Introduction
During embryonic and early postnatal development,
the axotomy of motorneurons or removal of their target results in
significant motorneuron cell loss. In adults however, axotomy can
result in either complete motorneuron survival or motorneuron
death. For patients this implies an incomplete or even complete
loss of function of the muscular targets of the lost motorneurons
(1).
It is well-established that injuries of the spinal
cord (2,3) as well as the peripheral nerves
(4–6)
lead to changes in gene and protein expression levels in
motorneurons and glial cells, which may result in neuronal
apoptosis. This is the basis for therapeutic strategies that aim to
enhance axonal regeneration and functional recovery following
peripheral nerve injury, including pharmacological treatments
(7).
In this physiological context, minocycline has been
widely used, but the advantages and disadvantages of this treatment
appear equal in number. Minocycline is a semi-synthetic second
generation tetracycline with broad spectrum anti-microbial activity
(8). The primary applications of
minocycline include treatment of pneumonia, rheumatoid arthritis,
acne and infections of the skin, the genital, and urinary systems
(9). There are also promising
preclinical studies for the treatment of stroke (10,11),
Alzheimer's disease (12),
Huntington's disease (13),
Parkinson's disease (14),
amyotrophic lateral sclerosis (15),
multiple sclerosis (15,16) and traumatic brain injury (17). Clinical trials with minocycline for
the treatment of spinal cord injury have been underway since the
early 2000s (18). The predominant
effect of minocycline is associated with its ability to modulate
microglia and immune cell activation and to reduce apoptosis
(19). There have however been
reports of conflicting results, with a number of previous studies
demonstrating that minocycline worsened spinal cord and brain
injuries (20–23).
Our previous investigations demonstrated that
minocycline impairs motorneuron survival in organotypic rat spinal
cord cultures (24) and inhibited
the regeneration of peripheral nerves (25). The present study was undertaken to
examine the effects of minocycline on the expression of selected
transcriptional and translational profiles in the rat spinal cord
following sciatic nerve transection and microsurgical coaptation.
In addition to the spinal cord in vivo, the present study
conducted in vitro experiments using NSC-34 motorneuron-like
cells. NSC-34 is a hybrid cell line produced by the fusion of
neuroblastoma with mouse motorneuron-enriched primary spinal cord
cells (26). These cells share
numerous morphological and physiological characteristics with
mature primary motorneurons, and thus are an accepted model for
studying the pathophysiology of motorneurons (26). Stress was induced by oxygen glucose
deprivation (OGD) or lipopolysaccharide (LPS) treatment. The mRNA
and protein expression levels of the following compounds were
examined: i) B cell lymphoma 2 (Bcl-2)-associated X protein (Bax),
which has been demonstrated to be upregulated in the spinal
motorneurons of newborn rats following sciatic nerve injury
(27) and in adult cats following
partial dorsal root ganglion ectomy (28); ii) caspase-3, which is activated in
adult spinal motorneurons during injury-induced apoptosis (29); iii) Bcl-2, which has been reported to
be activated in the adult spinal motorneurons of rats in the first
three weeks following sciatic nerve injury (30); iv) major histocompatibility complex
of class I (MHC I), which is upregulated in the spinal motorneurons
of neonatal rats following sciatic nerve injury (31); v) tumor necrosis factor (TNF-α),
released from astrocytes and microglia around motorneurons in rat
spinal cord in the first two weeks following sciatic nerve crush
(32); vi) activating transcription
factor (ATF3), which is a marker for regenerative response
following nerve root injury (33),
and its expression in neurons is closely associated with their
survival and the regeneration of their axons following axotomy
(34); vii) vascular endothelial
growth factor (VEGF), which has been demonstrated to be upregulated
in the spinal motorneurons of adult rats in response to neurotomy
(35); viii) matrix
metalloproteinase 9 (MMP9), immediately upregulated in adult mice
spinal motorneurons following nerve injury (36); and ix) growth-associated protein 43
(GAP-43), which is expressed at high levels during development
(37) and stressed by nerve injury
adult motorneurons (38).
Materials and methods
Ethical approval
The present study was conducted in accordance with
the European Commission regulations and those of the National Act
on the Use of Experimental Animals of Germany, and adhered to the
guidelines of the Committee for Research and Ethical Issues of the
International Association for the Study of Pain.
Animal model
Animals
A total of 51 female Wistar rats (10 weeks old,
200–230 g, strain-matched, inbred) were obtained from
Harlan-Winkelmann GmbH (Borchen, Germany). The rats were housed
under controlled laboratory conditions with a 12-h light/dark cycle
(lights on at 6 am) at 20±2°C with an air humidity of 55–60%. The
animals were provided with ad libitum access to commercial
rat pellets (Altromin 1324™; Altromin Spezialfutter GmbH & Co.
KG, Lage, Germany) and tap water. Following intervention the rats
were housed in pairs in Makrolon IIL cages (Bioscape GmbH,
Castrop-Rauxel, Germany). Every effort was made to minimize the
amount of suffering and the number of animals used in the
experiments.
A total of 46 rats were injured and divided into
four phosphate-buffered saline (PBS; Sigma-Aldrich Chemie GmbH,
Munich, Germany) and four minocycline treatment groups with
survival times of 3, 5, 7 and 14 days post-intervention (DPI), with
five animals/group for semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR). An additional
three animals from the 7-day PBS-treated and from the the 7-day
minocycline-treated groups were used for immunohistochemical
analysis. For semi-quantitative RT-PCR the spinal cords of five
untreated animals were also prepared.
Minocycline treatment
Minocycline hydrochloride (Sigma-Aldrich, St. Louis,
MO, USA) was administered once daily for ≥7 consecutive days by
intraperitoneal injection at a dosage of 50 mg/kg body weight (~10
times the usual human dose), starting at 30 min following nerve
reconstruction. The drug was dissolved in saline (pH 7.2, freshly
prepared daily) at 37°C. A dosage of >20 mg/kg was selected to
induce the maximal anti-hyperalgesic effect, as lower doses are
unable to affect gene expression in a sufficiently stable manner
(39). Control rats were injected
with PBS (pH 7.2) using an identical treatment regime.
Surgical protocol
The surgical procedure protocol for nerve
reconstruction was the same for all groups, and consisted of
exposing the right sciatic nerve through a dorsal incision under
general anesthesia (60 mg/kg pentobarbital, intraperitoneal;
Sigma-Aldrich) and aseptic conditions using an SV8 operating
microscope (Zeiss GmbH, Jena, Germany). The nerve was transected at
the proximal origin of the gracilis muscle and immediately
microsurgically coaptated with respect to intraneuronal topography
using epineural sutures (Ethilon 11×0; Johnson & Johnson, New
Brunswick, NJ, USA) followed by closure of the dorsal incision.
Semi-quantitative RT-PCR
Following the respective survival times (3, 5, 7 and
14 days), the animals were sacrificed by an excess of anesthesia
(pentobarbital) via intraperitoneal injection. L3-L6 sections of
the spinal cord, divided into ipsilateral and contralateral sites
were harvested and homogenized in peqGOLD TriFast total RNA
isolation reagent (cat. no. 30–2030; PeqLab Biotechnologie GmbH,
Erlangen, Germany) using an Ultra-Turrax Homogenizer
(IKA® Werke GmbH & Co. KG, Staufen im Breisgau,
Germany). Total RNA was prepared according to the manufacturer's
instructions. Potentially contaminating DNA was removed by treating
5 µg total cell RNA with Turbo DNA-free (Ambion; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). RNA (4 µl; 2 µg input RNA) was
reverse transcribed using a RevertAid™ H Minus First Strand cDNA
Synthesis kit primed with Oligo(dT)18 primers (cat. no.
K1631; Thermo Fisher Scientific, Inc.; primers listed in Table I). cDNA (1 µl) was then amplified by
PCR using Taq DNA polymerase (PeqLab Biotechnologie GmbH),
as previously described (40).
One-tenth of each reaction product was electrophoresed on a 1%
agarose gel (Serva Electrophoresis GmbH, Heidelberg, Germany)
(excluding TNF-α, which required a 2% agarose gel). The PCR product
bands were quantified by densitometric analysis using a GeneGenius
bio-imaging system (Syngene, Cambridge, UK) and the ratio of their
expression levels to those of the GAPDH reference gene were
calculated. Each experiment was repeated in triplicate.
 | Table I.Sequences of primers used for
semi-quantitative reverse transcription-polymerase chain
reaction. |
Table I.
Sequences of primers used for
semi-quantitative reverse transcription-polymerase chain
reaction.
| Gene | Sequence | Primer
location | Product size (base
pairs) | Cycle no. | Annealing
temperature (°C) | Accession
number |
|---|
| ATF3 |
5′-TTCCGAGAGTTTGGGGGTCTGCC-3′ |
1,285–1,307 | 320 | 36 | 60 | NM 012912 |
|
|
5′-CCAGTCTCCACGGGCTGTGGTT-3′ |
1,604–1,583 |
|
|
|
|
| Bax |
5′-GGATGGTTGCTGATGTGGATAC-3′ |
86–107 | 342 | 40 | 58 | XM_001081479 |
|
|
5′-CCATCTTCTTCCAGATGGTGAG-3′ |
427–406 |
|
|
|
|
| Bcl-2 |
5′-CGACTTTGCAGAGATGTCCAG-3′ |
555–575 | 392 | 40 | 58 | NM_016993 |
|
|
5′-CTCACTTGTGGCCCAGGTATG-3′ |
946–926 |
|
|
|
|
| Caspase-3 |
5′-CCTCAGAGAGACATTCATGGC-3′ |
275–295 | 247 | 40 | 56 | NM_012922 |
|
|
5′-TCGGCTTTCCAGTCAGACTC-3′ |
522–503 |
|
|
|
|
| GAP-43 |
5′-AGCGCAGCCTCCAACGGAGAC-3′ |
759–779 | 247 | 36 | 60 | NM 017195 |
|
|
5′-GCTCACACACGTGAGCAGGACA-3′ | 1,005–984 |
|
|
|
|
| MHC I |
5′-GGCTCACACTCGCTGCGGTATTT-3′ |
82–104 | 626 | 36 | 60 | M 31018 |
|
|
5′-TAGAAGCCCAGGGCCCAGCA-3′ |
707–688 |
|
|
|
|
| MMP9 |
5′-AGTTTGGTGTCGCGGAGCAC-3′ |
509–528 | 754 | 40 | 60 | NM_031055 |
|
|
5′-TACATGAGCGCTTCCGGCAC-3′ |
1,262–1,243 |
|
|
|
|
| TNF-α |
5′-CCCAGACCCTCACACTCAGAT-3′ |
389–413 | 214 | 38 | 56 | NM 012675 |
|
|
5′-TTTTCCCTTGAAGAGAACCTG-3′ |
563–541 |
|
|
|
|
| VEGF |
5′-GAAGTTCATGGACGTCTACC-3′ |
121–139 | 237 | 40 | 55 | NM_031836 |
|
|
5′-CATCTCTCCTATGTGCTGGC-3′ |
357–338 |
|
|
|
|
| GAPDH |
5′-TTAGCACCCCTGGCCAAGG-3′ |
802–820 | 531 | 24 | 55 | XM_228411 |
|
|
5′-CTTACTCCTTGGAGGCCATG-3′ |
1,332–1,314 |
|
|
|
|
Statistical analysis of all groups was conducted
using a non-parametric Kruskal-Wallis test
Dunn's multiple comparison test was used as a
post-hoc test. For statistical analysis of the groups within one
survival time, analysis of variance with Tukey's post-hoc test was
performed. Graph Pad Prism 4 software (GraphPad Software Inc., La
Jolla, CA, USA) was used to conduct the statistical analyses.
P<0.05 was considered to indicate a statistically significant
result.
Immunohistochemical evaluation
At 5 DPI, anesthetized rats administered an excess
of intraperitoneal pentobarbital were transcardially perfused with
4% paraformaldehyde (PFA; Sigma-Aldrich). L3-L6 sections of the
spinal cord were removed and postfixed for 24 h, cryo-protected in
30% sucrose (in 0.4% buffered PFA) for 24 h, rapidly frozen, and
sectioned using a cryostat (Jung Frigocut 2800 E; Leica
Microsystems GmbH, Wetzlar, Germany; 20 µm). The tissue sections
were subsequently immunostained for mouse monoclonal
anti-pan-neuronal neurofilament marker (neuronal marker; pan-NF
SMI311; non-phosphoneurofilament specific; 1:1,000; Covance Inc.,
Princeton, NJ, USA) combined with the following antibodies: i)
Rabbit polyclonal anti-glial fibrillary acidic protein (GFAP;
astroglial marker; 1:1,000; cat. no. 10555; Progen Biotechnik GmbH,
Heidelberg, Germany); ii) goat polyclonal anti-ionized calcium
binding adaptor molecule 1 (IBA1; microglia marker; 1:1,000; cat.
no. ab5076; Abcam, Cambridge, UK); iii) rabbit monoclonal anti-Bax
(1:200; cat. no. ab32503; Abcam); iv) rabbit polyclonal
anti-caspase-3 (1:100; cat. no. AB3623; EMD Millipore, Billerica,
MA, USA); v) rabbit polyclonal anti-Bcl-2 (1:1,000; cat. no.
AB1722; EMD Millipore); vi) rat monoclonal anti-MHC I (1:100; cat.
no. ab15680; Abcam); vii) rabbit polyclonal anti-TNF-α (1:500; cat.
no. ab9755; Abcam), and viii) rabbit polyclonal anti-MMP9 (1:100;
cat. no. ab7299; Abcam) or ix) rabbit polyclonal anti-GAP-43
(1:500; cat. no. AB5220;EMD Millipore). Co-staining of mouse
monoclonal anti-VEGF (1:500; cat. no. 05–1116; EMD Millipore) and
mouse monoclonal anti-ATF3 (1:200; cat. no. ab191513; Abcam) was
performed with rabbit polyclonal anti-β-III-tubulin (1:1,000; cat.
no. 802001; Biolegend, San Diego, CA, USA) as a neuronal marker.
All antibodies were diluted in 1% normal goat serum and 0.3% Triton
X-100 (Sigma-Aldrich) in PBS. The tissue sections were incubated
overnight at 7°C. PBS washing was conducted prior to secondary
antibody incubation for 3 h with goat anti-mouse Alexa 488 (1:500;
cat. no. A-11001; Invitrogen; Thermo Fisher Scientific, Inc.) and
donkey anti-rabbit Cy3 (1:250; cat. no. 711-165-152; Dianova
Vertriebs-Gesellschaft mbH, Hamburg, Germany) diluted in 1% normal
goat serum and 0.3% Triton in PBS. Cryosections were mounted on
slides, embedded with Immu-Mount (Thermo Fisher Scientific, Inc.)
and examined under a fluorescent AxioImager M1 microscope (Zeiss
GmbH, Jena, Germany) with rhodamine and fluorescein isothiocyanate
filters and a Plan-Neofluar objective (20/0.5).
Cell culture model
NSC-34 cell line culture
NSC-34 cells (cat. no. CLU140; Cedarlane,
Burlington, Canada) were stored at −80°C in cryo tubes. In
preparation for experiments, 106 cells were pre-cultured
in 15 ml pyruvate-free Dulbecco's modified Eagle's Medium (DMEM)
supplemented with 4.5 g/l glucose, 10% fetal calf serum (FCS) and
0.2% Ciprobay (all Gibco®; Thermo Fisher Scientific,
Inc.) for 7 days in vitro (DIV) in 75-cm2 flasks
at 37°C in a humidified atmosphere containing 5% CO2
(herein referred to as ‘normal conditions’). Thereafter, the cells
were harvested by scraping from the bottom, centrifuged for 10 min
at 360 × g at room temperature, resuspended in 10 ml DMEM
(constituents as above) and seeded (0 DIV) in 96-well plates
(1×104 cells/100 µl DMEM/well) for assessment of cell
proliferation/survival by MTT assay, and in 25-cm2
flasks (2×105 cells/5 ml DMEM/flask) for
semi-quantitative RT-PCR, or in φ 35-mm culture dishes
(5×104 cells/2 ml DMEM/dish) for
immunohistochemistry.
Stress induction
OGD was induced following 4 DIV. Briefly, the medium
was removed and replaced with normal medium under normal conditions
or OGD medium (glucose-free DMEM supplemented with 10% FCS and 0.2%
Ciprobay) under OGD conditions. OGD conditions were reached by
exposing the cultures to an atmosphere composed of 5%
CO2 and 1% O2, using nitrogen gas to displace
ambient air in a C200 incubator (Labotect Technik-Göttingen GmbH,
Rosdorf, Germany) at 37°C for 6 h. For reoxygenation the incubator
atmosphere was reestablished to 5% CO2 and 21%
O2 and 4.5 mg/ml glucose was added.
Addition of LPS (Escherichia coli;
Sigma-Aldrich) was also performed at 4 DIV. Regarding OGD, the
medium was replaced with normal medium supplemented with 2 mg/ml
LPS for 24 h.
Minocycline treatment
Minocycline hydrochloride (molecular weight, 493.9
g/mol; Sigma-Aldrich) was dissolved in sterile PBS to obtain a
stock solution of 5 mg/ml (pH 6.5). From this stock solution, 1
µl/well, 20 µl/dish and 50 µl/flask were added to the respective
groups (control, OGD, LPS) following medium replacement in order to
start the stress induction (final minocycline concentration 100 µM,
final pH 7.0).
Assessment of cell proliferation/survival by MTT,
bromodeoxyuridine (BrdU) and vital staining
The specific turnover of MTT (6 mg/ml;
Sigma-Aldrich) to formazan by viable cells was analyzed 24 h
following OGD induction using photometry. Briefly, 8 µl MTT (6
mg/ml) was added to each well and incubated for 3 h prior to
complete removal of the medium. A total of 100 µl dimethyl
sulfoxide (DMSO; Merck Millipore, Darmstadt, Germany) was then
added to each well, and extinction coefficients in each well were
determined using an Infinite® M200 (Tecan GmbH,
Crailsheim, Germany) and calculated by subtracting the reference
absorbance at 690 nm from the absorbance at 570 nm. The absorbance
of the empty wells filled only with DMSO was subtracted.
Subsequently, the mean values of the respective treatment groups
were calculated and associated with the norm medium control. Each
experiment was performed with 12 repeats/treatment group.
As the MTT assay is a general assay for cell
viability and proliferation, the mitotic indices were additionally
determined using BrdU (Roche Diagnostics GmbH, Mannheim, Germany),
which was added at the same time as stress induction, and 24 h
prior to fixation with 4% PFA, as previously described (41). Fixed cell cultures were washed with
PBS, incubated with 2 N HCl at 37°C for 1 h, washed repeatedly with
borate buffer (pH 8.5) and PBS, and finally incubated at 7°C for 24
h with monoclonal rat anti-BrdU antibody (1:100; cat. no. OBT0030;
AbD Serotec; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
combined with mouse monoclonal anti-pan-NF (cat. no. 837802;
Biolegend). Subsequently, the washed cultures were then incubated
for 3 h with secondary antibodies goat anti-rat Alexa 546 (1:500;
cat. no. A11081; Thermo Fisher Scientific) and anti-mouse Alexa 488
(1:500; Invitrogen; Thermo Fisher Scientific, Inc.) prior to
examination using an AxioImager M1 fluorescence microscope with a
20× objective lens. Each treatment group consisted of three culture
dishes in which the BrdU-positive NSC-34 cells in three different
fields of view were counted. The three values/dish were combined,
and the percentage of BrdU-positive cells relative to the total
number of NSC-34 cells was calculated.
Cell viability
Cell viability was assessed by double-labeling with
fluorescein diacetate and propidium iodide (PI) (42). The assay is based on the ability of
living cells to hydrolyze fluorescein diacetate (10 µg/ml PBS, 5
min; Sigma-Aldrich) using intracellular esterases, resulting in a
green/yellow-colored fluorescence. Dead cells were labeled with PI
(5 µg/ml PBS, 5 min; Sigma-Aldrich), which interacts with DNA to
produce a red fluorescence of cell nuclei. The analysis procedure
was the same as described above for the BrdU assay.
For all assays, the respective mean values were
analyzed using a non-parametric Kruskal-Wallis test and Dunn's
multiple comparison test as post-hoc tests using Graph Pad
Prism 4 software. P≤0.05 was considered to indicate a statistically
significant result. All experiments were independently performed in
triplicate.
Semi-quantitative RT-PCR
Cells were harvested 24 h prior to OGD induction or
LPS treatment. The mRNA expression levels were determined as
described above for the spinal cord tissue sections. Five flasks
were prepared for each treatment group (control, control +
minocycline, OGD, OGD + minocycline, LPS and LPS + minocycline).
The experiment was repeated in duplicate. Statistical analysis was
performed with a non-parametric Kruskal-Wallis test and Dunn's
multiple comparison test as post-hoc test using Graph Pad
Prism 4 software. P<0.05 was considered to indicate a
statistically significant result.
Immunohistochemistry
At 5 DIV (24 h after OGD induction), the cell
cultures were fixed for 30 min in 4% buffered PFA, and unspecific
binding sites were blocked with 10% bovine serum albumin (BSA;
Sigma-Aldrich)/0.3% Triton X-100 in PBS for 1 h. Subsequently, the
cultures were incubated with the aforementioned primary antibodies:
Anti-Bax (1:200), anti-caspase-3 (1:100), anti-Bcl-2 (1:1,000),
anti-MHC I (1:100), anti-TNF-α (1:500), anti-MMP9 (1:100),
anti-GAP-43 (1:500), anti-VEGF (1:500), and anti-ATF3 (1:200)
co-stained with mouse monoclonal pan-NF (1:1,000) or with rabbit
polyclonal anti-β-III-tubulin (1:1,000) at 7°C overnight, followed
by a wash with PBS, then incubation with the secondary antibodies
goat anti-mouse Alexa 488 (1:500) and donkey anti-rabbit Cy3
(1:250) at room temperature for 3 h. All antibodies were diluted in
10% BSA/0.3% Triton in PBS. The specificity of the immunoreaction
was controlled by the application of buffer instead of primary
antibodies. Cell cultures were examined using a fluorescence
microscope (AxioImager M1; Plan-Neofluar objective; 20/0.5). For
each treatment group (control, control + minocycline, OGD, OGD +
minocycline, LPS, LPS + minocycline) and staining type two dishes
were examined, in total 108 dishes. The experiment was performed in
duplicate.
Results
Animal model
Surgical outcome/macroscopic assessment
The surgical procedure was well tolerated and wounds
healed well. The treatments were fatal to none of the animals. In
the first two post-operative weeks, clinical signs of hyperalgesia
or discomfort were observed. Compared with injured PBS-treated
rats, the minocycline-treated animals demonstrated diminished
peripheral nerve regeneration, as indicated by significantly lower
axon counts in the distal stump when compared with PBS-treated
animals. Functional outcome (response of animals to thermal stimuli
and muscle weight ratio of the gastrocnemius muscle) has been
previously described (25).
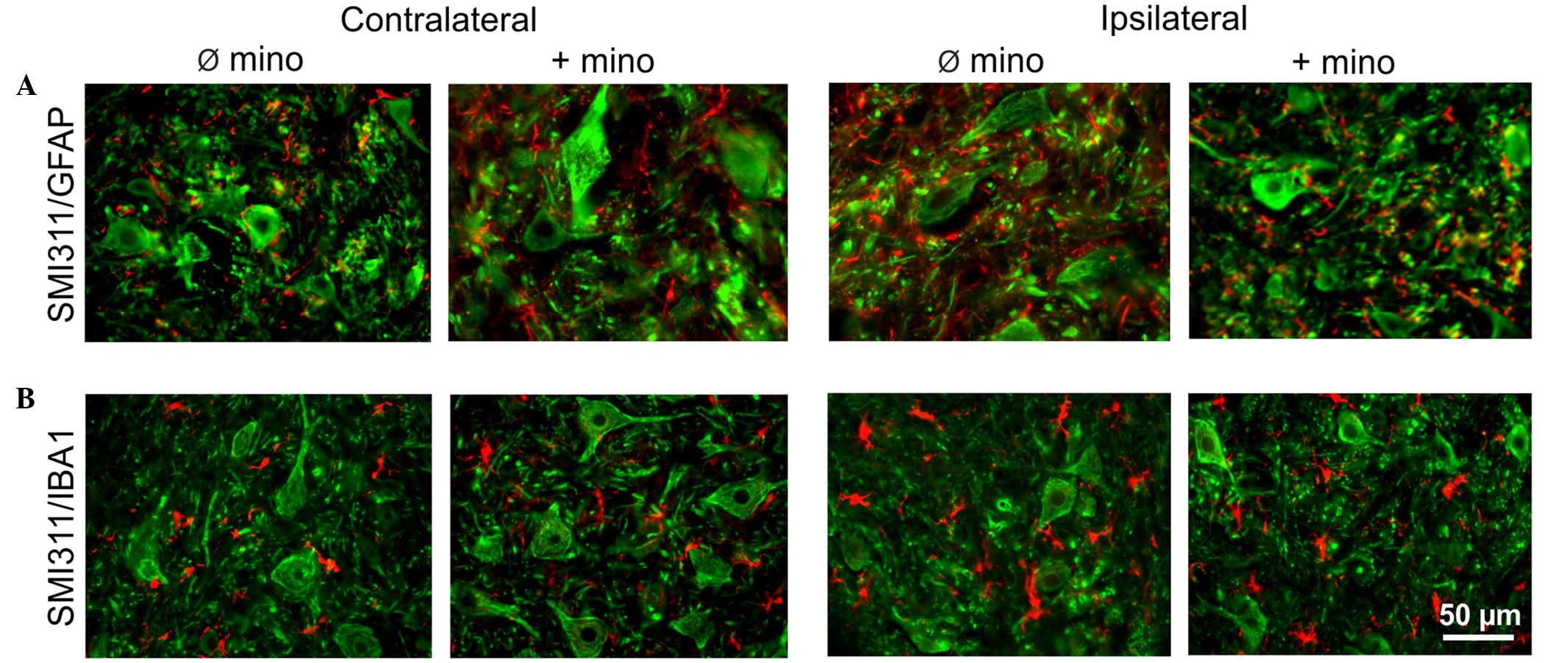
Microscopic assessment
Immunohistochemical assessment was performed at 5
DPI as at this DPI the PCR revealed the most marked alterations
(see below). At 5 DPI, the population of SMI311-expressing
motorneurons of the contralateral and ipsilateral ventral horns
(VH) was equal in number, form and staining intensity (Fig. 1). Astroglia-specific GFAP (Fig. 1A) and microglia-specific IBA1
(Fig. 1B) were expressed in the
contralateral VH. In the ipsilateral side, a marked induction of
both markers was evident (Fig. 1A and
B). Microglia activation could also be demonstrated by cell
morphology. The cells were altered from their ramified form, and
became thicker and retracted their branches. Glia activation
indicated ongoing neurodegenerative processes at the nerve fiber
level, which was not yet evident from SMI311 staining. With regards
to GFAP, treatment with minocycline was ineffective (Fig. 1A). However, microglia activity was
decreased by minocycline, and this effect was more marked in the
ipsilateral VH (Fig. 1B).
Semi-quantitative RT-PCR
In the spinal cord of untreated animals, all
experimental genes were constitutively expressed. GAP-43 possessed
the highest expression levels, which were increased at ≥7 DPI. The
expression levels of all other genes were significantly increased
by sciatic nerve injury at 3 DPI. This increase in expression
levels was evident >5 DPI. In the case of MMP9 a marked increase
in expression levels was observed at 3 and 5 DPI. At 7 DPI, the
expression levels of caspase-3, Bcl-2, ATF3, TNF-α and VEGF
returned to levels similar to those of the control, and the
expression levels of VEGF were already significantly reduced at 5
DPI. The expression levels of Bax, MHC I and MMP9 remained high at
≥14 DPI. With the exception of MMP9, no significant differences
were observed between ipsilateral and contralateral effects
following nerve injury. At 5 DPI, MMP9 was expressed at
significantly higher levels on the ipsilateral side (Fig. 2).
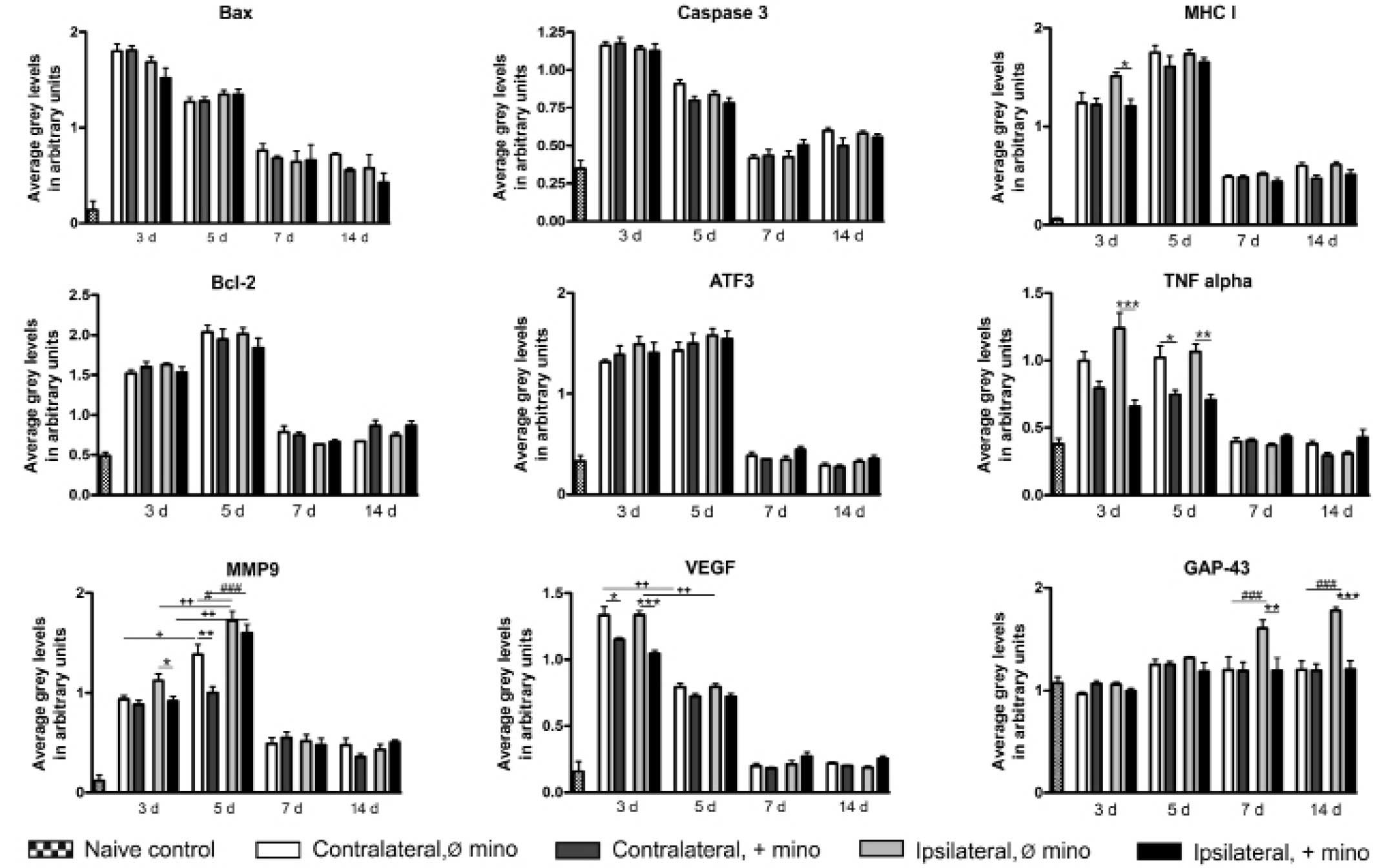 | Figure 2.Semi-quantitative reverse
transcription-polymerase chain reaction analysis of the mRNA
expression levels of various genes in rat L3-L6 spinal cord
sections in the naive control, contralateral (Ø and + mino) and
ipsilateral (Ø and + mino) groups. *,#,+P<0.05; **,++P<0.01;
***,###P<0.001. Bax, Bcl-2-associated X protein; MHC I, major
histocompatibility complex I; Bcl-2, B cell lymphoma 2; ATF3,
activating transcription factor 3; TNF-α, tumor necrosis factor-α;
MMP9, matrix metalloproteinase 9; VEGF, vascular endothelial growth
factor; GAP-43, growth associated protein-43; D, days; Ø mino, not
treated with minocycline; + mino, treated with minocycline. |
Only the expression levels of certain genes were
affected by minocycline. Treatment with minocycline reduced the
ipsilateral expression levels of MHC I at 3 DPI. The expression
levels of TNF-α were reduced ipsilaterally at 3 and 5 DPI. At 5
DPI, the contralateral expression levels of TNF-α were also
diminished. Minocycline reduced the ipsilateral expression levels
of MMP9 at 3 DPI and the contralateral expression levels at 5 DPI.
Treatment with minocycline decreased the ipsilateral and
contralateral expression levels of VEGF at 3 DPI. Furthermore, the
nerve injury-induced expression of GAP-43 was significantly
suppressed (Fig. 2).
Immunohistochemistry
The protein expression levels of the genes examined
by PCR were evaluated by fluorescence immunohistochemistry at 5 DPI
(Fig. 3). For caspase-3, no
fluorescence signal was detected in the contralateral VH (Fig. 3A). Bax, Bcl-2, TNF-α, MHC I, MMP9
(Fig. 3A), ATF3 and VEGF (Fig. 3B) were expressed in motorneurons of
the contralateral VH, although at relatively low levels. Only
GAP-43 was markedly expressed, indicating synaptic contacts
(Fig. 3A).
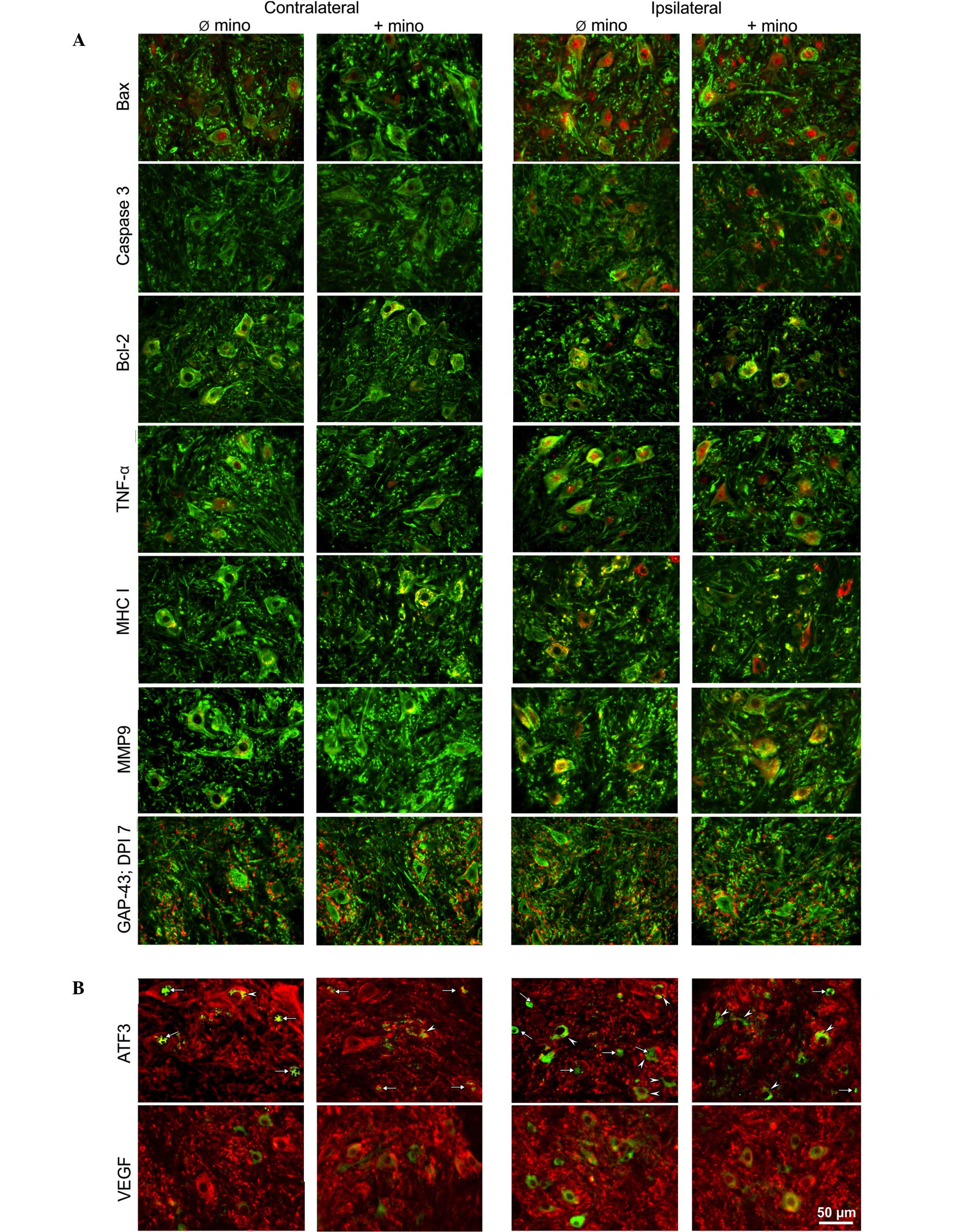 | Figure 3.Representative fluorescence images of
rat L4 and L5 ventral spinal cord sections at 5 DPI. (A) Tissue
sections were double-immunostained with SMI311 (green staining,
neuron-specific) and various antibodies (mentioned at the left, red
staining), resulting in a combined yellow immunosignal. (B) Tissue
sections were double-immunostained with β-III-tubulin (red
staining, neuron-specific) and ATF3 or VEGF (green staining),
resulting in a combined yellow immunosignal. Arrows indicate
nuclear expression, arrowheads indicate cytoplasmic expression. Ø
mino, not treated with minocycline; + mino, treated with
minocycline; Bax, Bcl-2-associated X protein; Bcl-2, B cell
lymphoma 2; TNF-α, tumor necrosis factor-α; MHC I, major
histocompatibility complex I; MMP9, matrix metalloproteinase 9;
GAP-43, growth associated protein-43; DPI, days post-intervention;
ATF3, activating transcription factor 3; VEGF, vascular endothelial
growth factor. |
The majority of immunofluorescence signals were
activated by unilateral nerve injury in the ipsilateral VH. In the
case of Bax, in addition to marked cytoplasmic staining of
motorneurons, marked nuclear fluorescence was visible (Fig. 3A). Such injury/hypoxia-induced
translocation of Bax to the nucleus has previously been described
for neonatal neurons of the spinal cord (27) and brain (43). The enhanced motorneuronal signals of
caspase-3 and Bcl-2 (Fig. 3A) were
located in the cytoplasm, and Bcl-2 was also markedly expressed in
the contralateral VH motorneurons (Fig.
3A). Intense TNF-α staining was observed in the motorneuronal
cytoplasm with compaction/concentration around and inside the
nucleus (Fig. 3A). The markedly
intense immunosignals of MHC I, MMP9 (Fig. 3A) and VEGF (Fig. 3B) were evenly distributed in the
cytoplasm of the motorneurons. In addition, the expression of the
ATF3 transcription factor was predominantly upregulated in the
cytoplasm (Fig. 3B). A similar
pattern with the majority of neurons exhibiting marked cytoplasmic
staining and only a minority also exhibiting nuclear translocation
was reported by Seijffers et al (44). GAP-43 immunostaining demonstrated a
reduction in synaptic contacts (Fig.
3A).
The effect of minocycline was marginal. MHC I
appeared to be upregulated by minocycline in the contralateral and
ipsilateral VH motorneurons (Fig.
3A). Conversely, the injury-induced upregulation of VEGF was
reversed by minocycline (Fig.
3B).
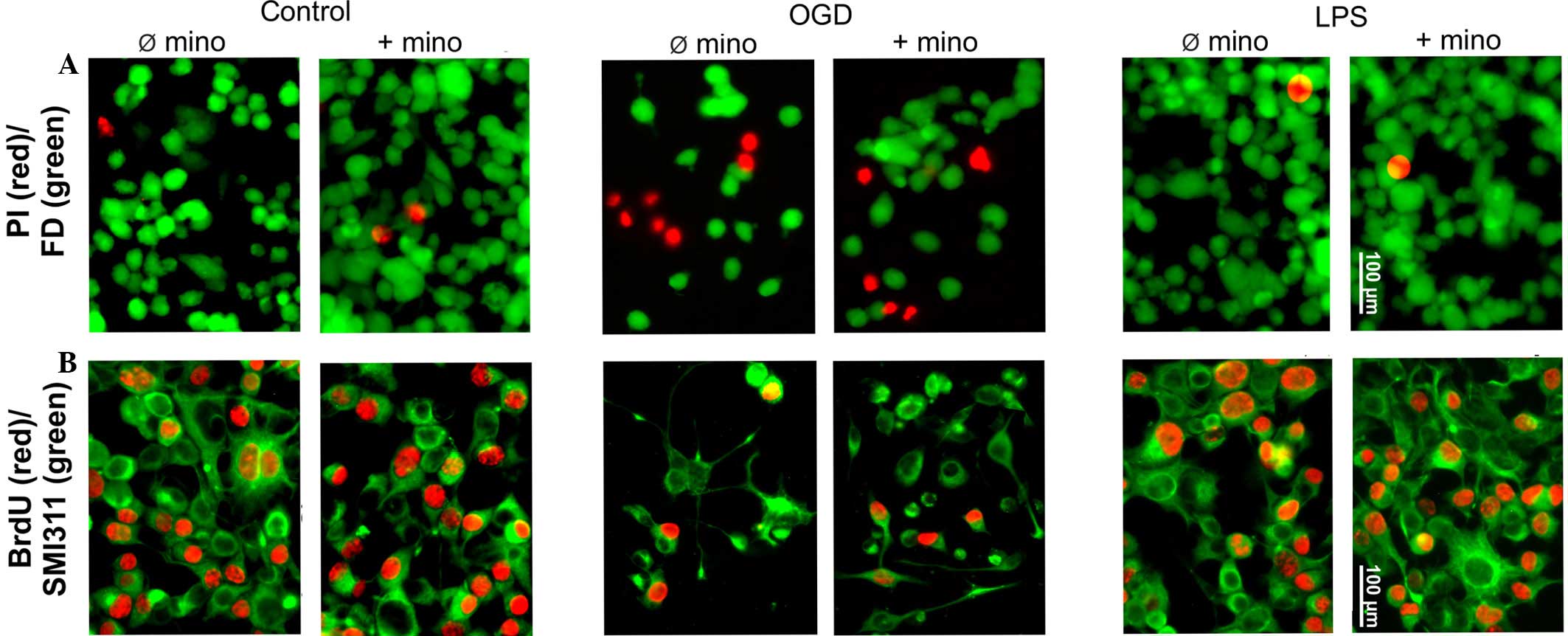
Cell culture model
Assessment of cell survival and
proliferation
MTT, bromodeoxyuridine (BrdU) and vital staining
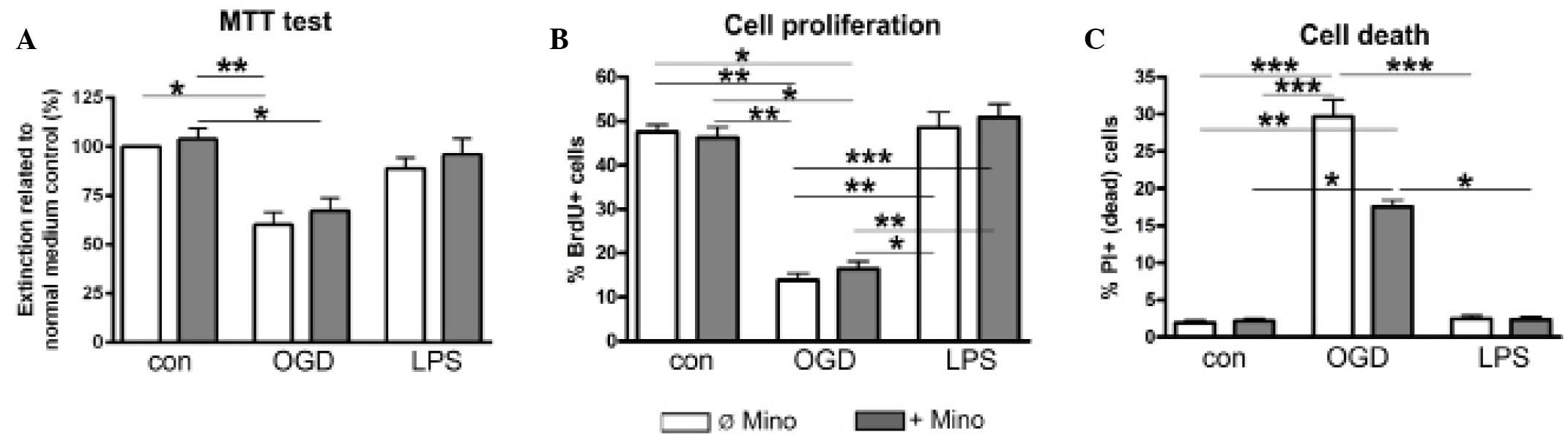
were used to assess cellular survival and proliferation (Figs. 4 and 5). The MTT assay is based on the specific
turnover of MTT to formazan, requiring viable cells. An increased
extinction coefficient indicates an enhanced MTT turnover rate and
thus a greater number of viable cells. The MTT assay demonstrated a
significant (P<0.05) OGD-induced reduction of the metabolic
activity of NSC-34 cells, whereas LPS had no effect on metabolic
activity levels (Fig. 5A).
Independent of treatment, minocycline did not alter the treatment
group-specific MTT turnover (Fig.
5A).
In contrast to post-mitotic motorneurons, NSC-34
cells are able to proliferate as they are neuroblastoma-spinal cord
hybrids. The basic mitotic index, determined by BrdU incorporation
in the control group, was 48±5% (Figs.
4B and 5B). OGD significantly
reduced the proliferation of NSC-34 cells (P<0.01); however, LPS
was ineffective (Figs. 4B and
5B). Minocycline had no effect on
NSC-34 cell proliferation, in either the control or the LPS group.
In addition, minocycline was not able to reverse the inhibitory
effect of OGD (Figs. 4B and 5B).
Vital staining of control cultures revealed only
scattered dead (PI-positive) cells, which were not affected by
minocycline (Figs. 4A and 5C). OGD induced significant neurotoxicity
(P<0.001), while minocycline was marginally able to reverse this
neurotoxicity (P<0.1; Fig. 4A and
5C). LPS alone or in combination
with minocycline had no effect on NSC-34 cell viability (Fig. 4A and 5C).
Semi-quantitative RT-PCR
In untreated control NSC-34 cell cultures, all
experimental genes were constitutively expressed, with low
expression levels observed for ATF3, caspase-3 and VEGF. OGD was
able to significantly increase the expression levels of Bcl-2
(P<0.05), TNF-α (P<0.01) and MMP9 (P<0.05). TNF-α and MMP9
were also significantly upregulated by LPS (P<0.05). Similarly
to the results observed following the analysis of the tissue
samples, the OGD stress-induced expression was significantly
(P<0.05) suppressed by minocycline. Furthermore, minocycline
also significantly reduced VEGF expression (P<0.05; Fig. 6).
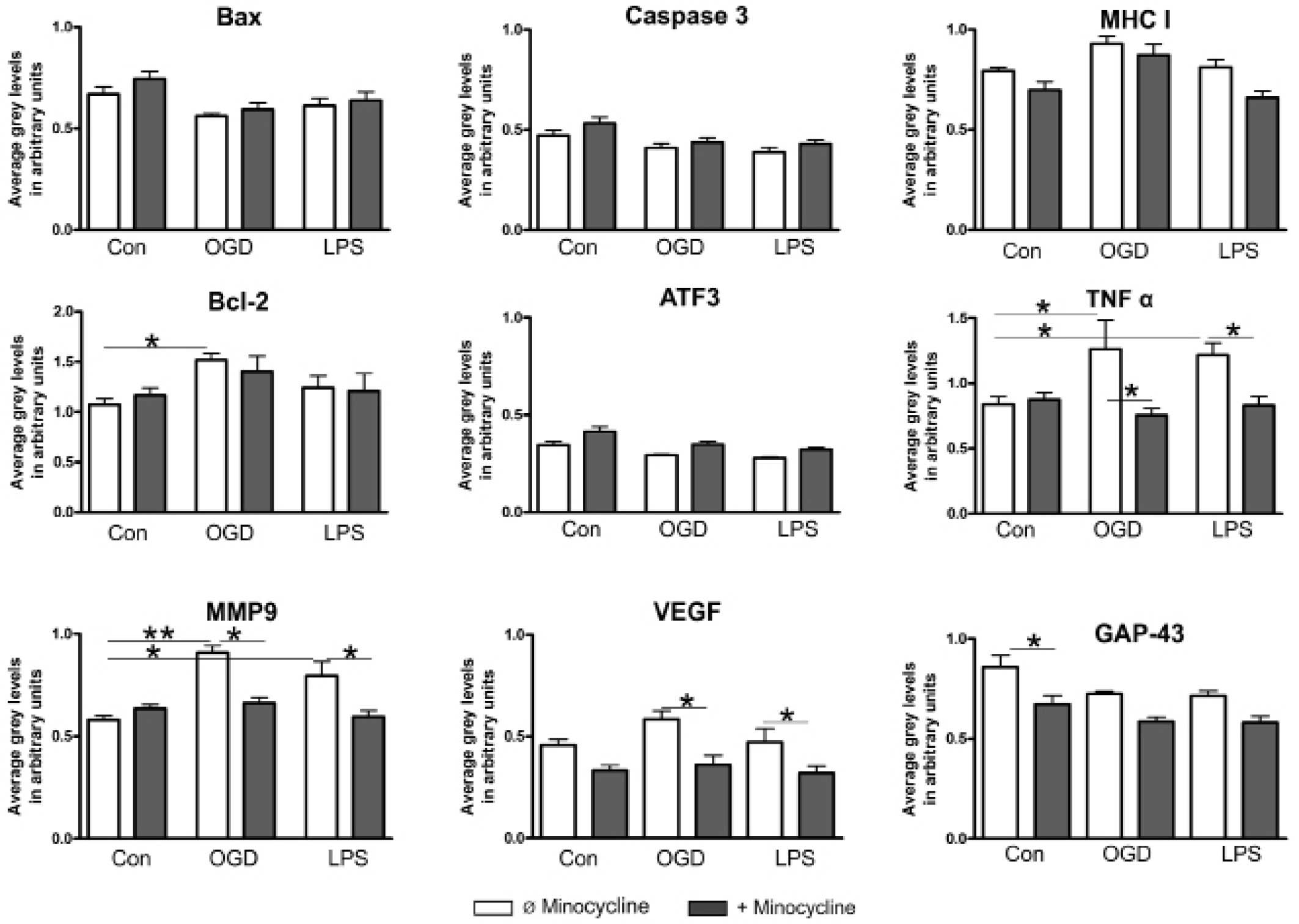 | Figure 6.Semi-quantitative reverse
transcription-polymerase chain reaction analysis of mRNA expression
levels in NSC-34 cells in the con, OGD or LPS groups treated with
or without minocycline. Data are presented as means ± standard
deviation; n=10 flask/group. *P<0.05; **P<0.01. Bax,
Bcl-2-associated X protein; Con, control group; OGD, oral glucose
deprivation; LPS, lipopolysaccharide; MHC I, major
histocompatibility complex I; Bcl-2, B cell lymphoma 2; ATF3,
activating transcription factor 3; TNF-α, tumor necrosis factor-α;
MMP9, matrix metalloproteinase 9; VEGF, vascular endothelial growth
factor; GAP-43, growth associated protein-43. |
Immunohistochemistry
The mRNA expression profile of NSC-34 cells was also
investigated by fluorescence immunohistochemical evaluation of
protein expression (Fig. 7). In
untreated control cell cultures (Fig. 7A
and B), all proteins were expressed endogenously. This result
was expected as primary cell cultures are usually characterized by
a low and constant cell death rate. The stressors OGD (Fig. 7A and B) and LPS (Fig. 7A and B) induced the activation of all
proteins. Minocycline had no effect on control cultures (Fig. 7A and B), but was able to reduce the
stress-induced upregulation of TNF-α and MMP9 (Fig. 7A) expression. Combined with LPS,
minocycline was able to inhibit VEGF expression (Fig. 7B). Conversely to in vivo
experiments, the expression of Bax was predominantly located in the
cytoplasm (Fig. 7A). Only in
stressed and minocycline-treated cells was nuclear expression
visible (Fig. 7A). The expression of
transcription factor ATF3 was upregulated following stress and
located in the nucleus (Fig. 7B),
and this expression was inhibited by minocycline (Fig. 7B). Furthermore, the expression
pattern of GAP-43 was different to those observed in vivo.
Under all experimental conditions, fluorescence signals were
present in the cytoplasm of NSC-34 cells, although not in the
fibers (Fig. 7A).
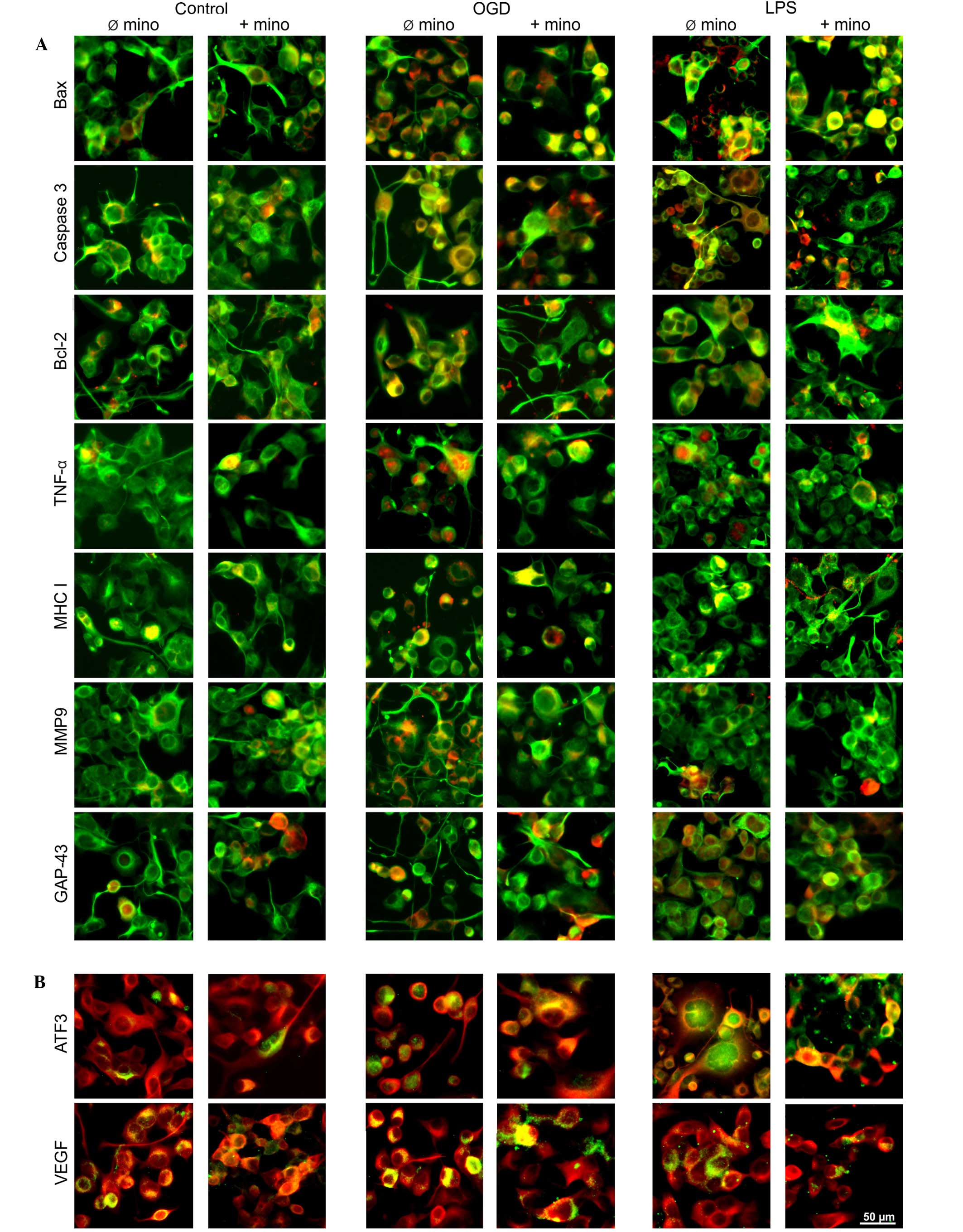 | Figure 7.Representative fluorescence images of
NSC-34 cells under various experimental conditions. (A) Cell
cultures were double-immunostained with SMI311 (green staining) and
various antibodies (red staining), often resulting in a combined
yellow immunosignal. (B) Cell cultures were double-immunostained
with β-III-tubulin (red staining) and ATF3 or VEGF (green
staining), resulting in a combined yellow immunosignal. OGD, oxygen
glucose deprivation; LPS, lipopolysaccharide; Ø mino, not treated
with minocycline; + mino, treated with minocycline; Bax,
Bcl-2-associated X protein; Bcl-2, B cell lymphoma 2; TNF-α, tumor
necrosis factor-α; MHC I, major histocompatibility complex I; MMP9,
matrix metalloproteinase 9; GAP-43, growth associated protein-43;
ATF3, activating transcription factor 3; VEGF, vascular endothelial
growth factor. |
Discussion
Transection of peripheral nerves induces a complex
cascade of reactions, including retrograde processes targeting the
axotomized spinal motorneurons. In neonatal rats, axotomized
motorneurons often die (45,46). However, in adult animals,
degeneration of spinal motorneurons following peripheral nerve
axotomy rarely occurs (47,48). Only severe nerve ventral root
avulsion has more severe effects and induces a significant loss of
axotomized motorneurons in the respective spinal cord segments
(49). In addition, less severely
injured adult ipsilateral motorneurons develop classical
post-traumatic signs, including synaptic terminal retraction
(48). Furthermore, microglia
activation is regularly observed (50,51).
In the rat model of sciatic nerve reconstruction in
the present study, a post-traumatic pattern in the VH was also
observed. Motorneurons did not exhibit visible signs of
neurodegeneration. Aside from that the sciatic nerve was
reconstructed immediately following transection, which is
neuroprotective (52). However,
microglia activation and synaptic terminal retraction were
detectable, and mRNA expression levels correlated with microglial
activation. In the untreated spinal cord, all experimental genes
were constitutively expressed at mRNA and protein levels. GAP-43,
as a crucial component of the axon and presynaptic terminals
exhibited, as expected, the highest expression levels. The genes
and proteins involved in inflammation (MHC I, MMP9, TNF-α),
apoptosis (Bax, Bcl-2, caspase-3), or stress response (ATF3, VEGF)
were expressed at basic levels. Sciatic nerve transection and
reconstruction significantly increased the expression levels of
these genes, although only in the first week. Similar time courses
have been described previously, including those for Bax (53), ATF3 (54) and MHC I (55). The coincident but temporary
upregulation of pro-apoptotic genes and proteins (Bax and
caspase-3) or anti-apoptotic genes and proteins (Bcl-2),
inflammation or stress demonstrated the presence of a
self-defensive response to injury, and a conflict between
injury-induced neurodegenerative signaling cascades and
neuroprotective mechanisms during the acute phase following injury.
In certain cases, this results in the survival and recovery of
stressed motorneurons, as was observed in the present model of
sciatic nerve reconstruction. In severe traumatic injuries, such as
nerve avulsion, significant motorneuronal death occurred,
accompanied by mitochondrial accumulation of Bax, cytochrome
c redistribution and activation of caspase-3 (29). Schwartz et al (56) termed this process a detrimental
cost-benefit ratio; inflammation, being primarily a positive
self-response eliminating or neutralizing injurious stimuli and
restoring tissue integrity, exceeds the threshold of tolerability,
and contributes to neuropathology. In this regard it appeared that
the immune response to nerve injury in neonatal rats is reversed
(57), thus offering one possible
explanation for the aforementioned enhanced neuro-vulnerability in
young animals.
Only MMP9 and GAP-43 demonstrated significantly
increased ipsilateral induction compared with the contralateral
side. All other genes reported no significant differences when the
ipsilateral and contralateral sides were compared. The absence of
ipsilateral vs. contralateral differences is in contrast to
findings of Tang et al (58),
which demonstrated that unilateral root-avulsion resulted in
significant alterations to microRNA expression only in the
ipsilateral spinal cord. However, the present results are in
agreement with Rotshenker and Tal (59), who revealed that sprouting and
synapse formation is enhanced by contralateral axotomy.
Furthermore, there is evidence for transneuronal correspondence
between ipsilateral and contralateral motorneurons. Transneuronal
labeling of the L4 and L5 VH neurons following pseudorabies virus
injection in the rat medial gastrocnemius muscle has been
previously described (60).
Neuropeptides, including peptide histidine isoleucine (61) and calcitonin gene-related peptide
(CGRP) (62), were also induced
bilaterally in rat spinal motor neurons following unilateral
sciatic nerve transection. CGRP has been proposed to be involved in
pain transmission and inflammation (63), as well as in repair mechanisms for
neural regeneration following brachial plexus (2) or sciatic nerve (5) injury, in which its anti-apoptotic
properties (64) were essential.
These results are concordant with the hypothesis that unilateral
sciatic nerve injury is able to induce bilateral stress and
self-defense.
Synapse stripping is a regular result of peripheral
axotomy, in which the extent of synaptic terminal retraction
depends on the distance between motorneuron and lesion [it is
lessened when the lesion side is further from the cell soma
(65)], and on the severity of the
lesion. This process occurs when neuronal cell death is not obvious
(66). This remodeling has been
suggested to be an adaptive mechanism of self-defense underlying
enhanced neuronal viability (67,68).
Although the microglia activation demonstrated in the present study
may be associated with synaptic stripping, a previous study
suggested that the activation of glia is not correlated with the
degree of synaptic stripping (65).
Numerous studies have demonstrated
minocycline-induced neuroprotection (69–71). For
axotomized motorneurons it influenced both the ipsilateral as well
as the contralateral site (72). In
the present investigation the effects of minocycline were
relatively low, but did induce marginal inhibition with a reduction
in the expression levels of MMP9, TNF-α, MHC I, VEGF and GAP-43.
The inhibitory effects of minocycline have previously been
described for MMP9 (73), MHC I
(74), VEGF (75,76),
TNF-α (77,78), and GAP-43 (79). One target of minocycline appears to
be the transcription factor NF-κB. Minocycline has been
demonstrated to inhibit the activation of NF-κB (80), as well as its translocation into the
cell nucleus (81). NF-κB however
induces the expression of MMP9 (82), MHC I (83) and VEGF (84). Furthermore, the activation of NF-κB
culminates in the release of TNF-α (85), which is a potent activator of NF-κB
(86), thus an escalation of the
minocycline effects can be assumed.
A late induction of motorneuronal GAP-43 expression
following sciatic nerve injury has been previously demonstrated
(87). GAP-43 is widely used as a
marker for the growth/regeneration state of motorneurons, including
synapse reconstruction, referred to as the ‘cell body response’
(88). The expression of GAP-43
requires acetylated p53 (89).
However, minocycline is able to downregulate the expression of p53
(90) and to inhibit acetylation
(91), which results in the
minocycline-induced downregulation of GAP-43 expression
demonstrated by the results of the present study.
Minocycline is able to downregulate or upregulate
Bax and Bcl-2, respectively, thereby resulting in an anti-apoptotic
ratio (76,92–94).
However, the present study did not demonstrate any significant
minocycline effects on Bax or Bcl-2. These non-concordant results
may be due to the heterogeneity of the models and minocycline
treatment regimes. Matsukawa et al (95) demonstrated that minocycline
attenuates experimentally-induced ischemic cell death by
upregulating Bcl-2 expression at low doses. However, high
minocycline doses exacerbated ischemic injury and reduced the
number of Bcl-2-expressing neurons. Furthermore, minocycline
targeted neurons alone, not astrocytes (95). The present results of the PCR
analysis on the spinal cord tissue samples revealed the expression
pattern of all spinal cord cell types including glial cells.
Experiments were conducted using the NSC-34 motorneuronal-like cell
line.
OGD, but not LPS, was highly toxic for NSC-34 cells
and minocycline reduced the OGD-induced cell death rate, although
these results were not significant (P>0.05). Notably,
stress-induced changes in apoptosis-associated Bax and caspase-3
expression were not observed. These results were concordant with an
in vivo study that demonstrated that dying lumbar
motorneurons did not always exhibit apoptotic morphology (96). There is also evidence that NSC-34
cells expressed the apoptotic markers only under specific
conditions (97). NSC-34 cell death
could be induced by various apoptotic agents, and when
intracellular protein inclusions containing mutant SOD1 existed,
dispersed SOD1 prevented NSC-34 cells from apoptotic cell death
(98). This may explain the observed
apoptotic death of NSC-34 cells following
H2O2-induced oxidative stress (99), as oxidative stress induced SOD1
aggregation (100). The absence of
Bax, caspase-3, MHC I and ATF3 activation in NSC-34 cells suggested
that motorneurons were not preferentially or even solely
responsible for the nerve injury-mediated upregulation of these
genes. PCR analysis demonstrated the expression pattern of neurons
and glial cells, and the expression and stress-mediated regulation
of these genes has been well-described: Astroglial Bax and
caspase-3 (101); MHC I (102); ATF3 (103); microglial Bax and caspase-3
(104); MHC I (105); ATF3 (34); oligodendroglial Bax and caspase-3
(106); MHC I (107); and ATF3 (108). The absence of GAP-43 activation in
NSC-34 cells may be a result of the in vitro absence of
retrograde signals, which in vivo originate from the distal
nerve stump and the disconnected nerve targets to initiate and
support axonal regeneration (109).
TNF-α and VEGF induced the expression of MMP9;
however, only OGD induced the expression of Bcl-2, and was able to
parallelize the activating potency of sciatic nerve reconstruction.
These results suggested that the expression level changes observed
in vivo may be induced by motorneurons. However, the
involvement of glia cells cannot be excluded. The glial expression
of the four genes has previously been described: MMP9 (110,111);
TNF-α (112); VEGF (113,114);
and Bcl-2 (92,115).
In NSC-34 cells minocycline exhibits inhibitory
effects, whereby the above-mentioned NF-κB signaling pathway may be
accepted due to the fact that NF-κB is expressed by NSC-34 cells,
and activated and translocated into the nucleus as a result of cell
stress (116,117).
The present study demonstrated a massive but
temporary SNR-mediated upregulation of all studied genes in L3-L6
sections of the spinal cord that was moderately affected by
minocycline. The results observed within NSC-34 cells indicate that
motorneurons are not significantly or solely responsible for these
SNR-mediated changes in gene expression. To further clarify the
cell-specific gene profiles, a more complex model of organotypic
cell cultures may be a helpful alternative. This model could mimic
tissue architecture of the spinal cord, which would allow an
understanding of cellular etiology of these processes.
Acknowledgements
The authors of the present study are grateful to
Ms. Leona Bück for technical assistance.
References
|
1
|
Oliveira AL, Risling M, Deckner M,
Lindholm T, Langone F and Cullheim S: Neonatal sciatic nerve
transection induces TUNEL labeling of neurons in the rat spinal
cord and DRG. Neuroreport. 8:2837–2840. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen LJ, Ren YH, Liu L, Zhang XQ, Zhao Y,
Wu WT and Li F: Upregulated expression of GAP-43 mRNA and protein
in anterior horn motoneurons of the spinal cord after brachial
plexus injury. Arch Med Res. 41:513–538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arocho LC, Figueroa JD, Torrado AI,
Santiago JM, Vera AE and Miranda JD: Expression profile and role of
EphrinA1 ligand after spinal cord injury. Cell Mol Neurobiol.
31:1057–1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobbert C and Thanos S: Topographic
representation of the sciatic nerve motor neurons in the spinal
cord of the adult rat correlates to region-specific activation
patterns of microglia. J Neurocytol. 29:271–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng LF, Wang R, Xu YZ, Yi XN, Zhang JW
and Zeng ZC: Calcitonin gene-related peptide dynamics in rat dorsal
root ganglia and spinal cord following different sciatic nerve
injuries. Brain Res. 1187:20–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu F, Miao X, Chen J, Sun Y, Liu Z, Tao Y
and Yu W: Down-regulation of GAP-43 by inhibition of caspases-3 in
a rat model of neuropathic pain. Int J Clin Exp Pathol. 5:948–955.
2012.PubMed/NCBI
|
|
7
|
Terenghi G, Hart A and Wiberg M: The nerve
injury and the dying neurons: Diagnosis and prevention. J Hand Surg
Eur Vol. 36:730–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amantea D and Bagetta G: Drug repurposing
for immune modulation in acute ischemic stroke. Curr Opin
Pharmacol. 26:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smilack JD: The tetracyclines. Mayo Clin
Proc. 74:727–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yrjanheikki J, Tikka T, Keinänen R,
Goldsteins G, Chan PH and Koistinaho J: A tetracycline derivative,
minocycline, reduces inflammation and protects against focal
cerebral ischemia with a wide therapeutic window. Proc Natl Acad
Sci USA. 96:13496–13500. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Fagan SC, Waller JL, Edwards D,
Borlongan CV, Zheng J, Hill WD, Feuerstein G and Hess DC: Low dose
intravenous minocycline is neuroprotective after middle cerebral
artery occlusion-reperfusion in rats. BMC Neurol. 4:72004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen SD, Yin JH, Hwang CS, Tang CM and
Yang DI: Anti-apoptotic and anti-oxidative mechanisms of
minocycline against sphingomyelinase/ceramide neurotoxicity:
Implication in Alzheimer's disease and cerebral ischemia. Free
Radic Res. 46:340–350. 2012. View Article : Google Scholar
|
|
13
|
Hodl AK and Bonelli RM: Huntington's
disease and minocycline. Mov Disord. 20:510–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zemke D and Majid A: The potential of
minocycline for neuroprotection in human neurologic disease. Clin
Neuropharmacol. 27:293–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pontieri FE, Ricci A, Pellicano C,
Benincasa D and Buttarelli FR: Minocycline in amyotrophic lateral
sclerosis: A pilot study. Neurol Sci. 26:285–287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giuliani F, Fu SA, Metz LM and Yong VW:
Effective combination of minocycline and interferon-beta in a model
of multiple sclerosis. J Neuroimmunol. 165:83–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wells JE, Hurlbert RJ, Fehlings MG and
Yong VW: Neuroprotection by minocycline facilitates significant
recovery from spinal cord injury in mice. Brain. 126:1628–1637.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monaco EA 3rd, Weiner GM and Friedlander
RM: Randomized-controlled trial of minocycline for spinal cord
injury shows promise. Neurosurgery. 72:N17–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stirling DP, Koochesfahani KM, Steeves JD
and Tetzlaff W: Minocycline as a neuroprotective agent.
Neuroscientist. 11:308–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diguet E, Gross CE, Tison F and Bezard E:
Rise and fall of minocycline in neuroprotection: Need to promote
publication of negative results. Exp Neurol. 189:1–4. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuji M, Wilson MA, Lange MS and Johnston
MV: Minocycline worsens hypoxic-ischemic brain injury in a neonatal
mouse model. Exp Neurol. 189:58–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinzon A, Marcillo A, Quintana A, Stamler
S, Bunge MB, Bramlett HM and Dietrich WD: A re-assessment of
minocycline as a neuroprotective agent in a rat spinal cord
contusion model. Brain Res. 1243:146–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JH, Tigchelaar S, Liu J, Stammers AM,
Streijger F, Tetzlaff W and Kwon BK: Lack of neuroprotective
effects of simvastatin and minocycline in a model of cervical
spinal cord injury. Exp Neurol. 225:219–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinkernelle J, Fansa H, Ebmeyer U and
Keilhoff G: Prolonged minocycline treatment impairs motor neuronal
survival and glial function in organotypic rat spinal cord
cultures. PloS One. 8:e734222013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keilhoff G, Langnaese K, Wolf G and Fansa
H: Inhibiting effect of minocycline on the regeneration of
peripheral nerves. Dev Neurobiol. 67:1382–1395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cashman NR, Durham HD, Blusztajn JK, Oda
K, Tabira T, Shaw IT, Dahrouge S and Antel JP: Neuroblastoma ×
spinal cord (NSC) hybrid cell lines resemble developing motor
neurons. Dev Dyn. 194:209–221. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tiraihi T and Rezaie MJ: Apoptosis onset
and bax protein distribution in spinal motoneurons of newborn rats
following sciatic nerve axotomy. Int J Neurosci. 113:1163–1175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao W, Zhao Q, Liu J, Xu XY, Sun WW, Zhou
X, Liu S and Wang TH: Electro-acupuncture reduces neuronal
apoptosis linked to Bax and Bcl-2 expression in the spinal cords of
cats subjected to partial dorsal root ganglionectomy. Neurochem
Res. 33:2214–2221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin LJ and Liu Z: Injury-induced spinal
motor neuron apoptosis is preceded by DNA single-strand breaks and
is p53- and Bax-dependent. J Neurobiol. 50:181–197. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu XM, Shu YH, Qiu CH, Chen KT and Wang
YT: Protective effects and anti-apoptotic role of nerve growth
factor on spinal cord neurons in sciatic nerve-injured rats. Neurol
Res. 36:814–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Payés AC, Zanon RG, Pierucci A and
Oliveira AL: MHC class I upregulation is not sufficient to rescue
neonatal alpha motoneurons after peripheral axotomy. Brain Res.
1238:23–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohtori S, Takahashi K, Moriya H and Myers
RR: TNF-alpha and TNF-alpha receptor type 1 upregulation in glia
and neurons after peripheral nerve injury: Studies in murine DRG
and spinal cord. Spine (Phila Pa 1976). 29:1082–1088. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindå H, Sköld MK and Ochsmann T:
Activating transcription factor 3, a useful marker for regenerative
response after nerve root injury. Front Neurol. 2:302011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hunt D, Raivich G and Anderson PN:
Activating transcription factor 3 and the nervous system. Front Mol
Neurosci. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu CY, Hong GX and Wang FB: Expression of
vascular endothelial growth factor and its fetal liver kinase-1
receptor in spinal cord and dorsal root ganglia after neurotomy of
sciatic nerve in rats. Chin J Traumatol. 8:17–22. 2005.PubMed/NCBI
|
|
36
|
Liou JT, Sum DC, Liu FC, Mao CC, Lai YS
and Day YJ: Spatial and temporal analysis of nociception-related
spinal cord matrix metalloproteinase expression in a murine
neuropathic pain model. J Chin Med Assoc. 76:201–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jacobson RD, Virág I and Skene JH: A
protein associated with axon growth, GAP-43, is widely distributed
and developmentally regulated in rat CNS. J Neurosci. 6:1843–1855.
1986.PubMed/NCBI
|
|
38
|
Bulsara KR, Iskandar BJ, Villavicencio AT
and Skene JH: A new millenium for spinal cord regeneration:
Growth-associated genes. Spine (Phila Pa 1976). 27:1946–1949. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raghavendra V, Tanga F and DeLeo JA:
Inhibition of microglial activation attenuates the development but
not existing hypersensitivity in a rat model of neuropathy. J
Pharmacol Exp Ther. 306:624–630. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Langnaese K, John R, Schweizer H, Ebmeyer
U and Keilhoff G: Selection of reference genes for quantitative
real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol
Biol. 9:532008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lucas B, Pinkernelle J, Fansa H and
Keilhoff G: Effects of cerebrolysin on rat Schwann cells in vitro.
Acta Histochem. 116:820–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Keilhoff G and Wolf G: Comparison of
double fluorescence staining and LDH-test for monitoring cell
viability in vitro. Neuroreport. 5:129–132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Infante SK, Oberhauser AF and Perez-Polo
JR: Bax phosphorylation association with nucleus and
oligomerization after neonatal hypoxia-ischemia. J Neurosci Res.
91:1152–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seijffers R, Mills CD and Woolf CJ: ATF3
increases the intrinsic growth state of DRG neurons to enhance
peripheral nerve regeneration. J Neurosci. 27:7911–7920. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wolff JR and Missler M: Synaptic
reorganization in developing and adult nervous systems. Ann Anat.
174:393–403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vieira AS, de Rezende AC and Rogerio F:
Evaluating motor neuron death in neonatal rats subjected to sciatic
nerve lesion. Methods Mol Biol. 846:383–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carlson J, Lais AC and Dyck PJ: Axonal
atrophy from permanent peripheral axotomy in adult cat. J
Neuropathol Exp Neurol. 38:579–585. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Victório SC, Havton LA and Oliveira AL:
Absence of IFNγ expression induces neuronal degeneration in the
spinal cord of adult mice. J Neuroinflammation. 7:772010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ohlsson M, Nieto JH, Christe KL and Havton
LA: Long-term effects of a lumbosacral ventral root avulsion injury
on axotomized motor neurons and avulsed ventral roots in a
non-human primate model of cauda equina injury. Neuroscience.
250:129–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen T, Koga K, Li XY and Zhuo M: Spinal
microglial motility is independent of neuronal activity and
plasticity in adult mice. Mol Pain. 6:192010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chew DJ, Carlstedt T and Shortland PJ: A
comparative histological analysis of two models of nerve root
avulsion injury in the adult rat. Neuropathol Appl Neurobiol.
37:613–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hart AM, Terenghi G and Wiberg M: Neuronal
death after peripheral nerve injury and experimental strategies for
neuroprotection. Neurol Res. 30:999–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gillardon F, Klimaschewski L, Wickert H,
Krajewski S, Reed JC and Zimmermann M: Expression pattern of
candidate cell death effector proteins Bax, Bcl-2, Bcl-X and c-Jun
in sensory and motor neurons following sciatic nerve transection in
the rat. Brain Res. 739:244–250. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tsujino H, Kondo E, Fukuoka T, Dai Y,
Tokunaga A, Miki K, Yonenobu K, Ochi T and Noguchi K: Activating
transcription factor 3 (ATF3) induction by axotomy in sensory and
motoneurons: A novel neuronal marker of nerve injury. Mol Cell
Neurosci. 15:170–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sabha M Jr, Emirandetti A, Cullheim S and
De Oliveira AL: MHC I expression and synaptic plasticity in
different mice strains after axotomy. Synapse. 62:137–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schwartz M, Butovsky O, Brück W and
Hanisch UK: Microglial phenotype: Is the commitment reversible?
Trends Neurosci. 29:68–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vega-Avelaira D, Moss A and Fitzgerald M:
Age-related changes in the spinal cord microglial and astrocytic
response profile to nerve injury. Brain Behav Immun. 21:617–623.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang Y, Ling ZM, Fu R, Li YQ, Cheng X,
Song FH, Luo HX and Zhou LH: Time-specific microRNA changes during
spinal motoneuron degeneration in adult rats following unilateral
brachial plexus root avulsion: Ipsilateral vs. contralateral
changes. BMC Neurosci. 15:922014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rotshenker S and Tal M: The transneuronal
induction of sprouting and synapse formation in intact mouse
muscles. J Physiol. 360:387–396. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rotto-Percelay DM, Wheeler JG, Osorio FA,
Platt KB and Loewy AD: Transneuronal labeling of spinal
interneurons and sympathetic preganglionic neurons after
pseudorabies virus injections in the rat medial gastrocnemius
muscle. Brain Res. 574:291–306. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Villar MJ, Cortés R, Theodorsson E,
Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC and
Hökfelt T: Neuropeptide expression in rat dorsal root ganglion
cells and spinal cord after peripheral nerve injury with special
reference to galanin. Neuroscience. 33:587–604. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Piehl F, Arvidsson U, Johnson H, Cullheim
S, Villar M, Dagerlind A, Terenius L, Hökfelt T and Ulfhake B:
Calcitonin Gene-related Peptide (CGRP)-like Immunoreactivity and
CGRP mRNA in rat spinal cord motoneurons after different types of
lesions. Eur J Neurosci. 3:737–757. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Benemei S, Nicoletti P, Capone JG and
Geppetti P: CGRP receptors in the control of pain and inflammation.
Curr Opin Pharmacol. 9:9–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sueur S, Pesant M, Rochette L and Connat
JL: Antiapoptotic effect of calcitonin gene-related peptide on
oxidative stress-induced injury in H9c2 cardiomyocytes via the
RAMP1/CRLR complex. J Mol Cell Cardiol. 39:955–963. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Spejo AB and Oliveira AL: Synaptic
rearrangement following axonal injury: Old and new players.
Neuropharmacology. 96:113–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guntinas-Lichius O, Neiss WF, Gunkel A and
Stennert E: Differences in glial, synaptic and motoneuron responses
in the facial nucleus of the rat brainstem following facial nerve
resection and nerve suture reanastomosis. Eur Arch
Otorhinolaryngol. 251:410–417. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hardingham GE: Coupling of the NMDA
receptor to neuroprotective and neurodestructive events. Biochem
Soc Trans. 37:1147–1160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tyzack GE, Sitnikov S, Barson D,
Adams-Carr KL, Lau NK, Kwok JC, Zhao C, Franklin RJ, Karadottir RT,
Fawcett JW and Lakatos A: Astrocyte response to motor neuron injury
promotes structural synaptic plasticity via STAT3-regulated TSP-1
expression. Nat Commun. 5:42942014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Garrido-Mesa N, Zarzuelo A and Gálvez J:
Minocycline: Far beyond an antibiotic. Br J Pharmacol. 169:337–352.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liao TV, Forehand CC, Hess DC and Fagan
SC: Minocycline repurposing in critical illness: Focus on stroke.
Curr Top Med Chem. 13:2283–2290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li C, Yuan K and Schluesener H: Impact of
minocycline on neurodegenerative diseases in rodents: A
meta-analysis. Rev Neurosci. 24:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Teng YD, Choi H, Onario RC, Zhu S,
Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME and
Friedlander RM: Minocycline inhibits contusion-triggered
mitochondrial cytochrome c release and mitigates functional
deficits after spinal cord injury. Proc Natl Acad Sci USA.
101:3071–3076. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chaturvedi M and Kaczmarek L: Mmp-9
inhibition: A therapeutic strategy in ischemic stroke. Mol
Neurobiol. 49:563–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Enose-Akahata Y, Matsuura E, Tanaka Y, Oh
U and Jacobson S: Minocycline modulates antigen-specific CTL
activity through inactivation of mononuclear phagocytes in patients
with HTLV-I associated neurologic disease. Retrovirology. 9:162012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jung HJ, Seo I, Jha BK, Suh SI, Suh MH and
Baek WK: Minocycline inhibits angiogenesis in vitro through the
translational suppression of HIF-1α. Arch Biochem Biophys.
545:74–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li CH, Liao PL, Yang YT, Huang SH, Lin CH,
Cheng YW and Kang JJ: Minocycline accelerates hypoxia-inducible
factor-1 alpha degradation and inhibits hypoxia-induced
neovasculogenesis through prolyl hydroxylase, von
Hippel-Lindau-dependent pathway. Arch Toxicol. 88:659–671.
2014.PubMed/NCBI
|
|
77
|
Sun C, Li XX, He XJ, Zhang Q and Tao Y:
Neuroprotective effect of minocycline in a rat model of branch
retinal vein occlusion. Exp Eye Res. 113:105–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Levkovitz Y, Fenchel D, Kaplan Z, Zohar J
and Cohen H: Early post-stressor intervention with minocycline, a
second-generation tetracycline, attenuates post-traumatic stress
response in an animal model of PTSD. Eur Neuropsychopharmacol.
25:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM,
Choi JY, Hwang DH and Kim BG: Contribution of macrophages to
enhanced regenerative capacity of dorsal root ganglia sensory
neurons by conditioning injury. J Neurosci. 33:15095–15108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ataie-Kachoie P, Badar S, Morris DL and
Pourgholami MH: Minocycline targets the NF-kB Nexus through
suppression of TGF-β1-TAK1-IkB signaling in ovarian cancer. Mol
Cancer Res. 11:1279–1291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pang T, Wang J, Benicky J and Saavedra JM:
Minocycline ameliorates LPS-induced inflammation in human monocytes
by novel mechanisms including LOX-1, Nur77 and LITAF inhibition.
Biochim Biophys Acta. 1820:503–510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li H, Xu H and Sun B: Lipopolysaccharide
regulates MMP-9 expression through TLR4/NF-kB signaling in human
arterial smooth muscle cells. Mol Med Rep. 6:774–778.
2012.PubMed/NCBI
|
|
83
|
Pick M, Ronen D, Yanuka O and Benvenisty
N: Reprogramming of the MHC-I and its regulation by NFkB in
human-induced pluripotent stem cells. Stem Cells. 30:2700–2708.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xie TX, Xia Z, Zhang N, Gong W and Huang
S: Constitutive NF-kB activity regulates the expression of VEGF and
IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep.
23:725–732. 2010.PubMed/NCBI
|
|
85
|
Zhao M, Zhou A, Xu L and Zhang X: The role
of TLR4-mediated PTEN/PI3K/AKT/NF-kB signaling pathway in
neuroinflammation in hippocampal neurons. Neuroscience. 269:93–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Pozniak PD, White MK and Khalili K:
TNF-α/NF-κB signaling in the CNS: Possible connection to EPHB2. J
Neuroimmune Pharmacol. 9:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Matsuura Y, Ochi M, Uchio Y, Suzuki G and
Iwata A: The time-dependent difference of GAP-43 expression between
sensory neurons and motoneurons after peripheral nerve transection.
Scand J Plast Reconstr Surg Hand Surg. 33:267–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gordon T, You S, Cassar SL and Tetzlaff W:
Reduced expression of regeneration associated genes in chronically
axotomized facial motoneurons. Exp Neurol. 264:26–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tedeschi A, Nguyen T, Puttagunta R, Gaub P
and Di Giovanni S: A p53-CBP/p300 transcription module is required
for GAP-43 expression, axon outgrowth and regeneration. Cell Death
Differ. 16:543–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jiang BP, Le L, Xu LJ and Xiao PG:
Minocycline inhibits ICAD degradation and the NF-kB activation
induced by 6-OHDA in PC12 cells. Brain Res. 1586:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kadiyala CS, Zheng L, Du Y, Yohannes E,
Kao HY, Miyagi M and Kern TS: Acetylation of retinal histones in
diabetes increases inflammatory proteins: Effects of minocycline
and manipulation of histone acetyltransferase (HAT) and histone
deacetylase (HDAC). J Biol Chem. 287:25869–25880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Levkovitch-Verbin H, Waserzoog Y, Vander
S, Makarovsky D and Piven I: Minocycline upregulates pro-survival
genes and downregulates pro-apoptotic genes in experimental
glaucoma. Graefes Arch Clin Exp Ophthalmol. 252:761–772. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hassanzadeh K, Habibi-asl B, Farajnia S
and Roshangar L: Minocycline prevents morphine-induced apoptosis in
rat cerebral cortex and lumbar spinal cord: A possible mechanism
for attenuating morphine tolerance. Neurotox Res. 19:649–659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Filipovic R and Zecevic N: Neuroprotective
role of minocycline in co-cultures of human fetal neurons and
microglia. Exp Neurol. 211:41–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Matsukawa N, Yasuhara T, Hara K, Xu L,
Maki M, Yu G, Kaneko Y, Ojika K, Hess DC and Borlongan CV:
Therapeutic targets and limits of minocycline neuroprotection in
experimental ischemic stroke. BMC Neurosci. 10:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kerr DA, Larsen T, Cook SH, Fannjiang YR,
Choi E, Griffin DE, Hardwick JM and Irani DN: BCL-2 and BAX protect
adult mice from lethal Sindbis virus infection but do not protect
spinal cord motor neurons or prevent paralysis. J Virol.
76:10393–10400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hemendinger RA, Armstrong EJ 3rd, Radio N
and Brooks BR: Neurotoxic injury pathways in differentiated mouse
motor neuron-neuroblastoma hybrid (NSC-34D) cells in vitro -
limited effect of riluzole on thapsigargin, but not staurosporine,
hydrogen peroxide and homocysteine neurotoxicity. Toxicol Appl
Pharmacol. 258:208–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Soo KY, Atkin JD, Horne MK and Nagley P:
Recruitment of mitochondria into apoptotic signaling correlates
with the presence of inclusions formed by amyotrophic lateral
sclerosis-associated SOD1 mutations. J Neurochem. 108:578–590.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee SH, Choi NY, Yu HJ, Park J, Choi H,
Lee KY, Huh YM, Lee YJ and Koh SH: Atorvastatin protects NSC-34
motor neurons against oxidative stress by activating PI3K, ERK and
free radical scavenging. Mol Neurobiol. Jan 11–2015.(Epub ahead of
print).
|
|
100
|
Ezzi SA, Urushitani M and Julien JP:
Wild-type superoxide dismutase acquires binding and toxic
properties of ALS-linked mutant forms through oxidation. J
Neurochem. 102:170–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu YX, Tai JL, Li GQ, Zhang ZW, Xue JH,
Liu HS, Zhu H, Cheng JD, Liu YL, Li AM and Zhang Y: Exposure to
1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of
astrocytes via caspase-3-dependent pathway. PLoS One. 7:e423322012.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ransohoff RM and Estes ML: Astrocyte
expression of major histocompatibility complex gene products in
multiple sclerosis brain tissue obtained by stereotactic biopsy.
Arch Neurol. 48:1244–1246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kim KH, Jeong JY, Surh YJ and Kim KW:
Expression of stress-response ATF3 is mediated by Nrf2 in
astrocytes. Nucleic Acids Res. 38:48–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sunkaria A, Wani WY, Sharma DR and Gill
KD: Dichlorvos exposure results in activation induced apoptotic
cell death in primary rat microglia. Chem Res Toxicol.
25:1762–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tambuyzer BR, Ponsaerts P and Nouwen EJ:
Microglia: Gatekeepers of central nervous system immunology. J
Leukoc Biol. 85:352–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Simonishvili S, Jain MR, Li H, Levison SW
and Wood TL: Identification of Bax-interacting proteins in
oligodendrocyte progenitors during glutamate excitotoxicity and
perinatal hypoxia-ischemia. ASN Neuro. 5:e001312013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ji Q, Castelli L and Goverman JM: MHC
class I-restricted myelin epitopes are cross-presented by Tip-DCs
that promote determinant spreading to CD8+ T cells. Nat
Immunol. 14:254–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Goldberg J, Daniel M, van Heuvel Y, Victor
M, Beyer C, Clarner T and Kipp M: Short-term cuprizone feeding
induces selective amino acid deprivation with concomitant
activation of an integrated stress response in oligodendrocytes.
Cell Mol Neurobiol. 33:1087–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Johanson SO, Crouch MF and Hendry IA:
Retrograde axonal transport of signal transduction proteins in rat
sciatic nerve. Brain Res. 690:55–63. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang HH, Hsieh HL, Wu CY and Yang CM:
Oxidized low-density lipoprotein-induced matrix metalloproteinase-9
expression via PKC-delta/p42/p44 MAPK/Elk-1 cascade in brain
astrocytes. Neurotox Res. 17:50–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lively S and Schlichter LC: The microglial
activation state regulates migration and roles of matrix-dissolving
enzymes for invasion. J Neuroinflammation. 10:752013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Monnet-Tschudi F, Defaux A, Braissant O,
Cagnon L and Zurich MG: Methods to assess neuroinflammation. Curr
Protoc Toxicol Unit. 12:192011.
|
|
113
|
Krum JM and Khaibullina A: Inhibition of
endogenous VEGF impedes revascularization and astroglial
proliferation: Roles for VEGF in brain repair. Exp Neurol.
181:241–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shin YJ, Riew TR, Park JH, Pak HJ and Lee
MY: Expression of vascular endothelial growth factor-C (VEGF-C) and
its receptor (VEGFR-3) in the glial reaction elicited by human
mesenchymal stem cell engraftment in the normal rat brain. J
Histochem Cytochem. 63:170–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Keshavarz M, Emamghoreishi M, Nekooeian
AA, Warsh JJ and Zare HR: Increased bcl-2 protein levels in rat
primary astrocyte culture following chronic lithium treatment. Iran
J Med Sci. 38:255–262. 2013.PubMed/NCBI
|
|
116
|
Barber SC, Higginbottom A, Mead RJ, Barber
S and Shaw PJ: An in vitro screening cascade to identify
neuroprotective antioxidants in ALS. Free Radic Biol Med.
46:1127–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Prell T, Lautenschläger J, Weidemann L,
Ruhmer J, Witte OW and Grosskreutz J: Endoplasmic reticulum stress
is accompanied by activation of NF-kB in amyotrophic lateral
sclerosis. J Neuroimmunol. 270:29–36. 2014. View Article : Google Scholar : PubMed/NCBI
|