Introduction
Adenomyomatosis of the gallbladder (ADM) is defined
as the epithelial proliferation and hypertrophy of the muscles of
the gallbladder wall (1). An
outpouching of the gallbladder mucosa into the thickened muscular
layer is termed Rokitansky-Aschoff sinus (2). ADM may be further divided into three
subtypes: Fundal, segmental and diffuse (3). A major challenge in ADM diagnosis
arises from the difficulty in distinguishing the disease from
gallbladder cancer (4).
Recent advances in diagnostic imaging have improved
the distinction between ADM and gallbladder cancer.
Contrast-enhanced endoscopic ultrasonography (EUS) is beneficial
for the differential diagnosis of gallbladder wall thickening
(5). High-resolution ultrasound is
also advantageous for differentiating gallbladder cancers from ADM
(6); however, the challenge of
differentiating gallbladder cancer from ADM has yet to be resolved.
A primary cause of the challenge is intratumoral cystic components,
which create difficulty in discerning between gallbladder cancer
and ADM (7).
Diffusion-weighted whole body imaging with
background body signal suppression (DWIBS) images are acquired
through multiple-signal averaging, pre-pulse fat suppression, and
heavy diffusion weighting during free breathing (8); DWIBS is based upon diffusion-weighted
imaging (DWI), which visualizes and assesses the random movement of
water at the molecular level (Brownian motion) (9,10). An
advantage of DWIBS is that it provides a strong contrast of
cancerous tissues against the adjacent non-cancerous tissues, which
is useful for detection and staging, and for monitoring the
response to therapy (11). A major
limitation of DWIBS is that anatomical analysis may occasionally
prove difficult (12,13). Using a workstation, DWIBS images may
be overlapped with T2-weighed images (T2WIs) to create DWIBS/T2
fusion images (11,14,15);
these DWIBS/T2 images can clearly provide functional information
about the anatomy.
Therefore, DWIBS/T2 images of ADM were analyzed in
the present study in order to investigate the distinctions between
ADM and gallbladder cancer.
Patients and methods
Patients
Patient records and images from April 2012 to
October 2014 were analyzed retrospectively. The enrolled patients
included 6 men (64.2±13.1 years) and 4 women (57.3±12.4 years). Of
the 10 patients, 8 had fundal, and 2 had segmental ADM. The
patients underwent DWIBS/T2 as part of routine clinical practice. A
number of patients (n=3) underwent surgery due to a clinical
suspicion of gallbladder cancer. The surgical specimens confirmed
the diagnosis of ADM. All patients underwent regular follow-up
(duration, 1–27 months), and none of the patients demonstrated any
evidence of gallbladder cancer. The study was submitted to the
ethics committee of the National Hospital Organization Shimoshizu
Hospital (Yotsukaido, China) for review and was not considered a
clinical trial as it was conducted during routine clinical
practice. Patient anonymity was preserved.
Diagnosis of ADM
The diagnosis of ADM was based upon imaging
techniques, including computed tomography (CT; SOMATOM Emotion 16;
Siemens AG, Munich, Germany), transabdominal ultrasonography (US;
SSA-700A; Toshiba Medical Systems Corporation, Ohtawara, Japan),
EUS (GF-UCT260; Olympus Corporation, Tokyo, Japan) and magnetic
resonance cholangiopancreatography (MRCP; Philips Healthcare, DA
Best, The Netherlands), with Achieva Software version 3.2.2 The
diagnosis of ADM was predominately based on the visualization of
the Rokitansky-Aschoff sinus by MRCP, as represented by the pearl
necklace sign (16). Wall thickening
>10 mm, disruption of the normal layers of the gallbladder wall
and hypoechoic lesions in the wall were absent upon US and EUS
(17). Microcysts were observed
during examination using US and EUS, indicating the presence of the
Rokitansky-Aschoff sinus (18). CT
imaging was applied for the detection of the wall thickening
(19).
Magnetic resonance imaging (MRI)
All MRI examinations were performed using an 1.5
Tesla scanner (Achieva, software version 3.2.2; Philips Medical
Systems B.V., Eindhoven, The Netherlands). The T1-weighted images
(T1WIs), T2WIs and diffusion-weighted images were obtained with
pulse sequences, as depicted in Table
I. The DWIBS/T2 images were constructed with the Extended MR
WorkSpace (Philips Medical Systems B.V.), and the sequences are
displayed in Table I. DWI gradients
were applied along the x-, y- and z-axes prior to and after a 180°
inversion pre-pulse to obtain fat-saturated, isotropic images with
DWI sensitivity using the following parameters for a single stack:
b value, 0 mm2/sec and 800 mm2/sec;
repetition time/echo time/inversion recovery, 6,960/79/150 msec;
acquisition matrix, 176×115; reconstruction matrix, 256; field of
view, right/left, 530 mm, anterior/posterior, 349 mm, and
feet/head, 226 mm; slice thickness, 6 mm; size of reconstructed
voxel, 2.07×2.08×6 mm3; and 4 averages. An apparent diffusion
coefficient (ADC) map was produced from the recorded ADC values in
order to eliminate the possibility of T2 shine-through and to
differentiate malignant lesions from non-malignant causes of
restricted diffusion (20).
 | Table I.Pulse sequences used in the present
study. |
Table I.
Pulse sequences used in the present
study.
| Parameter | T1-weighted
imaging | T2-weighted
imaging | DWI (DWIBS) |
|---|
| Sequences | GRE | SE | EPI SE |
| TR, msec | Shortest | 1,000 | 11,250 |
| TE, msec | First, 2.3
(out-phase); second: 4.6 (in-phase) | 90 | 83 |
| Flip angle, ° | 75 | 90 | 90 |
| NSA | 1 | 1 | 4 |
| Slice thickness,
mm | 8 | 8 | 5 |
| Slice gap | 1 | 1 | 0 |
| Fat saturation | None | None | SPAIR |
| Phase encoding
direction |
Posterior-anterior |
Posterior-anterior |
Posterior-anterior |
Results
ADM is negative upon DWBS/T2
imaging
Patient characteristics are displayed in Table II. All variables were within normal
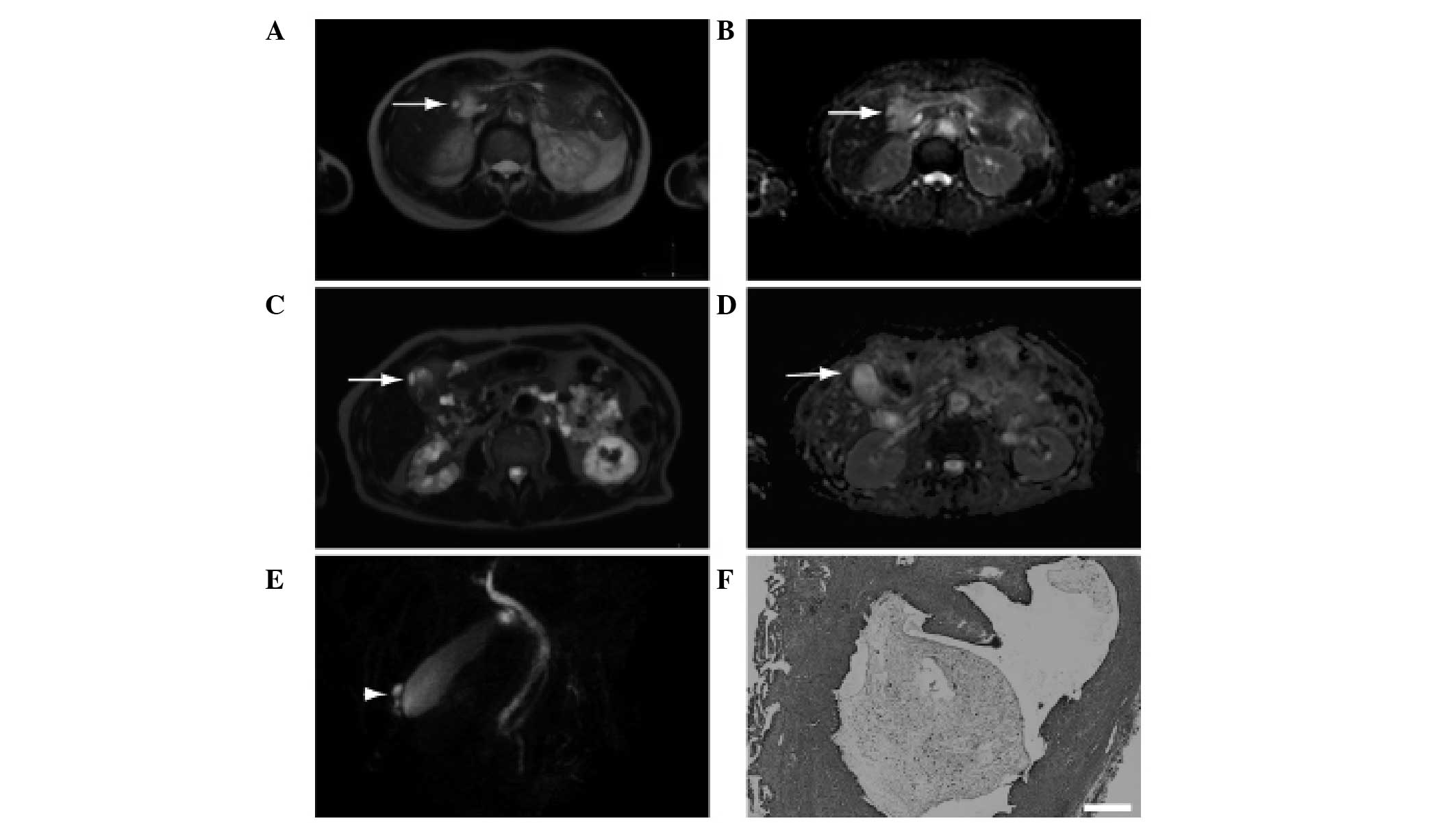
limits. Upon DWIBS/T2 imaging, only 1 patient had positive results.
Cystic lesions in the thickened wall exhibited a relatively high
signal intensity upon DWIBS/T2 imaging; however, the intensity was
not as strong as that from the spleen (Fig. 1A). The spleen displayed high signal
intensity upon DWIBS/T2 imaging. The intensity of the cystic lesion
was also high on the ADC map (Fig.
1B), indicating the presence of T2 shine-through. It was
determined that the cystic lesion was a Rokitansky-Aschoff sinus.
Low signal intensity was observed for the thickened wall. The
aforementioned results indicate that ADM may be negative upon
DWIBS/T2 imaging.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Variable | Value (mean ±
standard deviation) |
|---|
| WBC,
103/µl | 5.0±1.0 |
| Hb, g/dl | 14.3±1.2 |
| CRP, mg/dl | 0.15±0.10 |
| T-Bil, mg/dl | 0.98±0.33 |
| ALP, IU/l | 190±95 |
| AST, IU/l | 20.1±5.1 |
| ALT, IU/l | 17.6±5.4 |
| GGT, IU/l | 26.7±14.4 |
| CEA, ng/ml | 2.2±1.5 |
| CA19–9, U/ml | 12.0±8.4 |
ADM is concomitant with chronic
cholecystitis
A high signal intensity was observed upon DWIBS/T2
imaging for a second patient (Fig.
1C); however, the patient's ADC map did not reveal any high
intensity signals (Fig. 1D). It was
suggested that the patient may have gallbladder cancer; however,
the pearl necklace sign (21) was
observed during MRCP (Fig. 1E). Due
to the complexities involved in differentiating between ADM and
gallbladder cancer, the patient underwent surgery. The surgical
specimen revealed the presence of ADM concomitant with chronic
cholecystitis (Fig. 1F). On the
basis of the aforementioned findings, it was concluded that the
high signal intensity observed during DWIBS/T2 imaging was a false
positive.
Discussion
DWI is known to improve the accuracy of the
diagnosis of gallbladder cancer (22). Upon DWI, a high signal intensity is
observed for cystic lesions in ADM, indicating the presence of the
Rokitansky-Aschoff sinus (22).
Compared with benign lesions, gallbladder cancer displays
significantly lower signal intensity on the ADC map (23). These studies indicate that a
definitive diagnosis of ADM or gallbladder cancer is dependent upon
the ADC values. In the present study, the Rokitansky-Aschoff sinus
was negative upon DWIBS/T2 imaging, as was the thickened wall of
the ADM. The ADC values or ADC map were also valuable in diagnosing
T2 shine-through for the Rokitansky-Aschoff sinus, and in
confirming the ADM or gallbladder cancer diagnoses.
At present, few previous studies have reported the
use of DWI for the diagnosis of ADM, and no report has been found
regarding the use of DWIBS in ADM diagnosis. Ogawa et al
(24) reported the use of DWI in
gallbladder diseases; DWI was positive in 11% of their patients
(they determined a result to be positive when a high signal was
observed). There is a possibility that T2-shine through may be read
as a positive result. Therefore, in the present study, the
possibility of T2-shine through was omitted using a ADC map and ADM
was negative with DWIBS/T2. In addition, DWIBS/T2 enabled the
evaluation of signals in anatomical settings. These findings
suggested that negative DWIBS/T2 results may be considered useful
for the diagnosis of ADM.
The ADC values for gallbladder cancer are
significantly lower, as compared with those for benign lesions
(22). In the present study, only 1
of 10 patients displayed low signal intensity on the ADC map. It
was suggested that the patient had gallbladder cancer; however, the
surgical specimen revealed the presence of chronic cholecystitis
with ADM, highlighting the difficulty in differentiating between
chronic cholecystitis and ADM (4).
Chronic cholecystitis is known to cause false positives in DWI
(24). One patient with false
positive DWI findings was diagnosed with chronic cholecystitis in
the present study. Inflammation is also known to give rise to false
positives in DWI (25). It was
speculated that chronic cholecystitis may be the underlying cause
of the false positive findings in the present case.
A limitation of the present study is that it was
based upon a small number of patients. Another limitation was that
the present study did not include patients with gallbladder cancer.
In the future, we propose to include greater numbers of patients,
in particular, those with gallbladder cancer.
In conclusion, the majority of the patients with ADM
displayed negative findings upon DWIBS/T2 imaging. A patient with a
false positive finding had accompanying chronic cholecystitis.
References
|
1
|
Colquhoun J: Adenomyomatosis of the
gall-bladder (intramural diverticulosis). Br J Radiol. 34:101–112.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Williams I, Slavin G, Cox A, Simpson P and
de Lacey G: Diverticular disease (adenomyomatosis) of the
gallbladder: A radiological-pathological survey. Br J Radiol.
59:29–34. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JH, Jeong IH, Han JH, Kim JH, Hwang
JC, Yoo BM, Kim JH, Kim MW and Kim WH: Clinical/pathological
analysis of gallbladder adenomyomatosis; Type and pathogenesis.
Hepatogastroenterology. 57:420–425. 2010.PubMed/NCBI
|
|
4
|
Kim BS, Oh JY, Nam KJ, Cho JH, Kwon HJ,
Yoon SK, Jeong JS and Noh MH: Focal thickening at the fundus of the
gallbladder: Computed tomography differentiation of fundal type
adenomyomatosis and localized chronic cholecystitis. Gut Liver.
8:219–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imazu H, Mori N, Kanazawa K, Chiba M,
Toyoizumi H, Torisu Y, Koyama S, Hino S, Ang TL and Tajiri H:
Contrast-enhanced harmonic endoscopic ultrasonography in the
differential diagnosis of gallbladder wall thickening. Dig Dis Sci.
59:1909–1916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joo I, Lee JY, Kim JH, Kim SJ, Kim MA, Han
JK and Choi BI: Differentiation of adenomyomatosis of the
gallbladder from early-stage, wall-thickening-type gallbladder
cancer using high-resolution ultrasound. Eur Radiol. 23:730–738.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimitsu K, Irie H, Aibe H, Tajima T,
Nishie A, Asayama Y, Matake K, Yamaguchi K, Matsuura S and Honda H:
Well-differentiated adenocarcinoma of the gallbladder with
intratumoral cystic components due to abundant mucin production: A
mimicker of adenomyomatosis. Eur Radiol. 15:229–233. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahara T, Imai Y, Yamashita T, Yasuda S,
Nasu S and Van Cauteren M: Diffusion weighted whole body imaging
with background body signal suppression (DWIBS): Technical
improvement using free breathing, STIR and high resolution 3D
display. Radiat Med. 22:275–282. 2004.PubMed/NCBI
|
|
9
|
Sehy JV, Ackerman JJ and Neil JJ: Apparent
diffusion of water, ions and small molecules in the Xenopus
oocyte is consistent with Brownian displacement. Magn Reson Med.
48:42–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koike N, Cho A, Nasu K, Seto K, Nagaya S,
Ohshima Y and Ohkohchi N: Role of diffusion-weighted magnetic
resonance imaging in the differential diagnosis of focal hepatic
lesions. World J Gastroenterol. 15:5805–5812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwee TC, Takahara T, Ochiai R, Nievelstein
RA and Luijten PR: Diffusion-weighted whole-body imaging with
background body signal suppression (DWIBS): Features and potential
applications in oncology. Eur Radiol. 18:1937–1952. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohno Y, Koyama H, Onishi Y, Takenaka D,
Nogami M, Yoshikawa T, Matsumoto S, Kotani Y and Sugimura K:
Non-small cell lung cancer: Whole-body MR examination for M-stage
assessment-utility for whole-body diffusion-weighted imaging
compared with integrated FDG PET/CT. Radiology. 248:643–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fischer MA, Nanz D, Hany T, Reiner CS,
Stolzmann P, Donati OF, Breitenstein S, Schneider P, Weishaupt D,
von Schulthess GK and Scheffel H: Diagnostic accuracy of whole-body
MRI/DWI image fusion for detection of malignant tumours: A
comparison with PET/CT. Eur Radiol. 21:246–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sommer G, Wiese M, Winter L, Lenz C,
Klarhöfer M, Forrer F, Lardinois D and Bremerich J: Preoperative
staging of non-small-cell lung cancer: Comparison of whole-body
diffusion-weighted magnetic resonance imaging and
18F-fluorodeoxyglucose-positron emission
tomography/computed tomography. Eur Radiol. 22:2859–2867. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nechifor-Boilă IA, Bancu S, Buruian M,
Charlot M, Decaussin-Petrucci M, Krauth JS, Nechifor-Boilă AC and
Borda A: Diffusion weighted imaging with background body signal
suppression/T2 image fusion in magnetic resonance mammography for
breast cancer diagnosis. Chirurgia (Bucur). 108:199–205.
2013.PubMed/NCBI
|
|
16
|
Stunell H, Buckley O, Geoghegan T, O'Brien
J, Ward E and Torreggiani W: Imaging of adenomyomatosis of the gall
bladder. J Med Imaging Radiat Oncol. 52:109–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HJ, Park JH, Park DI, Cho YK, Sohn CI,
Jeon WK, Kim BI and Choi SH: Clinical usefulness of endoscopic
ultrasonography in the differential diagnosis of gallbladder wall
thickening. Dig Dis Sci. 57:508–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akatsu T, Aiura K, Shimazu M, Ueda M,
Wakabayashi G, Tanabe M, Kawachi S and Kitajima M: Can endoscopic
ultrasonography differentiate nonneoplastic from neoplastic
gallbladder polyps? Dig Dis Sci. 51:416–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ching BH, Yeh BM, Westphalen AC, Joe BN,
Qayyum A and Coakley FV: CT differentiation of adenomyomatosis and
gallbladder cancer. AJR Am J Roentgenol. 189:62–66. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Miller FH, Chen ZE, Merrick L,
Mortele KJ, Hoff FL, Hammond NA, Yaghmai V and Nikolaidis P:
Diffusion-weighted MR imaging of solid and cystic lesions of the
pancreas. Radiographics. 31:E47–E64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haradome H, Ichikawa T, Sou H, Yoshikawa
T, Nakamura A, Araki T and Hachiya J: The pearl necklace sign: An
imaging sign of adenomyomatosis of the gallbladder at MR
cholangiopancreatography. Radiology. 227:80–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee NK, Kim S, Kim TU, Kim DU, Seo HI and
Jeon TY: Diffusion-weighted MRI for differentiation of benign from
malignant lesions in the gallbladder. Clin Radiol. 69:e78–e85.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshioka M, Watanabe G, Uchinami H,
Miyazawa H, Abe Y, Ishiyama K, Hashimoto M, Nakamura A and Yamamoto
Y: Diffusion-weighted MRI for differential diagnosis in gallbladder
lesions with special reference to ADC cut-off values.
Hepatogastroenterology. 60:692–698. 2013.PubMed/NCBI
|
|
24
|
Ogawa T, Horaguchi J, Fujita N, Noda Y,
Kobayashi G, Ito K, Koshita S, Kanno Y, Masu K and Sugita R: High
b-value diffusion-weighted magnetic resonance imaging for
gallbladder lesions: Differentiation between benignity and
malignancy. J Gastroenterol. 47:1352–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Padhani AR, Koh DM and Collins DJ:
Whole-body diffusion-weighted MR imaging in cancer: Current status
and research directions. Radiology. 261:700–718. 2011. View Article : Google Scholar : PubMed/NCBI
|