture. At 3 days following MCAO, the rats were
sacrificed by cervical dislocation following anesthetization by
intraperitoneal injection with 10% chloral hydrate (0.35 ml/100 g).
Under sterile conditions, tibias and femurs were harvested, the
adherent soft tissue was removed and the ends of the bones were
excised toward the start of the marrow cavity. Fresh bone marrow
was harvested aseptically by flushing the cavity of the bone with
needles filled with Dulbecco's modified Eagle's medium-low glucose
(DMEM-LG; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone). A single
cell suspension was prepared by gentle pipetting several times and
passing the cell suspension through a 200 mesh metal strainer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were
seeded into each tissue culture flask at a density of
106 cells/ml and cultured in an incubator (Forma
Scientific; Thermo Fisher Scientific, Inc.) containing 5%
CO2 at 37°C. After 48 h, non-adherent cells were removed
and the medium renewed and changed every 2 days thereafter. Once
cells reached ~90% confluency, as determined by phase contrast
microscopy (Eclipse 80i; Nikon Corporation, Tokyo, Japan), they
were passaged into culture flasks at a 1:2 ratio.
Cell labeling
In order to monitor the distribution of bone
marrow-derived MSCs in rat brains, 5-(and 6)-carboxyfluorescein
diacetate succinimidyl ester (CFSE; Fluka, Buchs, Switzerland) was
used to label the MSCs. Briefly, after trypsinization (Invitrogen;
Thermo Fisher Scientific, Inc.), cells at passage 3 were washed
twice and resuspended in 200 µl DMEM. The cell suspension was then
mixed with 200 µl labeling solution in a tube for 10 min at 37°C,
with periodical tapping of the tube. The reaction was terminated by
the addition of FBS, and the CFSE labeled cells were washed twice
and resuspended in 0.01 ml PBS. Cell viability was evaluated by
Trypan blue staining (Sigma-Aldrich). Only single cell suspensions
with 95% viability were used for implantation. Approximately
3×106 cells/ml of MSCs in 1 ml PBS were transplanted
into each rat.
Experimental groups and
transplantation
All rats (n=90) were randomly divided into 2 groups
at 24 h following the induction of MCAO or sham operation: MCAO
group (n=40), and MSCs group (n=50). Rats in the MCAO group
received 1 ml 0.01 M PBS via an intravenous injection. Rats in the
MSCs group received CFSE-labeled 3×106 MSCs in 1 ml 0.01
M PBS via an intravenous injection. All transplantation procedures
were performed under aseptic conditions and rats that received
transplantation were selected at random.
Distribution of CFSE-labeled bone
marrow derived MSCs
Three days subsequent to the establishment of MCAO,
10 rats in the MSCs group were sacrificed, and their brains were
removed and stored in liquid nitrogen. Coronal blocks (2 mm)
containing brain tissues were cut anterior and posterior to the
optic chiasm, and cryostat sections were prepared. Subsequently,
the survival and distribution of CFSE-labelled MSCs were observed
using an inverted fluorescence microscope (Eclipse 80i).
Behavioral testing
Modified neurological severity scores (mNSS)
described by Chen et al (9)
were employed to evaluate neurological deficits in rats. In all
rats, a series of NSS tests was performed on days 3, 7 and 14
following the establishment of MCAO by 2 investigators who were
blind to the experimental groups. Neurological function was scored
on a scale of 0 to 11 (normal score, 0; maximal deficit score, 11).
The severity of injury was scored by awarding 1 point each time the
rat was unable to perform the assessment or for the lack of a
tested reflex; thus, the higher the score, the more severe the
injury. This modified version of mNSS is composite of the motor,
sensory and reflex assessments.
Immunohistochemical assessment
Rats were anesthetized with 10% chloral hydrate
(0.35 ml/100 g, intraperitoneal injection). The rat brains were
removed and were postfixed in 4% paraformaldehyde (Sigma-Aldrich)
for 24 h, and embedded in paraffin. Sections were deparaffinized
and incubated with 3% H2O2 for 10 min to
block endogenous peroxidase activity. Brain sections were placed in
citrate buffer (pH 6) at 80 kPa for 2 min. Tissues were treated
with primary antibodies and incubated at 4°C for 12 h. Tissues were
incubated at room temperature for 20 min with horseradish
peroxidase-labeled goat anti-rat IgG antibody (1:200; cat. no.
ZB2307; ZSGB-BIO, Beijing, China). Stable 3,3′-diaminobenzidine
(ZSGB-BIO) was then used as a chromogen for light microscopy
(Eclipse 80i). Counterstaining of sections was performed with
hematoxylin and eosin (Shanghai BetterBioChem Co., Ltd., Shanghai,
China). The primary antibodies used in the study were as follows:
Rabbit anti-Caspase-3 polyclonal antibody (1:100; PA5-16335;
LabVision/Neomarkers; Thermo Fisher Scientific, Inc.), rabbit
anti-Bcl-2 antibody (1:80; sc-492; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit anti-Nogo-A antibody (1:30; PA1060; Wuhan
Boster Biological Technology, Ltd., Wuhan, China), rabbit anti-OMgp
antibody (1:80; BS-1200R; BIOSS, Beijing, China) and rabbit
anti-MAG antibody (1:100; BS-0257R; BIOSS). Photomicrographs were
acquired at ×200 magnification under an inverted microscope
(Eclipse 80i). The distribution of Nogo-A, OMgp, MAG, Caspase-3, in
addition to Bcl-2 expression levels, were assessed by counting the
number of positively stained cells in 10 distinct regions of
ischemic penumbra in each section.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the rat brain tissue was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Brain tissue was homogenized in ~1 ml TRIzol
reagent. After homogenization, insoluble material from the
homogenate was removed by centrifuging at 10,387 × g for 10 min at
4°C. Following isopropyl alcohol precipitation, total RNA was
treated with DNase (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, in order to remove
contaminating DNA. cDNA was synthesized from 1 µg total RNA using
the SuperScript III Reverse-Transcriptase kit (Invitrogen; Thermo
Fisher Scientific, Inc.) in a final volume of 20 µl. The reactions
were incubated in 96-well optical plates at 95°C for 10 min,
followed by 40 cycles of 2 min at 95°C, 45 sec at 54°C, 45 sec at
68°C, and extension at 68°C for 8 min. qPCR was performed using the
SYBR Prime Script kit (Invitrogen; Thermo Fisher Scientific, Inc.)
and the Applied Biosystems 7500 Fluorescent Quantitative PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer
sequences used were as follows: Caspase-3 forward,
5′-CATGGCCCTGAAATACGAAGTC-3′ and reverse,
5′-CCGTCCCTTGAATTTCTCCAG-3′; Bcl-2 forward,
5′-GGGAGATCGTGATGAAGTACATCC-3′ and reverse,
5′-AGTTCCACAAAGGCATCCCAG-3′; β-actin forward,
5′-AAGAGAGGCATCCTGACCCTG-3′ and reverse, 5′-TCCAGACGCAGGATGGCATG-3′
(GenePharma Co., Ltd., Shanghai, China). Relative quantification
was performed using the 2−ΔΔCq method (24), with normalization to the β-actin
gene. As a negative control, RT-qPCR was performed using all
reagents except for the template, and for an RT-minus control,
RT-qPCR was conducted in the absence of reverse transcriptase.
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Statistical
significance of mNSS was analyzed using one-way analysis of
variance. All data are presented as the mean ± standard deviation
and analyzed for statistical significance by Pearson's
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
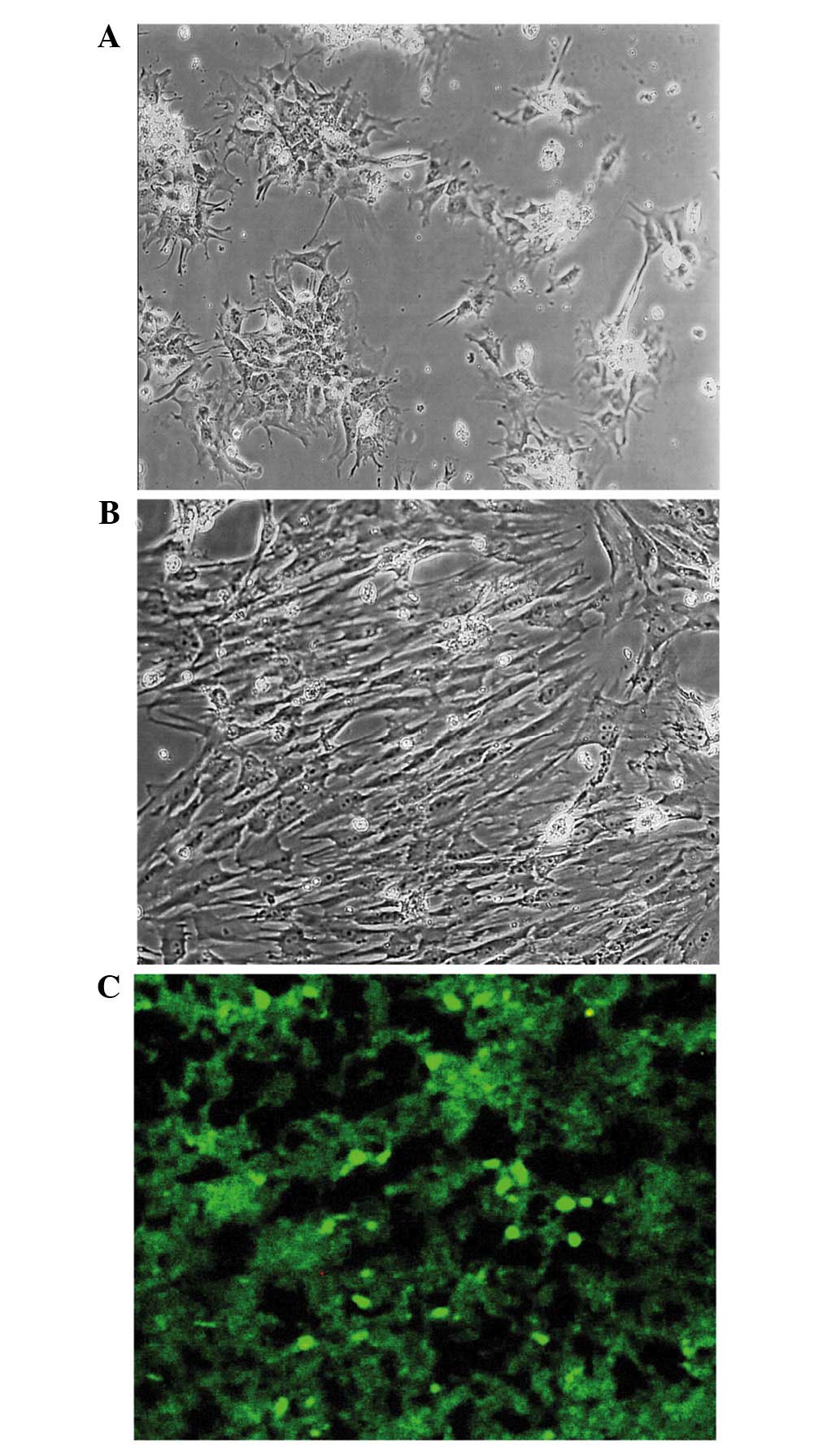
Morphology and MSC migration
MSCs were collected from the bone marrow of femurs
and tibias of SD rats and were evaluated morphologically using a
phase contrast microscope. Subsequent to 14 days of culture,
primary bone marrow derived MSCs reached 70% confluence and
exhibited a fibroblast-like morphology (Fig. 1A). The third passage of MSCs also
exhibited a fibroblastic morphology and reached 80–90% confluency
after 7 days of culture (Fig. 1B). A
model of MCAO was then established in rats to detect the effect of
transplantation of MSCs by intravenous injection to cerebral
ischemia. Green fluorescent CFSE-labeled MSCs were observed 3 days
following the establishment of MCAO using a fluorescence
microscope. CFSE-labeled MSCs migrated to the brains of rats and
survived in the ischemic brain (Fig.
1C).
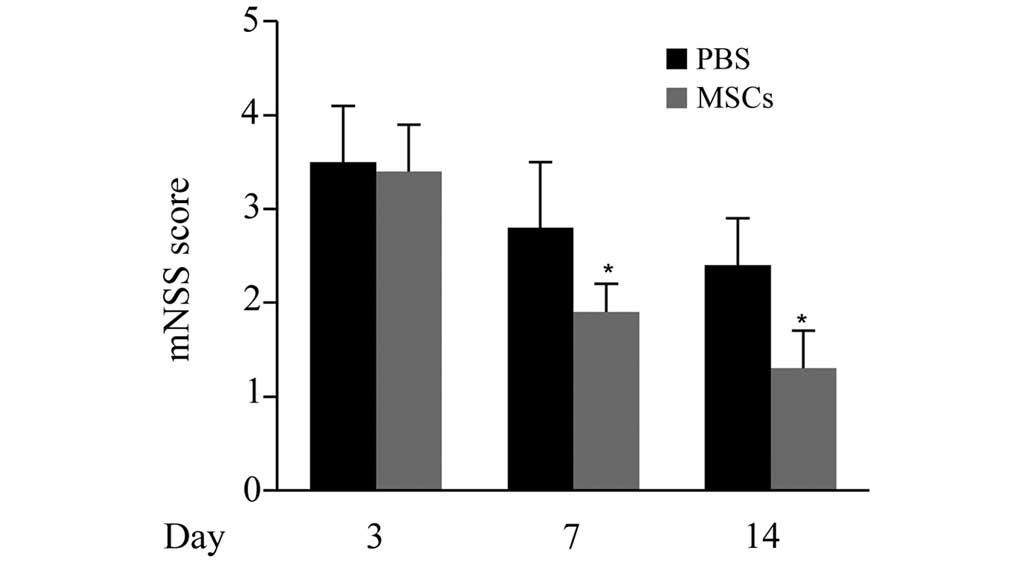
Evaluation of neurological
deficit
The cerebral ischemia-induced neurological deficits
were determined by mNSS assessments at 3, 7 and 14 days. As
displayed in Fig. 2, no significant
differences in mNSS scores were observed between the MSCs group and
the PBS group on day 3. However, on days 7 and 14, the mNSS scores
of the MSCs group were significantly lower compared with those of
the PBS group (P<0.05), indicating that the transplantation of
MSCs promoted the functional recovery of the CNS following cerebral
ischemia.
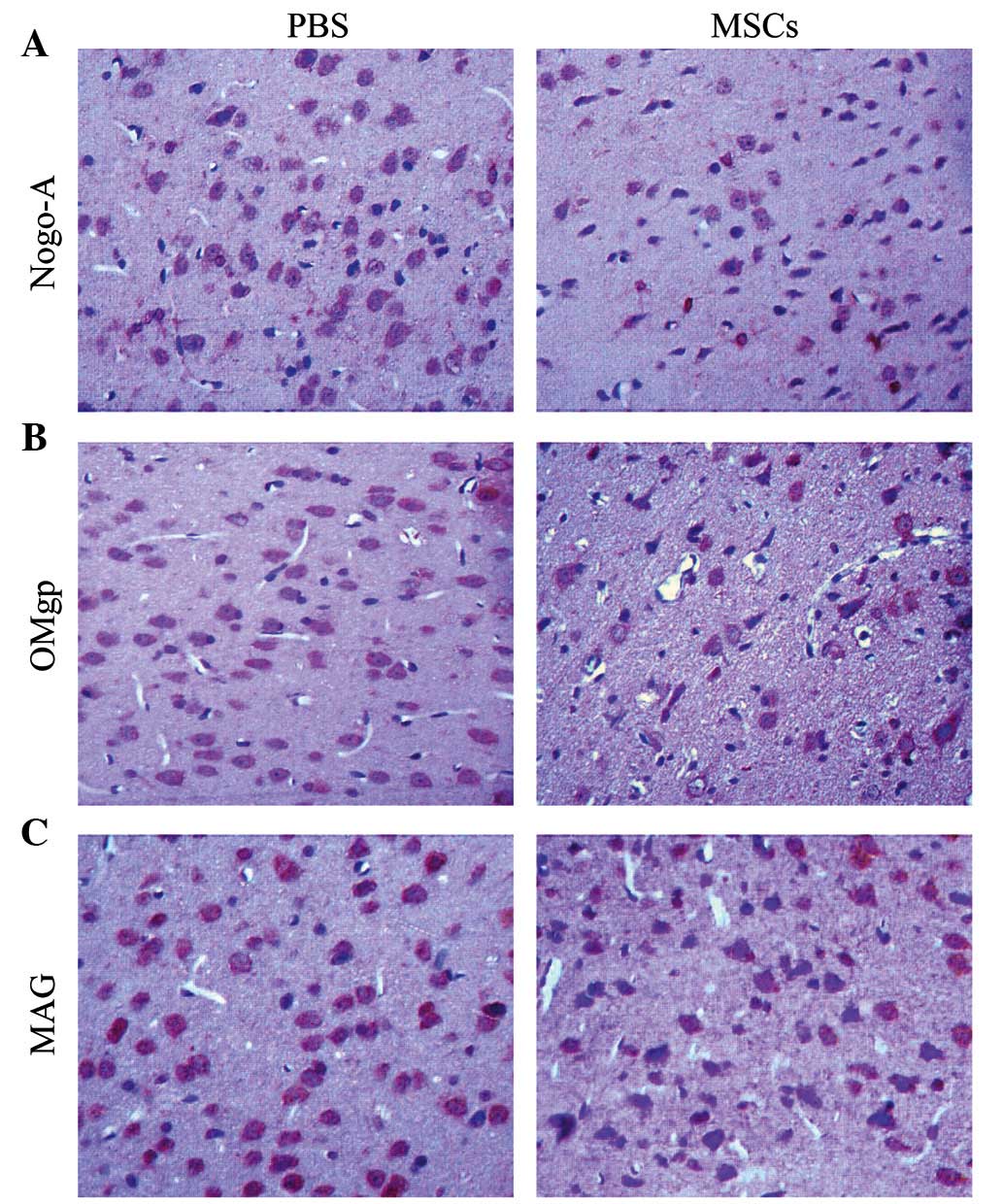
Expression of Nogo-A, MAG and
OMgp
As Nogo-A, MAG and OMgp are involved in the
prevention of CNS regeneration following brain injury, we
subsequently investigated whether transplantation of MSCs was able
to affect their cellular expression. The MSCs group displayed a
significantly reduced number of Nogo-A positive cells compared with
the PBS group on days 3 and 14 (P<0.05); however, the difference
was not significant on day 7 (Fig. 3
and Table I). With regard to OMgp,
the PBS and the MCAO group displayed a similar number of
OMgp-positive cells on day 3. However, the MSCs group displayed a
significantly lower number of OMgp-positive cells compared with the
MCAO group on days 7 and 14 (P<0.05; Table II). Furthermore, the MSCs group
displayed a significantly lower number of MAG-positive cells
compared with the MCAO group between days 3 and 14 (P<0.05;
Table III). The aforementioned
results suggest that the transplantation of MSCs reduced the
cellular expression levels of Nogo-A, OMgp and MAG and thus
contributed to the functional recovery of the CNS following
cerebral ischemia in rats.
 | Table I.Nogo-A-positively stained cells at
different time points (n=10). |
Table I.
Nogo-A-positively stained cells at
different time points (n=10).
| Group | Day 3 | Day 7 | Day 14 |
|---|
| PBS | 31.02±3.85 | 25.07±3.00 | 52.28±4.55 |
| MSCs |
24.97±4.20a | 21.93±2.68 |
40.77±6.68a |
 | Table II.Oligodendrocyte myelin
glycoprotein-positively stained cells at different time points
(n=10). |
Table II.
Oligodendrocyte myelin
glycoprotein-positively stained cells at different time points
(n=10).
| Group | Day 3 | Day 7 | Day 14 |
|---|
| PBS | 32.60±5.14 | 40.87±3.12 | 46.02±3.35 |
| MSCs | 30.42±3.58 |
31.22±3.22a |
32.55±5.94a |
 | Table III.Myelin-associated
glycoprotein-positively stained cells at different time points
(n=10). |
Table III.
Myelin-associated
glycoprotein-positively stained cells at different time points
(n=10).
| Group | Day 3 | Day 7 | Day 14 |
|---|
| MCAO | 68.48±6.35 | 66.70±5.01 | 67.32±6.69 |
| MSCs |
51.68±7.21a |
55.28±9.91a |
53.52±7.70a |
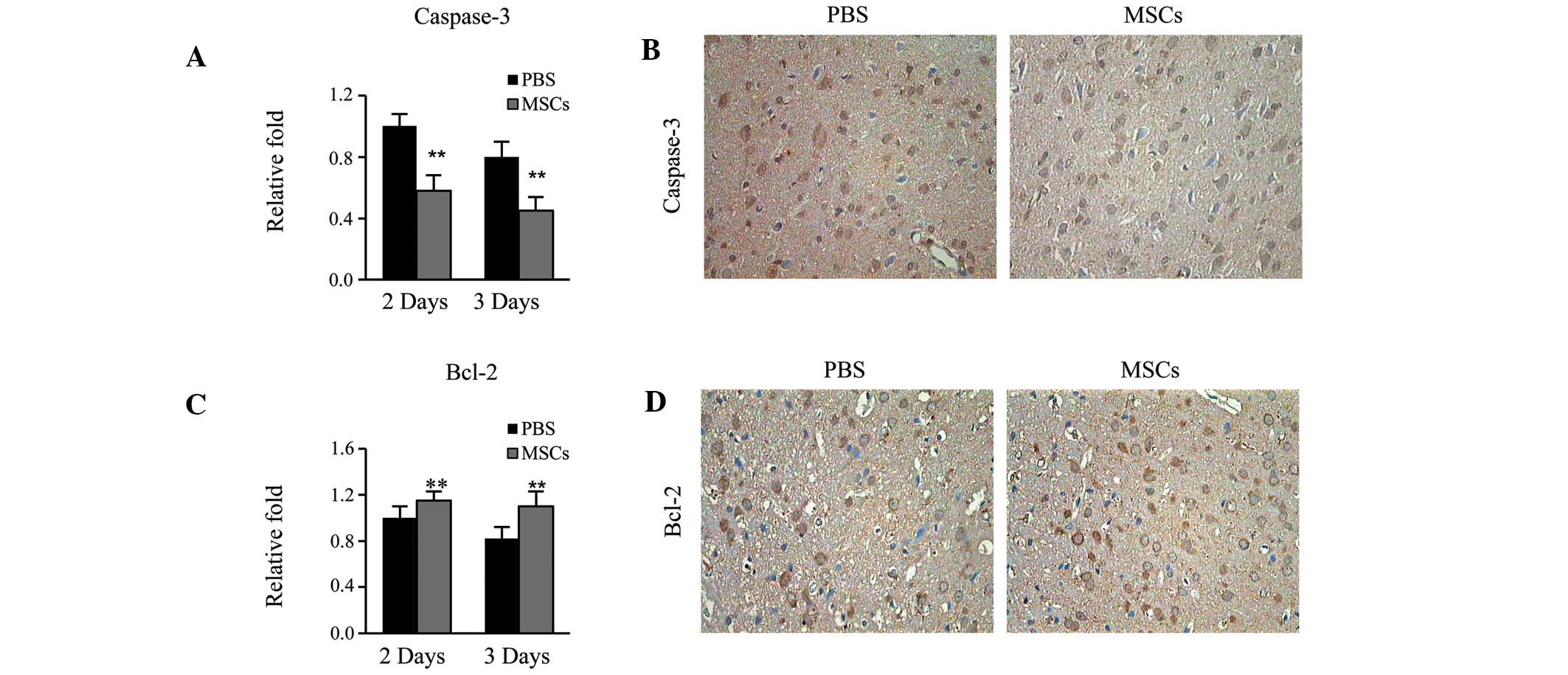
Expression of Caspase-3 and Bcl-2
Finally, it was determined whether the
transplantation of MSCs was able to affect cell apoptosis, which is
crucial factor in the functional recovery of CNS after cerebral
ischemia. MSCs group had significantly lower Caspase-3 mRNA
expression levels compared with the MCAO group on days 2 and 3
(P<0.01; Fig. 4A and B).
Conversely, the MSCs group displayed significantly higher Bcl-2
expression levels compared with the MCAO group on days 2 and 3
(P<0.01; Fig. 4C and D). Thus,
the present results indicate that the transplantation of MSCs is
able to reduce apoptosis, which was likely to have accounted for
the functional recovery of the CNS following cerebral ischemia.
Discussion
In the present study, the effect of transplantation
of bone marrow derived MSCs on functional recovery of CNS after
brain ischemia in a rat MCAO model was assessed, and the possible
underlying mechanisms investigated.
Initially, it was confirmed that transplantation of
MSCs provided therapeutic benefit to cerebral ischemia through the
establishment of a rat model of MCAO. MSCs were isolated from rats
and injected intravenously into rats with MCAO. The MSCs were able
to survive and migrate to the damaged brain, and the
transplantation of MSCs significantly improved the functional
recovery of the CNS.
The possible mechanisms by which transplantation of
MSCs promoted the functional recovery of the CNS following cerebral
ischemia were then examined. The present study determined that
transplantation of MSCs reduced the expression of axon regeneration
inhibitors, including Nogo-A, MAG and OMgp. Furthermore,
transplantation of MSCs inhibited neuronal apoptosis, decreasing
Caspase-3 and increasing Bcl-2 protein expression levels. The two
mechanisms function synergistically to promote the functional
recovery of the CNS following cerebral ischemia.
The underlying mechanisms by which transplantation
of MSCs reduces the expression of axon regeneration inhibitors and
inhibits neuronal apoptosis have yet to be elucidated. It has
previously been confirmed that MSCs constitutively secrete several
growth factors, including nerve growth factors (NGF), brain derived
neurotrophic factors (BDNF) and glioma derived neurotrophic factors
(GDNF) (25). Exogenous NGF has an
important role in neuronal plasticity and regenerative potential,
in addition to the inhibition of neural apoptosis (26). BDNF is able to support the survival
of injured CNS neurons in vitro and in vivo and
inducing neurite outgrowth (27).
GDNF is able to protect against neuronal death after brain injury
(28,29). Therefore, it is plausible that MSCs
secrete these growth factors, which in turn reduce the expression
of axon regeneration inhibitors and inhibit neuronal apoptosis.
In conclusion, the present study revealed that
transplantation of bone marrow derived MSCs promotes the functional
recovery of the CNS subsequent to cerebral ischemia through
decreasing the expression of myelin-associated inhibitors (MAIs)
and inhibiting neuronal apoptosis. We propose that inhibitors
targeting the aforementioned MAIs and the apoptotic pathway may
have potential for the treatment of cerebral ischemia.
Acknowledgements
This work was supported by grants from the Science
and Technology Project of the Education Department of Heilongjiang
Province (grant nos. 12531311 and 12521336) and the Science Project
of the Health Department of Heilongjiang Province (grant no.
2010-095).
References
|
1
|
DeGracia DJ, Sullivan JM, Neumar RW,
Alousi SS, Hikade KR, Pittman JE, White BC, Rafols JA and Krause
GS: Effect of brain ischemia and reperfusion on the localization of
phosphorylated eukaryotic initiation factor 2 alpha. J Cereb Blood
Flow Metab. 17:1291–1302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar R, Azam S, Sullivan JM, Owen C,
Cavener DR, Zhang P, Ron D, Harding HP, Chen JJ, Han A, et al:
Brain ischemia and reperfusion activates the eukaryotic initiation
factor 2alpha kinase, PERK. J Neurochem. 77:1418–1421. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White BC, Sullivan JM, DeGracia DJ, O'Neil
BJ, Neumar RW, Grossman LI, Rafols JA and Krause GS: Brain ischemia
and reperfusion: Molecular mechanisms of neuronal injury. J Neurol
Sci. 179:1–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krumholz HM, Brindis RG, Brush JE, Cohen
DJ, Epstein AJ, Furie K, Howard G, Peterson ED, Rathore SS, Smith
SC Jr, et al: Standards for statistical models used for public
reporting of health outcomes: An American Heart Association
scientific statement from the Quality of Care and Outcomes Research
Interdisciplinary Writing Group: Cosponsored by the Council on
Epidemiology and Prevention and The Stroke Council. Endorsed by the
American College of Cardiology Foundation. Circulation.
113:456–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eglitis MA, Dawson D, Park KW and
Mouradian MM: Targeting of marrow-derived astrocytes to the
ischemic brain. Neuroreport. 10:1289–1292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prockop DJ: Marrow stromal cells as stem
cells for continual renewal of nonhematopoietic tissues and as
potential vectors for gene therapy. J Cell Biochem. (Suppl 30–31):
284–285. 1998. View Article : Google Scholar
|
|
7
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eglitis MA and Mezey E: Hematopoietic
cells differentiate into both microglia and macroglia in the brains
of adult mice. Proc Natl Acad Sci USA. 94:4080–4085. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Li Y, Wang L, Lu M, Zhang X and
Chopp M: Therapeutic benefit of intracerebral transplantation of
bone marrow stromal cells after cerebral ischemia in rats. J Neurol
Sci. 189:49–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao LR, Duan WM, Reyes M, Keene CD,
Verfaillie CM and Low WC: Human bone marrow stem cells exhibit
neural phenotypes and ameliorate neurological deficits after
grafting into the ischemic brain of rats. Exp Neurol. 174:11–20.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honmou O, Onodera R, Sasaki M, Waxman SG
and Kocsis JD: Mesenchymal stem cells: Therapeutic outlook for
stroke. Trends Mol Med. 18:292–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie F and Zheng B: White matter inhibitors
in CNS axon regeneration failure. Exp Neurol. 209:302–312. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grados-Munro EM and Fournier AE:
Myelin-associated inhibitors of axon regeneration. J Neurosci Res.
74:479–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwab ME: Nogo and axon regeneration.
Curr Opin Neurobiol. 14:118–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prinjha R, Moore SE, Vinson M, Blake S,
Morrow R, Christie G, Michalovich D, Simmons DL and Walsh FS:
Inhibitor of neurite outgrowth in humans. Nature. 403:383–384.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Domeniconi M, Cao Z, Spencer T,
Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y,
et al: Myelin-associated glycoprotein interacts with the Nogo66
receptor to inhibit neurite outgrowth. Neuron. 35:283–290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vourc'h P and Andres C: Oligodendrocyte
myelin glycoprotein (OMgp): Evolution, structure and function.
Brain Res Brain Res Rev. 45:115–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lipton P: Ischemic cell death in brain
neurons. Physiol Rev. 79:1431–1568. 1999.PubMed/NCBI
|
|
19
|
Mattson MP and Chan SL: Calcium
orchestrates apoptosis. Nat Cell Biol. 5:1041–1043. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohan S, Abdul AB, Abdelwahab SI,
Al-Zubairi AS, Sukari MA, Abdullah R, Taha Elhassan MM, Ibrahim MY
and Syam S: Typhonium flagelliforme induces apoptosis in CEMss
cells via activation of caspase-9, PARP cleavage and cytochrome c
release: Its activation coupled with G0/G1 phase cell cycle arrest.
J Ethnopharmacol. 131:592–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cleary ML, Smith SD and Sklar J: Cloning
and structural analysis of cDNAs for bcl-2 and a hybrid
bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Otake Y, Soundararajan S, Sengupta TK, Kio
EA, Smith JC, Pineda-Roman M, Stuart RK, Spicer EK and Fernandes
DJ: Overexpression of nucleolin in chronic lymphocytic leukemia
cells induces stabilization of bcl2 mRNA. Blood. 109:3069–3075.
2007.PubMed/NCBI
|
|
23
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salgado AJ, Sousa JC, Costa BM, Pires AO,
Mateus-Pinheiro A, Teixeira FG, Pinto L and Sousa N: Mesenchymal
stem cells secretome as a modulator of the neurogenic niche: Basic
insights and therapeutic opportunities. Front Cell Neurosci.
9:2492015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Wu F, Kong X, Yang J, Chen H,
Deng L, Cheng Y, Ye L, Zhu S, Zhang X, et al: Nerve growth factor
improves functional recovery by inhibiting endoplasmic reticulum
stress-induced neuronal apoptosis in rats with spinal cord injury.
J Transl Med. 12:1302014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson JE, Barde YA, Schwab M and Thoenen
H: Brain-derived neurotrophic factor supports the survival of
cultured rat retinal ganglion cells. J Neurosci. 6:3031–3038.
1998.
|
|
28
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen
S, Perez-Polo JR and Yang K: Protective effects of bone marrow
stromal cell transplantation in injured rodent brain: Synthesis of
neurotrophic factors. J Neurosci Res. 80:611–619. 2005. View Article : Google Scholar : PubMed/NCBI
|