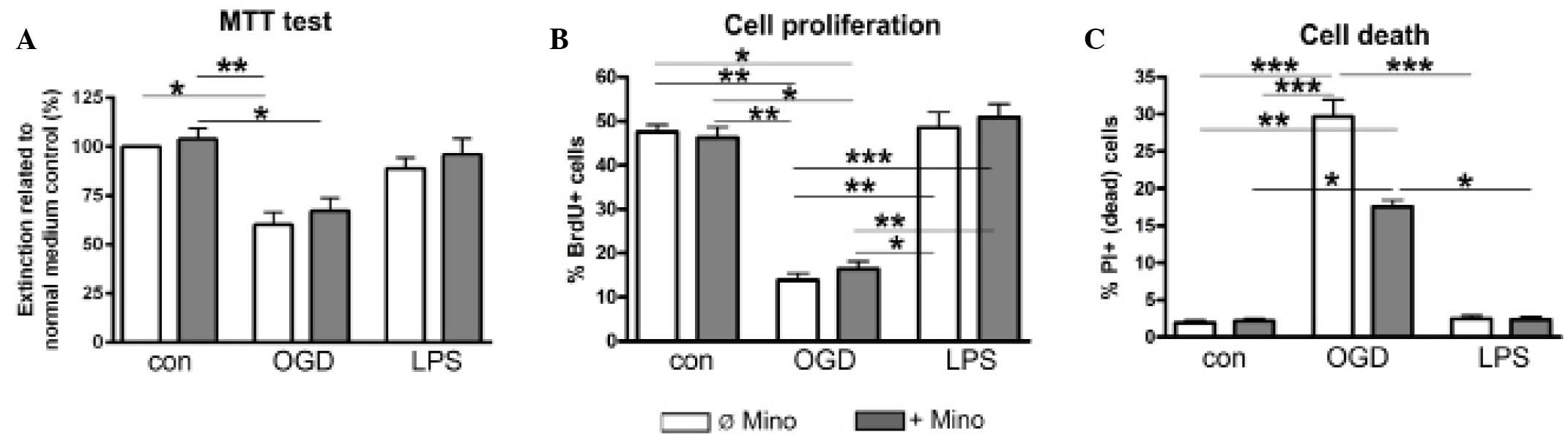
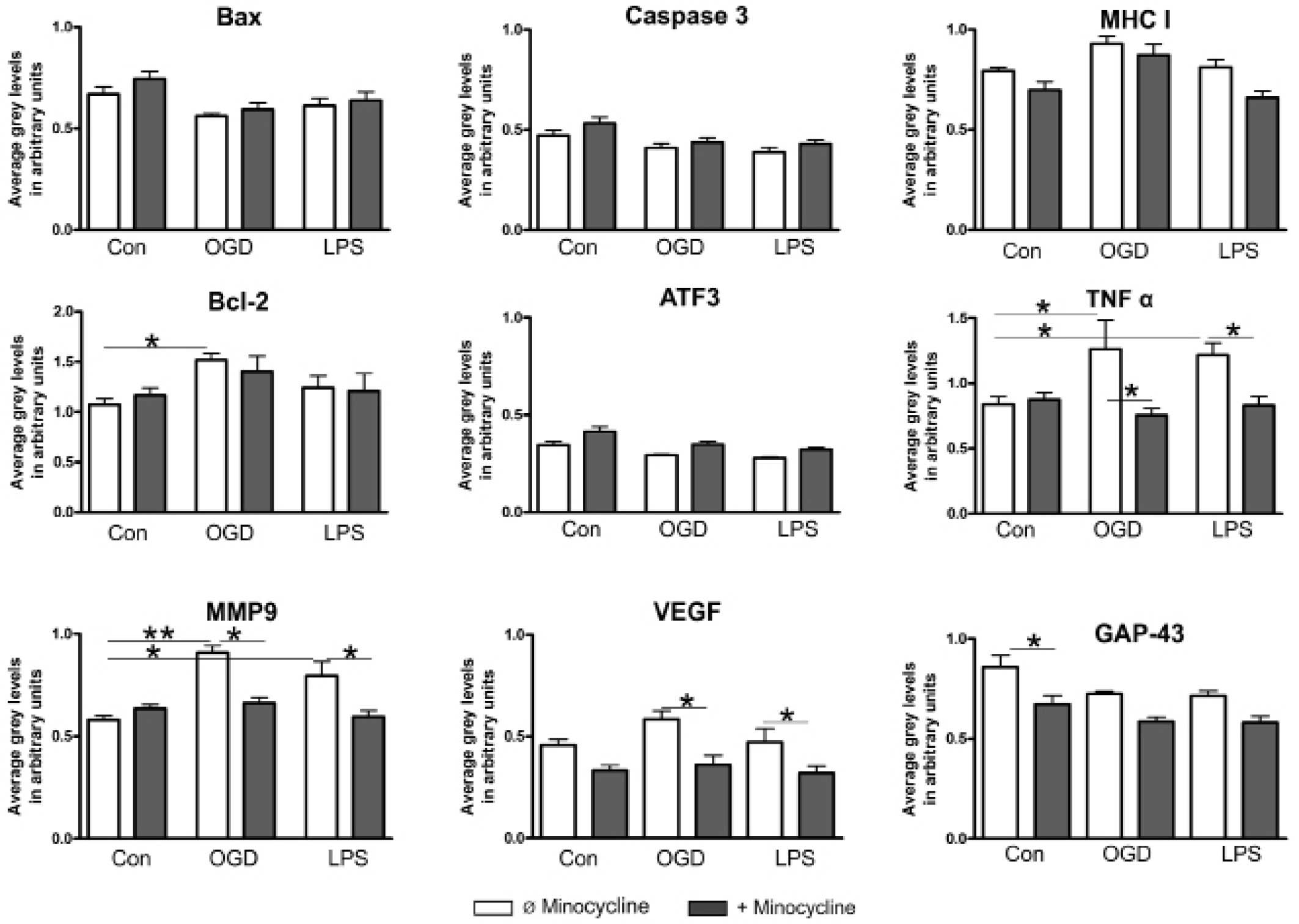
|
1
|
Oliveira AL, Risling M, Deckner M,
Lindholm T, Langone F and Cullheim S: Neonatal sciatic nerve
transection induces TUNEL labeling of neurons in the rat spinal
cord and DRG. Neuroreport. 8:2837–2840. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen LJ, Ren YH, Liu L, Zhang XQ, Zhao Y,
Wu WT and Li F: Upregulated expression of GAP-43 mRNA and protein
in anterior horn motoneurons of the spinal cord after brachial
plexus injury. Arch Med Res. 41:513–538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arocho LC, Figueroa JD, Torrado AI,
Santiago JM, Vera AE and Miranda JD: Expression profile and role of
EphrinA1 ligand after spinal cord injury. Cell Mol Neurobiol.
31:1057–1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobbert C and Thanos S: Topographic
representation of the sciatic nerve motor neurons in the spinal
cord of the adult rat correlates to region-specific activation
patterns of microglia. J Neurocytol. 29:271–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng LF, Wang R, Xu YZ, Yi XN, Zhang JW
and Zeng ZC: Calcitonin gene-related peptide dynamics in rat dorsal
root ganglia and spinal cord following different sciatic nerve
injuries. Brain Res. 1187:20–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu F, Miao X, Chen J, Sun Y, Liu Z, Tao Y
and Yu W: Down-regulation of GAP-43 by inhibition of caspases-3 in
a rat model of neuropathic pain. Int J Clin Exp Pathol. 5:948–955.
2012.PubMed/NCBI
|
|
7
|
Terenghi G, Hart A and Wiberg M: The nerve
injury and the dying neurons: Diagnosis and prevention. J Hand Surg
Eur Vol. 36:730–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amantea D and Bagetta G: Drug repurposing
for immune modulation in acute ischemic stroke. Curr Opin
Pharmacol. 26:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smilack JD: The tetracyclines. Mayo Clin
Proc. 74:727–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yrjanheikki J, Tikka T, Keinänen R,
Goldsteins G, Chan PH and Koistinaho J: A tetracycline derivative,
minocycline, reduces inflammation and protects against focal
cerebral ischemia with a wide therapeutic window. Proc Natl Acad
Sci USA. 96:13496–13500. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Fagan SC, Waller JL, Edwards D,
Borlongan CV, Zheng J, Hill WD, Feuerstein G and Hess DC: Low dose
intravenous minocycline is neuroprotective after middle cerebral
artery occlusion-reperfusion in rats. BMC Neurol. 4:72004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen SD, Yin JH, Hwang CS, Tang CM and
Yang DI: Anti-apoptotic and anti-oxidative mechanisms of
minocycline against sphingomyelinase/ceramide neurotoxicity:
Implication in Alzheimer's disease and cerebral ischemia. Free
Radic Res. 46:340–350. 2012. View Article : Google Scholar
|
|
13
|
Hodl AK and Bonelli RM: Huntington's
disease and minocycline. Mov Disord. 20:510–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zemke D and Majid A: The potential of
minocycline for neuroprotection in human neurologic disease. Clin
Neuropharmacol. 27:293–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pontieri FE, Ricci A, Pellicano C,
Benincasa D and Buttarelli FR: Minocycline in amyotrophic lateral
sclerosis: A pilot study. Neurol Sci. 26:285–287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giuliani F, Fu SA, Metz LM and Yong VW:
Effective combination of minocycline and interferon-beta in a model
of multiple sclerosis. J Neuroimmunol. 165:83–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wells JE, Hurlbert RJ, Fehlings MG and
Yong VW: Neuroprotection by minocycline facilitates significant
recovery from spinal cord injury in mice. Brain. 126:1628–1637.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monaco EA 3rd, Weiner GM and Friedlander
RM: Randomized-controlled trial of minocycline for spinal cord
injury shows promise. Neurosurgery. 72:N17–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stirling DP, Koochesfahani KM, Steeves JD
and Tetzlaff W: Minocycline as a neuroprotective agent.
Neuroscientist. 11:308–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diguet E, Gross CE, Tison F and Bezard E:
Rise and fall of minocycline in neuroprotection: Need to promote
publication of negative results. Exp Neurol. 189:1–4. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuji M, Wilson MA, Lange MS and Johnston
MV: Minocycline worsens hypoxic-ischemic brain injury in a neonatal
mouse model. Exp Neurol. 189:58–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinzon A, Marcillo A, Quintana A, Stamler
S, Bunge MB, Bramlett HM and Dietrich WD: A re-assessment of
minocycline as a neuroprotective agent in a rat spinal cord
contusion model. Brain Res. 1243:146–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JH, Tigchelaar S, Liu J, Stammers AM,
Streijger F, Tetzlaff W and Kwon BK: Lack of neuroprotective
effects of simvastatin and minocycline in a model of cervical
spinal cord injury. Exp Neurol. 225:219–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinkernelle J, Fansa H, Ebmeyer U and
Keilhoff G: Prolonged minocycline treatment impairs motor neuronal
survival and glial function in organotypic rat spinal cord
cultures. PloS One. 8:e734222013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keilhoff G, Langnaese K, Wolf G and Fansa
H: Inhibiting effect of minocycline on the regeneration of
peripheral nerves. Dev Neurobiol. 67:1382–1395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cashman NR, Durham HD, Blusztajn JK, Oda
K, Tabira T, Shaw IT, Dahrouge S and Antel JP: Neuroblastoma ×
spinal cord (NSC) hybrid cell lines resemble developing motor
neurons. Dev Dyn. 194:209–221. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tiraihi T and Rezaie MJ: Apoptosis onset
and bax protein distribution in spinal motoneurons of newborn rats
following sciatic nerve axotomy. Int J Neurosci. 113:1163–1175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao W, Zhao Q, Liu J, Xu XY, Sun WW, Zhou
X, Liu S and Wang TH: Electro-acupuncture reduces neuronal
apoptosis linked to Bax and Bcl-2 expression in the spinal cords of
cats subjected to partial dorsal root ganglionectomy. Neurochem
Res. 33:2214–2221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin LJ and Liu Z: Injury-induced spinal
motor neuron apoptosis is preceded by DNA single-strand breaks and
is p53- and Bax-dependent. J Neurobiol. 50:181–197. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu XM, Shu YH, Qiu CH, Chen KT and Wang
YT: Protective effects and anti-apoptotic role of nerve growth
factor on spinal cord neurons in sciatic nerve-injured rats. Neurol
Res. 36:814–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Payés AC, Zanon RG, Pierucci A and
Oliveira AL: MHC class I upregulation is not sufficient to rescue
neonatal alpha motoneurons after peripheral axotomy. Brain Res.
1238:23–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohtori S, Takahashi K, Moriya H and Myers
RR: TNF-alpha and TNF-alpha receptor type 1 upregulation in glia
and neurons after peripheral nerve injury: Studies in murine DRG
and spinal cord. Spine (Phila Pa 1976). 29:1082–1088. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindå H, Sköld MK and Ochsmann T:
Activating transcription factor 3, a useful marker for regenerative
response after nerve root injury. Front Neurol. 2:302011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hunt D, Raivich G and Anderson PN:
Activating transcription factor 3 and the nervous system. Front Mol
Neurosci. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu CY, Hong GX and Wang FB: Expression of
vascular endothelial growth factor and its fetal liver kinase-1
receptor in spinal cord and dorsal root ganglia after neurotomy of
sciatic nerve in rats. Chin J Traumatol. 8:17–22. 2005.PubMed/NCBI
|
|
36
|
Liou JT, Sum DC, Liu FC, Mao CC, Lai YS
and Day YJ: Spatial and temporal analysis of nociception-related
spinal cord matrix metalloproteinase expression in a murine
neuropathic pain model. J Chin Med Assoc. 76:201–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jacobson RD, Virág I and Skene JH: A
protein associated with axon growth, GAP-43, is widely distributed
and developmentally regulated in rat CNS. J Neurosci. 6:1843–1855.
1986.PubMed/NCBI
|
|
38
|
Bulsara KR, Iskandar BJ, Villavicencio AT
and Skene JH: A new millenium for spinal cord regeneration:
Growth-associated genes. Spine (Phila Pa 1976). 27:1946–1949. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raghavendra V, Tanga F and DeLeo JA:
Inhibition of microglial activation attenuates the development but
not existing hypersensitivity in a rat model of neuropathy. J
Pharmacol Exp Ther. 306:624–630. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Langnaese K, John R, Schweizer H, Ebmeyer
U and Keilhoff G: Selection of reference genes for quantitative
real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol
Biol. 9:532008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lucas B, Pinkernelle J, Fansa H and
Keilhoff G: Effects of cerebrolysin on rat Schwann cells in vitro.
Acta Histochem. 116:820–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Keilhoff G and Wolf G: Comparison of
double fluorescence staining and LDH-test for monitoring cell
viability in vitro. Neuroreport. 5:129–132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Infante SK, Oberhauser AF and Perez-Polo
JR: Bax phosphorylation association with nucleus and
oligomerization after neonatal hypoxia-ischemia. J Neurosci Res.
91:1152–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seijffers R, Mills CD and Woolf CJ: ATF3
increases the intrinsic growth state of DRG neurons to enhance
peripheral nerve regeneration. J Neurosci. 27:7911–7920. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wolff JR and Missler M: Synaptic
reorganization in developing and adult nervous systems. Ann Anat.
174:393–403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vieira AS, de Rezende AC and Rogerio F:
Evaluating motor neuron death in neonatal rats subjected to sciatic
nerve lesion. Methods Mol Biol. 846:383–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carlson J, Lais AC and Dyck PJ: Axonal
atrophy from permanent peripheral axotomy in adult cat. J
Neuropathol Exp Neurol. 38:579–585. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Victório SC, Havton LA and Oliveira AL:
Absence of IFNγ expression induces neuronal degeneration in the
spinal cord of adult mice. J Neuroinflammation. 7:772010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ohlsson M, Nieto JH, Christe KL and Havton
LA: Long-term effects of a lumbosacral ventral root avulsion injury
on axotomized motor neurons and avulsed ventral roots in a
non-human primate model of cauda equina injury. Neuroscience.
250:129–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen T, Koga K, Li XY and Zhuo M: Spinal
microglial motility is independent of neuronal activity and
plasticity in adult mice. Mol Pain. 6:192010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chew DJ, Carlstedt T and Shortland PJ: A
comparative histological analysis of two models of nerve root
avulsion injury in the adult rat. Neuropathol Appl Neurobiol.
37:613–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hart AM, Terenghi G and Wiberg M: Neuronal
death after peripheral nerve injury and experimental strategies for
neuroprotection. Neurol Res. 30:999–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gillardon F, Klimaschewski L, Wickert H,
Krajewski S, Reed JC and Zimmermann M: Expression pattern of
candidate cell death effector proteins Bax, Bcl-2, Bcl-X and c-Jun
in sensory and motor neurons following sciatic nerve transection in
the rat. Brain Res. 739:244–250. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tsujino H, Kondo E, Fukuoka T, Dai Y,
Tokunaga A, Miki K, Yonenobu K, Ochi T and Noguchi K: Activating
transcription factor 3 (ATF3) induction by axotomy in sensory and
motoneurons: A novel neuronal marker of nerve injury. Mol Cell
Neurosci. 15:170–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sabha M Jr, Emirandetti A, Cullheim S and
De Oliveira AL: MHC I expression and synaptic plasticity in
different mice strains after axotomy. Synapse. 62:137–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schwartz M, Butovsky O, Brück W and
Hanisch UK: Microglial phenotype: Is the commitment reversible?
Trends Neurosci. 29:68–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vega-Avelaira D, Moss A and Fitzgerald M:
Age-related changes in the spinal cord microglial and astrocytic
response profile to nerve injury. Brain Behav Immun. 21:617–623.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang Y, Ling ZM, Fu R, Li YQ, Cheng X,
Song FH, Luo HX and Zhou LH: Time-specific microRNA changes during
spinal motoneuron degeneration in adult rats following unilateral
brachial plexus root avulsion: Ipsilateral vs. contralateral
changes. BMC Neurosci. 15:922014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rotshenker S and Tal M: The transneuronal
induction of sprouting and synapse formation in intact mouse
muscles. J Physiol. 360:387–396. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rotto-Percelay DM, Wheeler JG, Osorio FA,
Platt KB and Loewy AD: Transneuronal labeling of spinal
interneurons and sympathetic preganglionic neurons after
pseudorabies virus injections in the rat medial gastrocnemius
muscle. Brain Res. 574:291–306. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Villar MJ, Cortés R, Theodorsson E,
Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC and
Hökfelt T: Neuropeptide expression in rat dorsal root ganglion
cells and spinal cord after peripheral nerve injury with special
reference to galanin. Neuroscience. 33:587–604. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Piehl F, Arvidsson U, Johnson H, Cullheim
S, Villar M, Dagerlind A, Terenius L, Hökfelt T and Ulfhake B:
Calcitonin Gene-related Peptide (CGRP)-like Immunoreactivity and
CGRP mRNA in rat spinal cord motoneurons after different types of
lesions. Eur J Neurosci. 3:737–757. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Benemei S, Nicoletti P, Capone JG and
Geppetti P: CGRP receptors in the control of pain and inflammation.
Curr Opin Pharmacol. 9:9–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sueur S, Pesant M, Rochette L and Connat
JL: Antiapoptotic effect of calcitonin gene-related peptide on
oxidative stress-induced injury in H9c2 cardiomyocytes via the
RAMP1/CRLR complex. J Mol Cell Cardiol. 39:955–963. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Spejo AB and Oliveira AL: Synaptic
rearrangement following axonal injury: Old and new players.
Neuropharmacology. 96:113–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guntinas-Lichius O, Neiss WF, Gunkel A and
Stennert E: Differences in glial, synaptic and motoneuron responses
in the facial nucleus of the rat brainstem following facial nerve
resection and nerve suture reanastomosis. Eur Arch
Otorhinolaryngol. 251:410–417. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hardingham GE: Coupling of the NMDA
receptor to neuroprotective and neurodestructive events. Biochem
Soc Trans. 37:1147–1160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tyzack GE, Sitnikov S, Barson D,
Adams-Carr KL, Lau NK, Kwok JC, Zhao C, Franklin RJ, Karadottir RT,
Fawcett JW and Lakatos A: Astrocyte response to motor neuron injury
promotes structural synaptic plasticity via STAT3-regulated TSP-1
expression. Nat Commun. 5:42942014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Garrido-Mesa N, Zarzuelo A and Gálvez J:
Minocycline: Far beyond an antibiotic. Br J Pharmacol. 169:337–352.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liao TV, Forehand CC, Hess DC and Fagan
SC: Minocycline repurposing in critical illness: Focus on stroke.
Curr Top Med Chem. 13:2283–2290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li C, Yuan K and Schluesener H: Impact of
minocycline on neurodegenerative diseases in rodents: A
meta-analysis. Rev Neurosci. 24:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Teng YD, Choi H, Onario RC, Zhu S,
Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME and
Friedlander RM: Minocycline inhibits contusion-triggered
mitochondrial cytochrome c release and mitigates functional
deficits after spinal cord injury. Proc Natl Acad Sci USA.
101:3071–3076. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chaturvedi M and Kaczmarek L: Mmp-9
inhibition: A therapeutic strategy in ischemic stroke. Mol
Neurobiol. 49:563–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Enose-Akahata Y, Matsuura E, Tanaka Y, Oh
U and Jacobson S: Minocycline modulates antigen-specific CTL
activity through inactivation of mononuclear phagocytes in patients
with HTLV-I associated neurologic disease. Retrovirology. 9:162012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jung HJ, Seo I, Jha BK, Suh SI, Suh MH and
Baek WK: Minocycline inhibits angiogenesis in vitro through the
translational suppression of HIF-1α. Arch Biochem Biophys.
545:74–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li CH, Liao PL, Yang YT, Huang SH, Lin CH,
Cheng YW and Kang JJ: Minocycline accelerates hypoxia-inducible
factor-1 alpha degradation and inhibits hypoxia-induced
neovasculogenesis through prolyl hydroxylase, von
Hippel-Lindau-dependent pathway. Arch Toxicol. 88:659–671.
2014.PubMed/NCBI
|
|
77
|
Sun C, Li XX, He XJ, Zhang Q and Tao Y:
Neuroprotective effect of minocycline in a rat model of branch
retinal vein occlusion. Exp Eye Res. 113:105–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Levkovitz Y, Fenchel D, Kaplan Z, Zohar J
and Cohen H: Early post-stressor intervention with minocycline, a
second-generation tetracycline, attenuates post-traumatic stress
response in an animal model of PTSD. Eur Neuropsychopharmacol.
25:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM,
Choi JY, Hwang DH and Kim BG: Contribution of macrophages to
enhanced regenerative capacity of dorsal root ganglia sensory
neurons by conditioning injury. J Neurosci. 33:15095–15108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ataie-Kachoie P, Badar S, Morris DL and
Pourgholami MH: Minocycline targets the NF-kB Nexus through
suppression of TGF-β1-TAK1-IkB signaling in ovarian cancer. Mol
Cancer Res. 11:1279–1291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pang T, Wang J, Benicky J and Saavedra JM:
Minocycline ameliorates LPS-induced inflammation in human monocytes
by novel mechanisms including LOX-1, Nur77 and LITAF inhibition.
Biochim Biophys Acta. 1820:503–510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li H, Xu H and Sun B: Lipopolysaccharide
regulates MMP-9 expression through TLR4/NF-kB signaling in human
arterial smooth muscle cells. Mol Med Rep. 6:774–778.
2012.PubMed/NCBI
|
|
83
|
Pick M, Ronen D, Yanuka O and Benvenisty
N: Reprogramming of the MHC-I and its regulation by NFkB in
human-induced pluripotent stem cells. Stem Cells. 30:2700–2708.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xie TX, Xia Z, Zhang N, Gong W and Huang
S: Constitutive NF-kB activity regulates the expression of VEGF and
IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep.
23:725–732. 2010.PubMed/NCBI
|
|
85
|
Zhao M, Zhou A, Xu L and Zhang X: The role
of TLR4-mediated PTEN/PI3K/AKT/NF-kB signaling pathway in
neuroinflammation in hippocampal neurons. Neuroscience. 269:93–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Pozniak PD, White MK and Khalili K:
TNF-α/NF-κB signaling in the CNS: Possible connection to EPHB2. J
Neuroimmune Pharmacol. 9:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Matsuura Y, Ochi M, Uchio Y, Suzuki G and
Iwata A: The time-dependent difference of GAP-43 expression between
sensory neurons and motoneurons after peripheral nerve transection.
Scand J Plast Reconstr Surg Hand Surg. 33:267–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gordon T, You S, Cassar SL and Tetzlaff W:
Reduced expression of regeneration associated genes in chronically
axotomized facial motoneurons. Exp Neurol. 264:26–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tedeschi A, Nguyen T, Puttagunta R, Gaub P
and Di Giovanni S: A p53-CBP/p300 transcription module is required
for GAP-43 expression, axon outgrowth and regeneration. Cell Death
Differ. 16:543–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jiang BP, Le L, Xu LJ and Xiao PG:
Minocycline inhibits ICAD degradation and the NF-kB activation
induced by 6-OHDA in PC12 cells. Brain Res. 1586:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kadiyala CS, Zheng L, Du Y, Yohannes E,
Kao HY, Miyagi M and Kern TS: Acetylation of retinal histones in
diabetes increases inflammatory proteins: Effects of minocycline
and manipulation of histone acetyltransferase (HAT) and histone
deacetylase (HDAC). J Biol Chem. 287:25869–25880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Levkovitch-Verbin H, Waserzoog Y, Vander
S, Makarovsky D and Piven I: Minocycline upregulates pro-survival
genes and downregulates pro-apoptotic genes in experimental
glaucoma. Graefes Arch Clin Exp Ophthalmol. 252:761–772. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hassanzadeh K, Habibi-asl B, Farajnia S
and Roshangar L: Minocycline prevents morphine-induced apoptosis in
rat cerebral cortex and lumbar spinal cord: A possible mechanism
for attenuating morphine tolerance. Neurotox Res. 19:649–659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Filipovic R and Zecevic N: Neuroprotective
role of minocycline in co-cultures of human fetal neurons and
microglia. Exp Neurol. 211:41–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Matsukawa N, Yasuhara T, Hara K, Xu L,
Maki M, Yu G, Kaneko Y, Ojika K, Hess DC and Borlongan CV:
Therapeutic targets and limits of minocycline neuroprotection in
experimental ischemic stroke. BMC Neurosci. 10:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kerr DA, Larsen T, Cook SH, Fannjiang YR,
Choi E, Griffin DE, Hardwick JM and Irani DN: BCL-2 and BAX protect
adult mice from lethal Sindbis virus infection but do not protect
spinal cord motor neurons or prevent paralysis. J Virol.
76:10393–10400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hemendinger RA, Armstrong EJ 3rd, Radio N
and Brooks BR: Neurotoxic injury pathways in differentiated mouse
motor neuron-neuroblastoma hybrid (NSC-34D) cells in vitro -
limited effect of riluzole on thapsigargin, but not staurosporine,
hydrogen peroxide and homocysteine neurotoxicity. Toxicol Appl
Pharmacol. 258:208–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Soo KY, Atkin JD, Horne MK and Nagley P:
Recruitment of mitochondria into apoptotic signaling correlates
with the presence of inclusions formed by amyotrophic lateral
sclerosis-associated SOD1 mutations. J Neurochem. 108:578–590.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee SH, Choi NY, Yu HJ, Park J, Choi H,
Lee KY, Huh YM, Lee YJ and Koh SH: Atorvastatin protects NSC-34
motor neurons against oxidative stress by activating PI3K, ERK and
free radical scavenging. Mol Neurobiol. Jan 11–2015.(Epub ahead of
print).
|
|
100
|
Ezzi SA, Urushitani M and Julien JP:
Wild-type superoxide dismutase acquires binding and toxic
properties of ALS-linked mutant forms through oxidation. J
Neurochem. 102:170–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu YX, Tai JL, Li GQ, Zhang ZW, Xue JH,
Liu HS, Zhu H, Cheng JD, Liu YL, Li AM and Zhang Y: Exposure to
1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of
astrocytes via caspase-3-dependent pathway. PLoS One. 7:e423322012.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ransohoff RM and Estes ML: Astrocyte
expression of major histocompatibility complex gene products in
multiple sclerosis brain tissue obtained by stereotactic biopsy.
Arch Neurol. 48:1244–1246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kim KH, Jeong JY, Surh YJ and Kim KW:
Expression of stress-response ATF3 is mediated by Nrf2 in
astrocytes. Nucleic Acids Res. 38:48–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sunkaria A, Wani WY, Sharma DR and Gill
KD: Dichlorvos exposure results in activation induced apoptotic
cell death in primary rat microglia. Chem Res Toxicol.
25:1762–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tambuyzer BR, Ponsaerts P and Nouwen EJ:
Microglia: Gatekeepers of central nervous system immunology. J
Leukoc Biol. 85:352–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Simonishvili S, Jain MR, Li H, Levison SW
and Wood TL: Identification of Bax-interacting proteins in
oligodendrocyte progenitors during glutamate excitotoxicity and
perinatal hypoxia-ischemia. ASN Neuro. 5:e001312013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ji Q, Castelli L and Goverman JM: MHC
class I-restricted myelin epitopes are cross-presented by Tip-DCs
that promote determinant spreading to CD8+ T cells. Nat
Immunol. 14:254–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Goldberg J, Daniel M, van Heuvel Y, Victor
M, Beyer C, Clarner T and Kipp M: Short-term cuprizone feeding
induces selective amino acid deprivation with concomitant
activation of an integrated stress response in oligodendrocytes.
Cell Mol Neurobiol. 33:1087–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Johanson SO, Crouch MF and Hendry IA:
Retrograde axonal transport of signal transduction proteins in rat
sciatic nerve. Brain Res. 690:55–63. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang HH, Hsieh HL, Wu CY and Yang CM:
Oxidized low-density lipoprotein-induced matrix metalloproteinase-9
expression via PKC-delta/p42/p44 MAPK/Elk-1 cascade in brain
astrocytes. Neurotox Res. 17:50–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lively S and Schlichter LC: The microglial
activation state regulates migration and roles of matrix-dissolving
enzymes for invasion. J Neuroinflammation. 10:752013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Monnet-Tschudi F, Defaux A, Braissant O,
Cagnon L and Zurich MG: Methods to assess neuroinflammation. Curr
Protoc Toxicol Unit. 12:192011.
|
|
113
|
Krum JM and Khaibullina A: Inhibition of
endogenous VEGF impedes revascularization and astroglial
proliferation: Roles for VEGF in brain repair. Exp Neurol.
181:241–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shin YJ, Riew TR, Park JH, Pak HJ and Lee
MY: Expression of vascular endothelial growth factor-C (VEGF-C) and
its receptor (VEGFR-3) in the glial reaction elicited by human
mesenchymal stem cell engraftment in the normal rat brain. J
Histochem Cytochem. 63:170–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Keshavarz M, Emamghoreishi M, Nekooeian
AA, Warsh JJ and Zare HR: Increased bcl-2 protein levels in rat
primary astrocyte culture following chronic lithium treatment. Iran
J Med Sci. 38:255–262. 2013.PubMed/NCBI
|
|
116
|
Barber SC, Higginbottom A, Mead RJ, Barber
S and Shaw PJ: An in vitro screening cascade to identify
neuroprotective antioxidants in ALS. Free Radic Biol Med.
46:1127–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Prell T, Lautenschläger J, Weidemann L,
Ruhmer J, Witte OW and Grosskreutz J: Endoplasmic reticulum stress
is accompanied by activation of NF-kB in amyotrophic lateral
sclerosis. J Neuroimmunol. 270:29–36. 2014. View Article : Google Scholar : PubMed/NCBI
|