Introduction
Allicin is the active compound in garlic, a
well-researched remedy that is widely used as a spice and food
(1,2). It has been reported that garlic may
reduce cholesterol levels, lower blood pressure, inhibit platelet
aggregation, activate fibrinolysis and prevent atherosclerosis,
while it also has antioxidant and anticancer effects (3–12).
Garlic has also been reported to have an antiarrhythmic effect
(13,14), which has been observed in ventricular
and supraventricular arrhythmias (13). The incidence of
ischemia/reperfusion-induced ventricular fibrillation in isolated
perfused rat hearts was found to be reduced by garlic powder
(15). Garlic significantly
decreases the upper limit of vulnerability of ventricular
fibrillation and improves defibrillation efficacy in a
dose-dependent pattern (16,17). Martín et al (18) revealed that allicin inhibited the
myocardial contraction and slowed the sinus rhythm. In a further
study, Martín et al (19)
demonstrated that garlic dialysate was able to prolong the
effective refractory period and the sinus node recovery time of
isolated rat atria, in addition to suppressing premature
ventricular contractions and ventricular tachycardia in
ouabain-intoxicated canines.
A study by Deng et al (20) revealed that allicin was able to
inhibit transient outward potassium currents (Ito) in
human atrial myocytes. However, the direct effect of allicin on
Ito in ventricular myocytes has yet to be elucidated.
Therefore, in the present study, the effects of allicin on
Ito in ventricular myocytes isolated from mice were
investigated, using the whole-cell patch clamp recording technique
to test the effect of allicin on Ito, as detected via
Ito amplitude and kinetics, including Ito
activation, inactivation and recovery.
Materials and methods
Ethical approval
All animal procedures were approved by the
Institutional Animal Care and Use Committee at Renmin Hospital of
Wuhan University (Wuhan, China). The animals used in the present
study were male C57 mice, aged 8–10 weeks.
Drugs and solution
Tyrode's solution was composed of the following: 130
mmol/l NaCl, 5.4 mmol/l KCl, 1.8 mmol/l CaCl2, 1 mmol/l
MgCl2, 0.3 mmol/l Na2HPO4, 10
mmol/l HEPES and 10 mmol/l glucose. The pH of the solution was
adjusted to pH 7.4 using NaOH. In addition, Ca2+-free Tyrode's
solution was used, without CaCl2. The collagenase
solution was composed of Ca2+-free Tyrode's solution
containing 0.6 mg/ml collagenase type II (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 0.1% bovine serum albumin, 20
mM taurine and 30 µM CaCl2. Kraft-Brühe (KB) solution
included 10 mmol/l taurine, 70 mmol/l glutamic acid, 25 mmol/l KCl,
10 mmol/l KH2PO4, 22 mmol/l glucose and 0.5
mmol/l ethylene glycol tetraacetic acid (EGTA). The pH of the KB
solution was adjusted to pH 7.2 using KOH. Tyrode's solution was
supplemented with 10, 30, 100 and 300 µmol/l allicin during allicin
treatment. Furthermore, the pipette solution used in the study
consisted of 110 mmol/l K-aspartate, 20 mmol/l KCl, 8 mmol/l NaCl,
1 mmol/l MgC12, 1 mmol/l CaC12, 4 mmol/l
MgATP, 0.1 mmol/l EGTA and 10 mmol/l HEPES, and was adjusted to pH
7.2 using KOH. Allicin was purchased from Xuzhou Ryen Pharm Co.,
Ltd (Xuzhou, China).
Isolation of ventricular cardiac
myocytes
A total of 36 C57BL/6 mice, weighing 25.1±3.4 g,
were heparinized [100 U; intraperitoneal injection (i.p.); Wangbang
Co., Xuzhou, China] 15 min prior to sacrifice, anaesthetized by
pentobarbital sodium (60 mg/kg; i.p.; Sigma-Aldrich, St. Louis, MO,
USA) and sacrificed by cervical dislocation. Hearts were rapidly
removed and retrogradely perfused at a temperature of 37°C with the
following solutions, according to Langendorff technique (21): i) Tyrode's solution (5 min); ii)
Ca2+-free Tyrode's solution (5 min); iii) collagenase solution (15
min); and iv) KB solution (5 min). Subsequent to the perfusion, the
left ventricular free wall was dissected from the heart and placed
in ice-cold KB solution. The tissue was then minced and titrated to
free individual myocytes. Isolated cardiac myocytes were stored in
KB solution at 4°C until required.
Electrophysiology recording
Whole-cell patch clamp was performed on the myocytes
using an EPC-9 amplifier (Heka Elektronik, Lambrecht, Germany), as
previously described (21), and data
was recorded and analyzed with a Pulse/Pulsefit software interface
(version 8.31; Heka Elektronik). During the experiments, 1.5 ml
myocytes were placed in the experimental chamber and mounted on the
stage of an inverted microscope (IX70; Olympus Corporation, Tokyo,
Japan) and perfused with Tyrode solution supplemented with 10, 30,
100 and 300 µmol/l allicin for 5 min at a rate of 2–3 ml/min at
room temperature. In order to elucidate the effect of allicin on
Ito in mouse ventricular myocytes, 6 cells were observed
per solution influx, in triplicate. Pipettes had resistances of
2.5–3.5 MΩ when filled with pipette solution. Series resistance
(Rs) was between 4–8 MΩ and was compensated by 80–90% to reduce the
Rs. Current signals were filtered at 3 kHz by an 8-pole Bessel
filter, digitized at a sampling rate of 1 kHz and recorded on a
computer running Pulse/Pulsefit software, which was additionally
used for the generation of voltage pulses and data analysis.
Ito recording
The total Ito was determined by 500 msec
depolarizing pulses varying from −50 to +60 mV in 10 mV increments
from a holding potential of −80 mV. In order to examine
Ito, pre-pulse (100 msec, −40 mV) was used to inactivate
Ito prior to activation steps with allicin, and
Ito was measured by subtracting the currents before and
after that pre-pulse. By dividing the measured current amplitude by
the membrane capacitance (pA/pF), Ito values were
reported as current densities.
The IC50 of allicin on
Ito was fitted with Hill function using OriginPro
version 8.0 software as follows: E = Emax[1+(D⁄C)b],
where E is the effect at concentration C, Emax is the
maximum effect, D is the concentration for half-maximum action
(IC50) and b is the Hill coefficient.
Steady-state activation curve of
Ito
Using the current-voltage (I–V) association for
Ito, the voltage-dependent of steady-state activation
curve for Ito was fitted to the Boltzmann equation as
follows: I/Imax = 1/[1+exp((VT -
V1/2)/k)], where Imax is maximum current,
VT is the membrane potential, V1/2 is the
midpoint potential for activation and K is a slope factor (22).
Steady-state inactivation of
Ito
The two-step voltage-clamp protocol was applied for
steady-state inactivation of Ito, as previously
described (21). The process
involved an inactivating pre-pulse period that varied from −110 mV
to +10 mV with a 1 sec pre-pulse, followed by a fixed 400 ms test
pulse to +40 mV. The test current amplitude of Ito at
each pulse potential was normalized to the maximal amplitude of
this current (I/Imax). Data were fitted to the Boltzmann
equation.
Recovery from inactivation of
Ito
The time-dependence of reactivation was measured
using an inactivating pulse (−40 mV, maintained for 500 msec).
Following this, at variable time intervals (10–200 msec), a 500
msec test pulse at +40 mV was performed. The ratio of the current
amplitude produced by the test pulse to the inactivating pulse
(P2/P1) was plotted as a function of the time intervals. The time
constant was calculated by data fitted to exponential
functions.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using a Student's t
test and analysis of variance, performed on SPSS version 17.0
software (SPSS, Inc. Chicago, IL, USA). Patch-clamp data were
analyzed using Origin version 8.0 (OriginLab Corporation,
Northampton, MA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of allicin on
voltage-dependent Ito
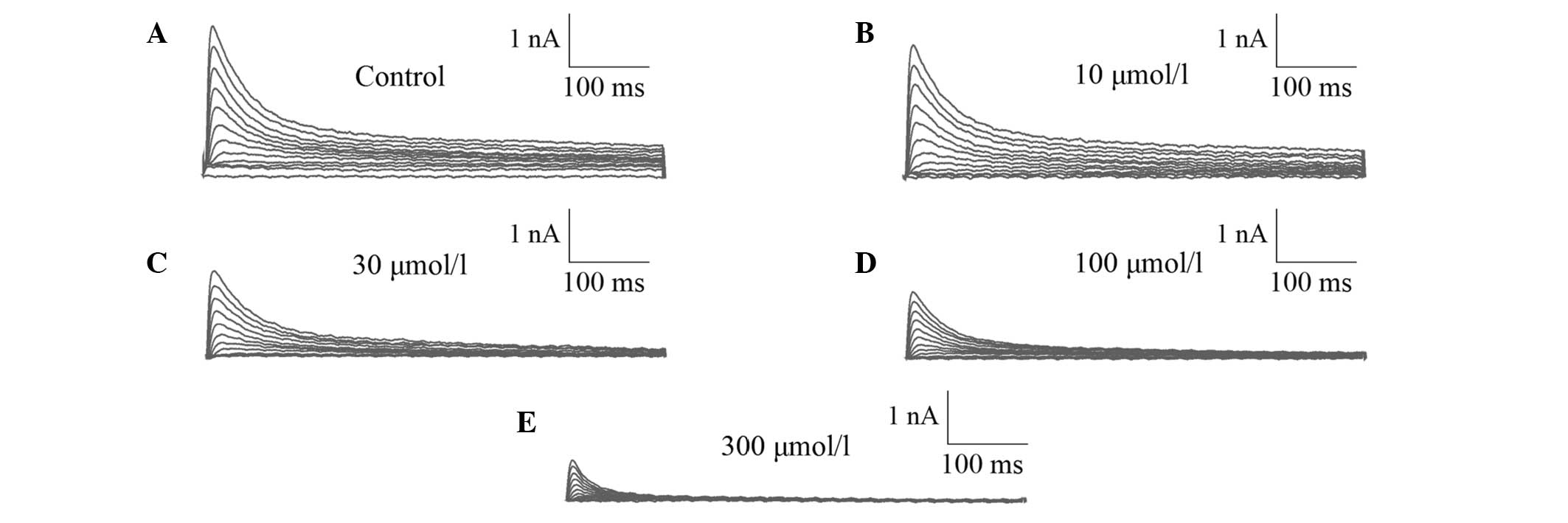
Allicin at 10, 30, 100 and 300 µmol/l was applied,
respectively. Ito was blocked by allicin in a
concentration-dependent manner. Currents were gradually decreased
with the increase of allicin concentration. The representative
current blocked by allicin at 10, 30, 100 and 300 µmol/l is shown
in Fig. 1.
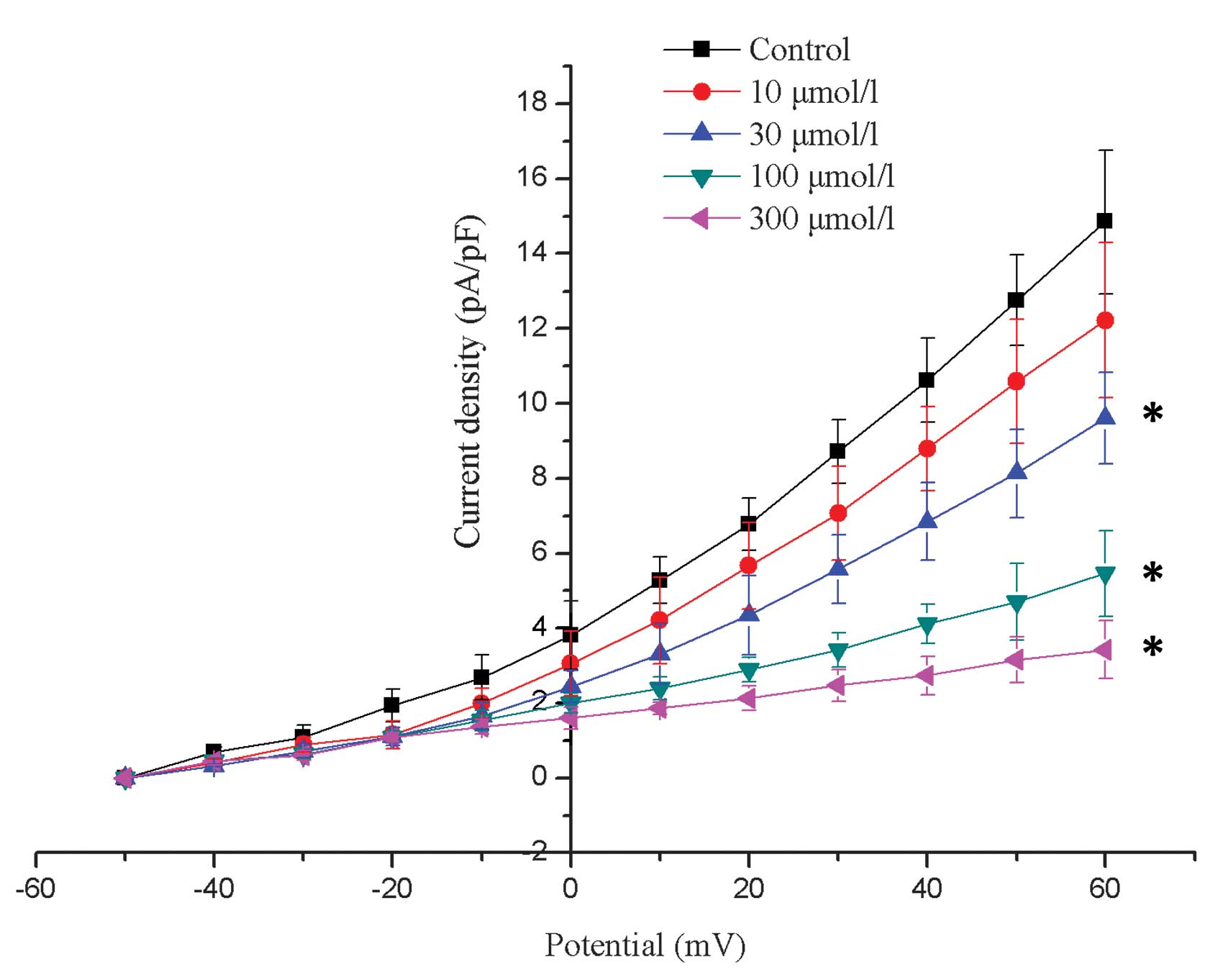
Fig. 2 displays the
I–V association for Ito density prior to and following
the application of 10, 30, 100 and 300 µmol/l allicin. The
Ito was not significantly suppressed by allicin in the
low dose (10 µmol/l; P>0.05); however, it was significantly
suppressed by higher doses (30, 100 and 300 µmol/l; P<0.05; n=6)
compared with the control.
In addition, Fig. 3
shows the dose-response association for the inhibition of
Ito by allicin. At a potential of +60 mv, treatment with
1, 10, 30, 100 and 300 µmol/l allicin decreased the peak
Ito by 1.5, 17.8, 35.3, 63.2 and 76.9%, respectively.
The IC50 of allicin on Ito was fitted with
Hill function and calculated as 41.6 µmol/l (n=6 cells in each
group) using OriginPro 8.0 software.
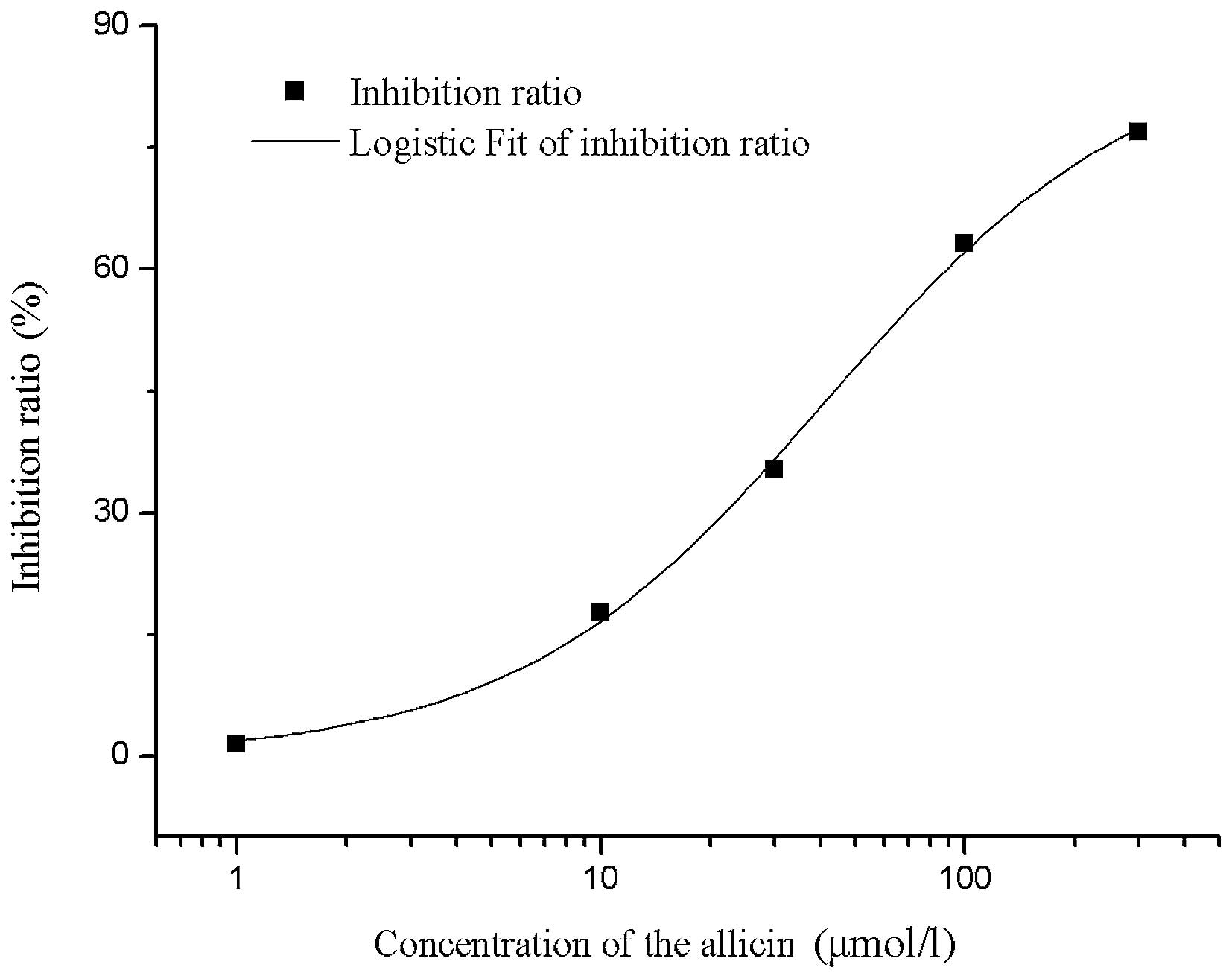 | Figure 3.Dose-response association for
inhibition of Ito by allicin. At a potential of +60 mV,
treatment with 1, 10, 30, 100 and 300 µmol/l allicin decreased the
peak Ito current by 1.5, 17.8, 35.3, 63.2 and 76.9%,
respectively. The IC50 of allicin on Ito was
fitted with Hill function and calculated to be 41.6 µmol/l, using
OriginPro version 8.0 software (n=6). Ito, transient
outward potassium current. |
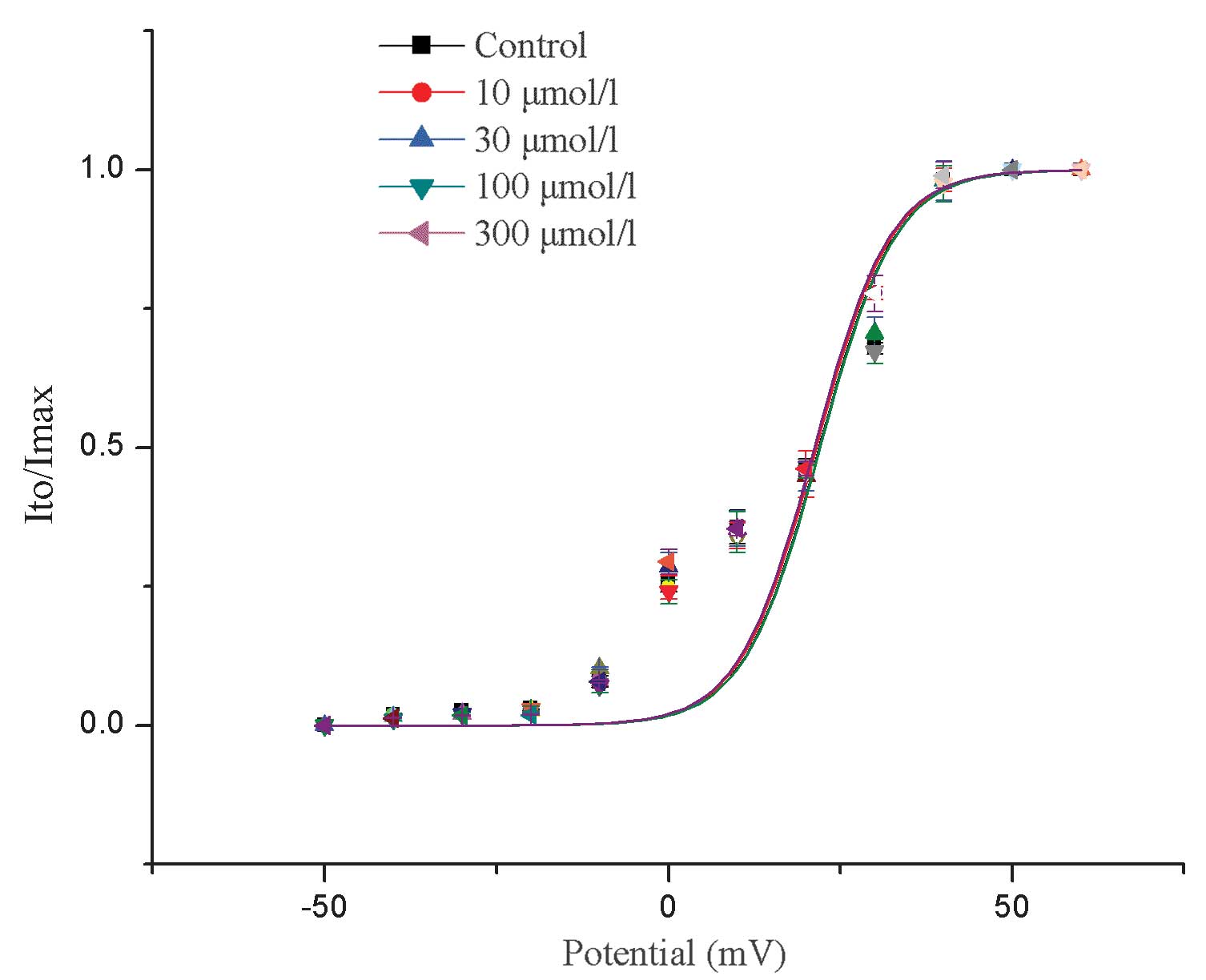
Effects of allicin on the steady-state
activation curve of Ito
Allicin was not found to have a significant effect
on the voltage-dependence of the steady-state activation curve of
Ito (P>0.05; Fig.
4).
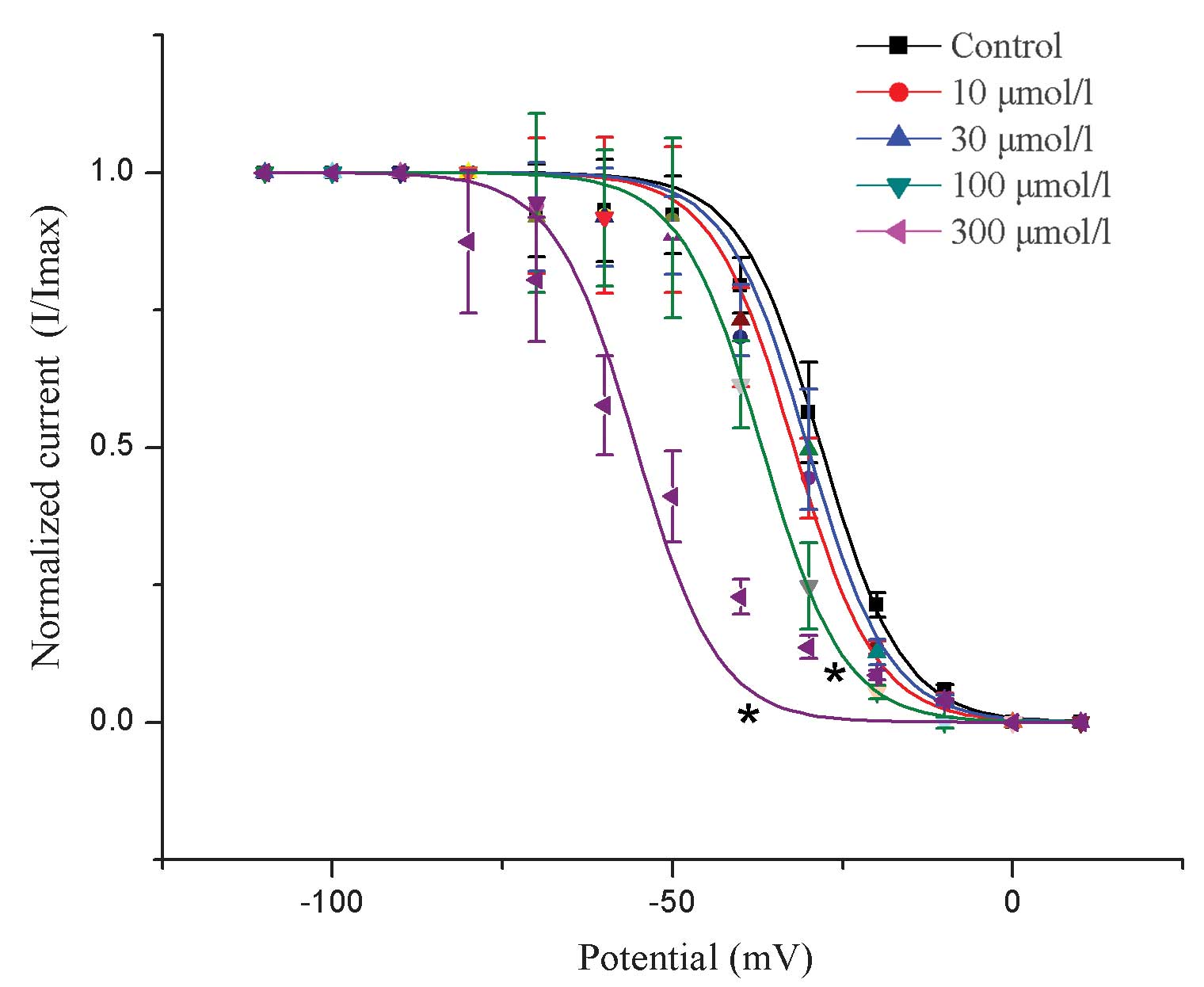
Effects of allicin on the steady-state
inactivation of Ito
The results revealed that a low dose of allicin had
no significant effect on the voltage-dependence of the inactivation
(I/Imax) of Ito (control, V1/2 =
−28.2±4.7 mV; 10 µmol/l allicin, V1/2 = −32.2±3.8 mV; 30
µmol/l allicin, V1/2 = −30.1±3.6 mV; n=6; P>0.05,
compared with the control). However, as shown in Fig. 5, high doses of allicin significantly
shifted the voltage-dependence of the inactivation of
Ito toward the negative potential (100 µmol/l allicin,
V1/2 = −36.9±4.1 mV; 300 µmol/l allicin, V1/2
= −55.3±5.0 mV; n=6; P<0.05 compared with the control).
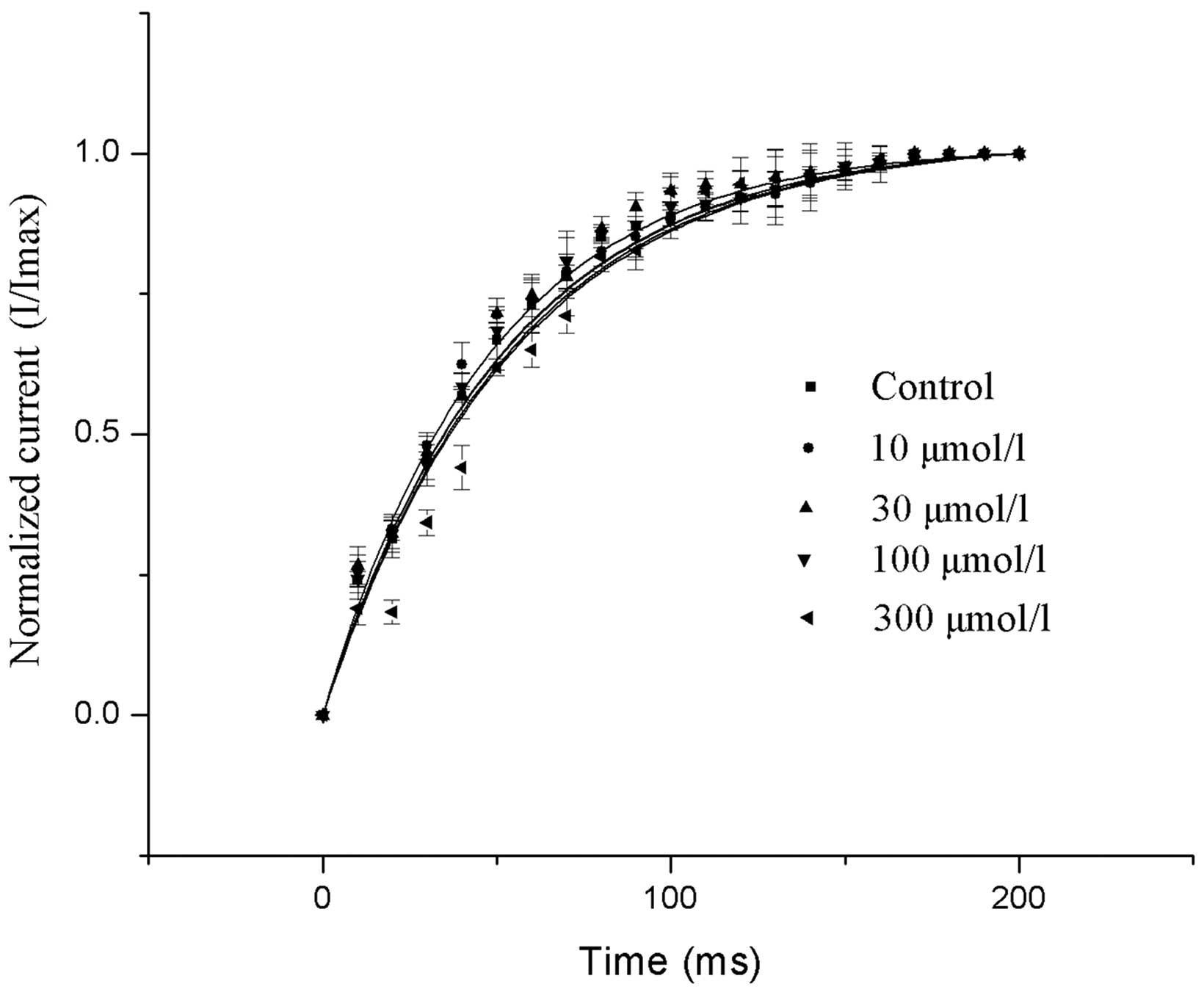
Effects of allicin on the recovery
from inactivation of Ito
Allicin was not found to have a significant effect
on the recovery from the inactivation of Ito following
allicin treatment (P>0.05; Fig.
6).
Discussion
In the present study, allicin significantly
inhibited Ito in mouse ventricular myocytes in a
concentration-dependent manner. High-dose allicin (≥100 µmol/l) was
able to significantly shift the voltage-dependence of the
steady-state inactivation curve of Ito towards an
increasingly negative potential. However, allicin did not have a
significant effect on steady-state activation, or recovery from the
inactivation of Ito.
Traditional Chinese medicine has been used for
thousands of years for the treatment of cardiovascular diseases
(23,24). In recent decades, garlic has been
found to possess antiarrhythmic effects (13,14).
Several reports (25–27) have indicated that allicin is the
predominant active component that is responsible for the majority
of the biological activities of garlic, including attenuating
ischemic injury, lowering blood pressure and antiarrhythmic effects
(6,28,29). The
chemical structure of allicin is
CH2=CH-CH2-S(O)-S-CH2-CH=CH2,
and it has been has been manufactured synthetically and produced
worldwide (18). Although garlic has
been discovered to be a significant antiarrhythmic agent, the exact
mechanism has yet to be elucidated.
In the present study, allicin significantly
inhibited Ito in mouse ventricular myocytes; however, it
had no significant effect on steady-state activation, or recovery
from inactivation of Ito, which is in agreement with
previous findings (20). In the
study by Deng et al (20),
the research target was human atrial myocytes, and it was
demonstrated that 30 µmol/l allicin was able to negatively shift
the voltage-dependence of the steady-state inactivation curve of
Ito. By contrast, in the present study, only high-dose
allicin (≥100 µmol/l) was able to significantly shift the
steady-state inactivation curve of Ito towards an
increasingly negative potential. This may be due to allicin having
different effects in different tissues and species. Allicin exerts
its suppressive effect on Ito by changing the quantity
and kinetic properties of Ito. In human atrial
monocytes, Ito contributes to cardiac repolarization,
whilst in the hearts of mice, Ito has a role in action
potential repolarization (30,31).
Notably, Ito is not uniformly distributed within the
left ventricle in humans, mice and certain other mammals (32–35). In
the left ventricular free wall, Ito is larger in
epicardial compared with endocardial regions, which contributes to
the regional variations of action potential (AP) profiles and
results in a prominent AP notch in the epicardium, but not in the
endocardium (36). It has been
confirmed that a prominent Ito is important in
physiological and pathophysiological process (37–41). The
high incidence of phase 2 reentry and ventricular fibrillation
during myocardial ischemia was partly due to the prominent
Ito-mediated epicardial AP dome (42). In patients with coronary heart
disease, the incidence of sudden mortality in men was significantly
higher compared with that in women (43,44).
This may be a result of a more prominent Ito in men
compared with women (39). Thus,
Ito block may be an effective therapy for arrhythmia
(37).
In the present study, it was revealed that allicin
was able to inhibit Ito, and may be the mechanism
through which allicin exerts its antiarrhythmic effect.
Antiarrhythmic therapeutics with low toxicity and low reverse
use-dependence (RUD) effects are a focal point in antiarrhythmic
drug research. Xing et al (23) confirmed that allicin has similar
effects to amiodarone on the conduction system and cardiac
electrophysiology. However, allicin possesses no RUD and this may
contribute to multi-channel blockers. Furthermore, allicin appears
to be safe for use in the majority of conditions (2) and is therefore likely to be a promising
antiarrhythmic therapy.
In conclusion, the present study revealed that
allicin inhibits Ito in mouse ventricular myocytes,
which may be the mechanism through which allicin exerts its
antiarrhythmic effect. Thus, allicin has demonstrated potential to
be a promising antiarrhythmic therapy in the future; however,
whether allicin exerts the same effect in other tissues or species
requires further investigation.
Acknowledgements
The present study was financially supported by the
Fundamental Research Funds for the Central Universities (grant no.
2012302020205).
References
|
1
|
Lan H and Lü YY: Allitridi induces
apoptosis by affecting Bcl-2 expression and caspase-3 activity in
human gastric cancer cells. Acta Pharmacol Sin. 25:219–225.
2004.PubMed/NCBI
|
|
2
|
Aviello G, Abenavoli L, Borrelli F,
Capasso R, Izzo AA, Lembo F, Romano B and Capasso F: Garlic:
Empiricism or science? Nat Prod Commun. 4:1785–1796.
2009.PubMed/NCBI
|
|
3
|
Seki T, Hosono T, Hosono-Fukao T, Inada K,
Tanaka R, Ogihara J and Ariga T: Anticancer effects of diallyl
trisulfide derived from garlic. Asia Pac J Clin Nutr. 17(Suppl 1):
S249–S252. 2008.
|
|
4
|
Qureshi AA, Abuirmeileh N, Din ZZ, Elson
CE and Burger WC: Inhibition of cholesterol and fatty acid
biosynthesis in liver enzymes and chicken hepatocytes by polar
fractions of garlic. Lipids. 18:343–348. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gebhardt R: Multiple inhibitory effects of
garlic extracts on cholesterol biosynthesis in hepatocytes. Lipids.
28:613–619. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malik ZA and Siddiqui S: Hypotensive
effect of freeze-dried garlic (Allium Sativum) sap in dog. J Pak
Med Assoc. 31:12–13. 1981.PubMed/NCBI
|
|
7
|
Boullin DJ: Garlic as a platelet
inhibitor. Lancet. 1:776–777. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaffen JD, Tavares IA and Bennett A: The
effect of garlic extracts on contractions of rat gastric fundus and
human platelet aggregation. J Pharm Pharmacol. 36:272–274. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srivastava KC: Evidence for the mechanism
by which garlic inhibits platelet aggregation. Prostaglandins
Leukot Med. 22:313–321. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Apitz-Castro R, Cabrera S, Cruz MR,
Ledezma E and Jain MK: Effects of garlic extract and of three pure
components isolated from it on human platelet aggregation,
arachidonate metabolism, release reaction and platelet
ultrastructure. Thromb Res. 32:155–169. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciplea AG and Richter KD: The protective
effect of Allium sativum and crataegus on isoprenaline-induced
tissue necroses in rats. Arzneimittelforschung. 38:1583–1592.
1988.PubMed/NCBI
|
|
12
|
Siegers CP, Röbke A and Pentz R: Effects
of garlic preparations on superoxide production by phorbol ester
activated granulocytes. Phytomedicine. 6:13–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Banerjee SK and Maulik SK: Effect of
garlic on cardiovascular disorders: A review. Nutr J. 1:42002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isensee H, Rietz B and Jacob R:
Cardioprotective actions of garlic (Allium sativum).
Arzneimittelforschung. 43:94–98. 1993.PubMed/NCBI
|
|
15
|
Rietz B, Belagyi J, Török B and Jacob R:
The radical scavenging ability of garlic examined in various
models. Boll Chim Farm. 134:69–76. 1995.PubMed/NCBI
|
|
16
|
Sungnoon R, Kanlop N, Chattipakorn SC,
Tawan R and Chattipakorn N: Effects of garlic on the induction of
ventricular fibrillation. Nutrition. 24:711–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sungnoon R, Shinlapawittayatorn K,
Chattipakorn SC and Chattipakorn N: Effects of garlic on
defibrillation efficacy. Int J Cardiol. 126:143–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martín N, Bardisa L, Pantoja C, Román R
and Vargas M: Experimental cardiovascular depressant effects of
garlic (Allium sativum) dialysate. J Ethnopharmacol. 37:145–149.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martín N, Bardisa L, Pantoja C, Vargas M,
Quezada P and Valenzuela J: Anti-arrhythmic profile of a garlic
dialysate assayed in dogs and isolated atrial preparations. J
Ethnopharmacol. 43:1–8. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng CY, Rao F, Kuang SJ, Wu SL, Shan ZX,
Li XH, Zhou ZL, Lin QX, Liu XY, Yang M, et al: Allitridi inhibits
transient outward potassium currents in human atrial myocytes. Clin
Exp Pharmacol Physiol. 38:323–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin M, Huang H, Wang T, Hu H, Liu Y, Cao
H, Li H and Huang C: Absence of Rgs5 prolongs cardiac
repolarization and predisposes to ventricular tachyarrhythmia in
mice. J Mol Cell Cardiol. 53:880–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brouillette J, Clark RB, Giles WR and
Fiset C: Functional properties of K+ currents in adult
mouse ventricular myocytes. J Physiol. 559:777–798. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xing Y, Chen J, Wang J, Gao Y, Niu W, Zhao
M, Zhu H, Guo L, Lu P and Wang S: The effects of allitridi and
amiodarone on the conduction system and reverse use-dependence in
the isolated hearts of rats with myocardial infarction. J
Ethnopharmacol. 141:674–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Wang X, Gu Y, Wang T and Huang C:
Wenxin Keli attenuates ischemia-induced ventricular arrhythmias in
rats: Involvement of L-type calcium and transient outward potassium
currents. Mol Med Rep. 7:519–524. 2013.PubMed/NCBI
|
|
25
|
Batirel HF, Naka Y, Kayano K, Okada K,
Vural K, Pinsky DJ and Oz MC: Intravenous allicin improves
pulmonary blood flow after ischemia-reperfusion injury in rats. J
Cardiovasc Surg (Torino). 43:175–179. 2002.PubMed/NCBI
|
|
26
|
Lawson LD, Ransom DK and Hughes BG:
Inhibition of whole blood platelet-aggregation by compounds in
garlic clove extracts and commercial garlic products. Thromb Res.
65:141–156. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prasad K, Laxdal VA, Yu M and Raney BL:
Antioxidant activity of allicin, an active principle in garlic. Mol
Cell Biochem. 148:183–189. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banerjee SK, Dinda AK, Manchanda SC and
Maulik SK: Chronic garlic administration protects rat heart against
oxidative stress induced by ischemic reperfusion injury. BMC
Pharmacol. 2:162002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin N, Bardisa L, Pantoja C, Vargas M,
Quezada P and Valenzuela J: Anti-arrhythmic profile of a garlic
dialysate assayed in dogs and isolated atrial preparations. J
Ethnopharmacol. 43:1–8. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barry DM, Xu H, Schuessler RB and Nerbonne
JM: Functional knockout of the transient outward current, long-QT
syndrome, and cardiac remodeling in mice expressing a
dominant-negative Kv4 alpha subunit. Circ Res. 83:560–567. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nerbonne JM and Kass RS: Molecular
physiology of cardiac repolarization. Physiol Rev. 85:1205–1253.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Näbauer M: Electrical heterogeneity in the
ventricular wall-and the M cell. Cardiovasc Res. 40:248–250.
1998.PubMed/NCBI
|
|
33
|
Guo W, Xu H, London B and Nerbonne JM:
Molecular basis of transient outward K+ current diversity in mouse
ventricular myocytes. J Physiol. 3:587–599. 1999. View Article : Google Scholar
|
|
34
|
Näbauer M, Beuckelmann DJ, Uberfuhr P and
Steinbeck G: Regional differences in current density and
rate-dependent properties of the transient outward current in
subepicardial and subendocardial myocytes of human left ventricle.
Circulation. 93:168–177. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wickenden AD, Jegla TJ, Kaprielian R and
Backx PH: Regional contributions of Kv1.4, Kv4.2 and Kv4.3 to
transient outward K+ current in rat ventricle. Am J
Physiol. 276:H1599–H1607. 1999.PubMed/NCBI
|
|
36
|
Akar FG, Wu RC, Deschenes I, Armoundas AA,
Piacentino V III, Houser SR and Tomaselli GF: Phenotypic
differences in transient outward K+ current of human and canine
ventricular myocytes: Insights into molecular composition of
ventricular Ito. Am J Physiol Heart Circ Physiol. 286:H602–H609.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan GX and Antzelevitch C: Cellular basis
for the Brugada syndrome and other mechanisms of arrhythmogenesis
associated with ST-segment elevation. Circulation. 100:1660–1666.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan GX, Lankipalli RS, Burke JF, Musco S
and Kowey PR: Ventricular repolarization components on the
electrocardiogram: Cellular basis and clinical significance. J Am
Coll Cardiol. 42:401–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Diego JM, Cordeiro JM, Goodrow RJ, Fish
JM, Zygmunt AC, Pérez GJ, Scornik FS and Antzelevitch C: Ionic and
cellular basis for the predominance of the Brugada syndrome
phenotype in males. Circulation. 106:2004–2011. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan GX and Antzelevitch C: Cellular basis
for the electrocardiographic J wave. Circulation. 93:372–379. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Di Diego JM, Sun ZQ and Antzelevitch C:
I(to) and action potential notch are smaller in left vs. right
canine ventricular epicardium. Am J Physiol. 271:H548–H561.
1996.PubMed/NCBI
|
|
42
|
Yan GX, Joshi A, Guo D, Hlaing T, Martin
J, Xu X and Kowey PR: Phase 2 reentry as a trigger to initiate
ventricular fibrillation during early acute myocardial ischemia.
Circulation. 110:1036–1041. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lerner DJ and Kannel WB: Patterns of
coronary heart disease morbidity and mortality in the sexes: A
26-year follow-up of the Framingham population. Am Heart J.
111:383–390. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Every N, Hallstrom A, McDonald KM, Parsons
L, Thom D, Weaver D and Hlatky MA: Risk of sudden versus nonsudden
cardiac death in patients with coronary artery disease. Am Heart J.
144:390–396. 2002. View Article : Google Scholar : PubMed/NCBI
|