Introduction
Otitis media (OM) is a common disease in infants and
may in certain cases develop into acute otitis media (AOM) or
chronic otitis media. Teele et al conducted a prospective,
cohort study in 1989 and estimated that ~62% of infants suffered at
least one episode of AOM by the age of 1 year, and ~83% of infants
by the age of 3 years (1). However,
studies from several other countries, summarized in a report on OM
research published between 2003 and 2007, have indicated a lower
incidence of AOM over this age range (2). In a previous study, Ting et al
(3) conducted >10,000
questionnaires between 2005 and 2010, which revealed that the
overall prevalence of AOM among Taiwanese children <5 years of
age was ~20%.
The pathogenesis of OM is complicated, involving
numerous factors associated with the anatomy, pathology and cell
biology of the middle ear, mastoid, Eustachian tube and nasopharynx
(4). Increasing attention is being
focused on investigating the role of infection-induced mucous cell
metaplasia/hyperplasia in the middle ear epithelium, and the
associated mucin hyperproduction that has been identified as a
fundamental occurrence of OM with effusion (5). Cellular proliferation and
differentiation are essential to this mucous cell
metaplasia/hyperplasia process. However, the precise mechanisms
that regulate these processes have yet to be fully elucidated, and
cellular interactions are a crucial factor during OM infection
The Notch signaling pathway is a highly conserved
network that regulates cell fate decisions in various tissues and
organisms (6). Notch proteins are
membrane-bound receptors, with the corresponding membrane bound
ligands Delta-like (Dll) and Jagged. Following the binding of a
ligand, the Notch intracellular domain (NICD) is cleaved by
γ-secretase and translocated to the nucleus, where it
transactivates target genes such as hairy and enhancer of split
(Hes) and Hes-related repressor protein (Hey). Hes and Hey function
as transcriptional repressors, suppressing the expression of
downstream target genes and thereby regulating cellular
proliferation and differentiation (7,8).
Notch signaling is involved in various aspects of
cellular regulation. Depending on the tissue and context, Notch may
either restrict or promote cell fate determination. In the
intestine, Notch and γ-secretase inhibitors block cellular
proliferation and induce secretory cell differentiation, and the
Notch signaling pathway is key to the differentiation or
self-renewal of intestinal stem cells (9–11). In
the human corneal epithelium,
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine
tert-butyl ester (DAPT), a specific inhibitor of γ-secretase,
has been shown to inhibit Notch signaling, thereby repressing cell
proliferation and promoting the differentiation of corneal
epithelial cells (12). A previous
study by Blanpain et al showed that Notch functions as a
regulator of epidermal differentiation, in addition to regulating
the balance between proliferative basal progenitor cells and
terminally differentiating suprabasal progeny cells (13). Furthermore, previous studies have
indicated that Notch signaling may be involved in the proliferation
and differentiation of airway epithelial cells and mammary cells
(14,15). However, the role of Notch signaling
and the expression of signaling pathway-associated genes in the
middle ear epithelium remains unclear, as does the role of Notch
signaling in the regulation of middle ear epithelial cell activity.
The aim of the present study was to identify the localization of
Notch receptors and their ligands, including Notch1-4, Jagged1,
Jagged2, Dll1, Dll3 and Dll4, in normal mouse middle ear epithelium
(NMMEE) cells. Furthermore, the study aimed to elucidate whether
the inhibition of Notch signaling by the γ-secretase inhibitor DAPT
was able to repress cellular proliferation and promote the
differentiation of NMMEE cells into mucous cells through inhibit
Notch signaling. Therefore, the mRNA expression levels of the
mucous cell-associated genes Spink4, Tff1, Spdef, Arg2 and Muc2
were evaluated.
Materials and methods
NMMEE cell culture and observation of
cell morphology
A total of 65 male BALB/c mice (age range, 4–6
weeks; weight, 20 g) were used as middle ear epithelial cell donors
(Shanghai Laboratory Animal Center, CAS, Shanghai, China). The
animal use protocol was approved by the Institutional Animal Care
and Animal Ethics Committee of Fudan University (Shanghai, China).
Mice were anesthetized with ketamine hydrochloride (100 mg/kg;
Hengrui Medicine Co., Ltd., Jiangsu, China) and xylazine (10 mg/kg;
Sangon Biotech Co., Ltd., Shanghai, China). The bullae were
immediately removed and rinsed in phosphate-buffered saline (PBS;
Thermo Fisher Scientific, Inc., Beijing, China). The middle ear
mucosa was aseptically dissociated from the bony part of the bullae
under a stereomicroscope (Stemi 2000-C; Carl Zeiss Jena GmbH, Jena,
Germany), cut into small pieces and incubated in 0.25% trypsin
(Invitrogen, Carlsbad, CA, USA) and 1.6 mM
ethylenediaminetetraacetic acid for 20 min at 37°C. Digestion was
terminated by adding Dulbeccos modified Eagles medium with 10%
fetal bovine serum (FBS; Invitrogen). The cellular suspension was
centrifuged at 160 × g for 5 min. The supernatant was discarded and
the pellet was resuspended in full growth medium. Cells were seeded
in culture dishes (~104 cells per dish) coated with
bovine collagen I (354231; BD Biosciences, Franklin Lakes, NJ, USA)
and coverslips. These primary cells were cultured at 37°C in a 5%
CO2 cell culture incubator. Full growth medium,
containing Ham's F-12K (Kaighn's) medium supplemented with 1.5 g/l
sodium bicarbonate, 2.0 mM L-glutamine, 10 ng/ml murine epidermal
growth factor (Peprotech Inc., Rocky Hill, NJ, USA), 10 mg/ml
insulin-transferrin-sodium selenite supplement (BD Biosciences),
2.7 g/l glucose, 500 ng/ml hydrocortisone (H0888; Sigma-Aldrich,
St. Louis, MO, USA), 0.1 mM non-essential amino acids (M7145;
Sigma-Aldrich) and 4% FBS, was changed every 2–3 days for 1 week.
The cells were used for experiments during an exponential growth
phase. Bright-field live cell images were acquired using a Nikon
Eclipse Ti-E microscope equipped with a Nikon DS-Fi1 digital camera
(Nikon Corporation, Tokyo, Japan).
Identification of the
immunolocalization of Notch receptors and their ligands using
immunofluorescence analysis
Complete bullae were removed and fixed in 4%
paraformaldehyde for 24 h, then the middle ear epithelial cells
were dissected out by the aforementioned method. The epithelial
cells were treated with 0.3% Triton X-100 (Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) in PBS for 30 min, then
blocked using 10% normal donkey serum (Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) in PBS for 1 h at room
temperature. Tissues were then incubated with the following primary
antibodies overnight at 4°C: Goat polyclonal anti-Jagged1 (1:200,
sc6011; Santa Cruz Biotechnology, Inc., La Jolla, CA, USA), rabbit
monoclonal anti-Notch1 (1:100; D6F11; Cell Signaling Technology,
Inc., Danvers, MA, USA) and rabbit monoclonal anti-Notch2 (1:100;
D76A6; Cell Signaling Technology, Inc.). After rinsing in PBS,
samples were incubated with fluorescence-labeled Alexa Fluor 488
(705–295-147) or 555 (711-545-152) secondary antibodies (1:1,000;
Thermo Fisher Scientific, Inc.) for 2 h at room temperature. For
nuclear labeling, samples were additionally incubated with Hoechst
stain (1:1,000; Thermo Fisher Scientific, Inc.) for 30 min. After
PBS washing, specimens were mounted on glass slides and observed
using a Leica SP5 confocal laser-scanning microscope (Leica
Microsystems GmbH, Wetzlar, Germany) and images were captured at a
magnification of ×40. Images were processed using Adobe Photoshop
software (Adobe Systems, Inc., Santa Clara, CA, USA).
Evaluation of the mRNA expression
levels of Notch receptors and ligands by reverse transcription
polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from primary cultures of
NMMEE cells by directly adding the RNA lysis buffer provided in an
RNeasy Micro kit (74004; Qiagen, Hilden, Germany) after rinsing
with PBS twice. RNA was purified using the kit according to the
manufacturer's instructions. RNA quality and quantity were assessed
using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific,
Inc.) and 500 ng total RNA was used for each sample. cDNA was
synthesized using PrimerScript RT Master mix (RR036A; Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's instructions.
PCR was conducted using an ABI-7500 Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA).
PCR primers for Notch 1–4 receptors and their
ligands (Dll1, Dll3, Dll4, Jagged1 and Jagged2) were designed
according to the gene sequences obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank/).
Primer sequences and the sizes of the expected PCR products are
detailed in Table I. PCR was
performed using Taq polymerase (DR001B; Takara Biotechnology, Co.,
Ltd., Dalian, China). PCR cycling consisted of 35 cycles, each
conducted as follows: Denaturation for 30 sec at 94°C, a 30-sec
annealing step at 57°C, 30 sec at 72°C and a final extension at 72°C
for 5 min. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was
used as a positive control. PCR products were subjected to
electrophoresis on 2.5% agarose gel (Sangon Biotech Co., Ltd.)
containing ethidium bromide and visualized under ultraviolet
light.
 | Table I.Reverse transcription primer
sequences. |
Table I.
Reverse transcription primer
sequences.
| Gene | Primer sequences 5′
to 3′ | Product size
(bp) |
|---|
| Notch1 |
TCAGGGTGTCTTCCAGATCCCAGCATCCACATTGTTCACC | 212 |
| Notch2 |
ATGTGGACGAGTGTCTGTTGCGGAAGCATAGGCACAGTCATC | 146 |
| Notch3 |
TGCCAGAGTTCAGTGGTGGCACAGGCAAATCGGCCATC | 157 |
| Notch4 |
CTCTTGCCACTCAATTTCCCTTTGCAGAGTTGGGTATCCCTG | 188 |
| Jagged1 |
CCTCGGGTCAGTTTGAGCTGCCTTGAGGCACACTTTGAAGTA | 150 |
| Jagged2 |
CAATGACACCACTCCAGATGAGGGCCAAAGAAGTCGTTGCG | 203 |
| Dll1 |
CAGGACCTTCTTTCGCGTATGAAGGGGAATCGGATGGGGTT | 168 |
| Dll3 |
CTGGTGTCTTCGAGCTACAAATTGCTCCGTATAGACCGGGAC | 199 |
| Dll4 |
TTCCAGGCAACCTTCTCCGAACTGCCGCTATTCTTGTCCC | 102 |
| GAPDH |
AGGTCGGTGTGAACGGATTTGTGTAGACCATGTAGTTGAGGTCA | 123 |
Evaluation of cellular proliferation
by labeling with 5-ethynyl-2′-deoxyuridine (EdU)
The γ-secretase inhibitor DAPT (565784; EMD
Chemicals, Inc., San Diego, CA, USA) was used to evaluate the
function of Notch signaling in the regulation of epithelial cell
differentiation and proliferation. NMMEE cells were cultured on
coverslips precoated with collagen I until subconfluent. The cells
were subsequently incubated in serum-free medium for 4 h, and then
incubated in medium containing 2.5 or 5 µM DAPT for 24 h. Control
wells were incubated in medium containing 0.2% dimethyl sulfoxide
(DMSO). EdU was used to label proliferating cells, and was detected
using an EdU labeling/detection kit (C10371-1; Guangzhou Ribobio,
Co., Ltd., Guangzhou, China) according to the manufacturer's
protocol. EdU was added to the culture media at a concentration of
10 µM simultaneously with DAPT for 24 h.
Evaluation of apoptosis and epithelial
cell proliferation using immunostaining analysis
Dual EdU and immunofluorescence staining was
conducted, with EdU staining performed first, followed by
immunostaining. Immunostaining was performed as described above.
Primary antibodies against caspase-3 (1:100; AF835; R&D
Systems, Inc., Minneapolis, MN, USA) and pan-cytokeratin (pan-CK)
(1:100; 4545; Cell Signaling Technology, Inc.) were used, overnight
at 4°C. Experiments, in triplicate, were repeated at least twice.
Images were captured digitally in at least six random microscope
fields from each sample, using a Leica DM 4000 B microscope
equipped with a DFC 500 camera (Leica, Mannheim, Germany).
Quantitative results were obtained by calculating
the ratio between the number of immunopositive cells for each
antibody and the total number of cells, which determined by
counting the nuclei revealed by Hoechst staining from six randomly
selected microscopic fields (magnification, ×400). Images were
processed using Adobe Photoshop software.
Detection of the expression of mucous
cell-associated genes in using quantitative RT-PCR (RT-qPCR)
Epithelial cells cultured in 6-cm dishes were
treated with DAPT as described in the cell proliferation assay.
Subsequently, 3–5 independent RNA pools were prepared for each DAPT
concentration (2.5 and 5 µM) and for the DMSO-treated control
cells. Total RNA extraction and cDNA synthesis protocols were
performed as described above. PCR primers for Arg2, Hes1, Muc2,
Spdef, Spink4 and Tff1 were designed according to the gene
sequences obtained from GenBank, as listed in Table II. qPCR was performed using SYBR
Green reagent and the Applied Biosystems 7500 Real-time PCR
System.
 | Table II.Quantitative polymerase chain reaction
primer sequences. |
Table II.
Quantitative polymerase chain reaction
primer sequences.
| Gene | Primer sequences 5′
to 3′ |
|---|
| Hes1 |
CGAGCGTGTTGGGGAAATAC |
|
|
GGTAGGTCATGGCGTTGATC |
| Spink4 |
GCTGAGCTTCCAAACTGTCC |
|
|
TTTTCATCCGGGTCAGGCAA |
| Tff1 |
CAGGCCCAGGAAGAAACATG |
|
|
AAACAGCAACCTCTCTCCGT |
| Spdef |
AACGTGCAGAAGTGGCTTTT |
|
|
ACTTCCAGATGTCCAGGTGG |
| Arg2 |
TCCCTGCCAATCATGTTCCT |
|
|
TAGCTTCTTCTGTCCCCGAG |
| Muc2 |
ATGTCCTGACCAAGAGCGAA |
|
|
GACAGTCTTCAGGCAGGTCT |
| GAPDH |
AGGTCGGTGTGAACGGATTTG |
|
|
TGTAGACCATGTAGTTGAGGTCA |
The 2−∆∆Cq method was used to quantify
the relative differences in mRNA expression levels following DAPT
treatment, as previously described (16). For each sample, ∆Cq was calculated as
the difference in threshold cycle number (Cq) between the target
gene and GAPDH. These values were averaged across the samples in a
group (n=3). The fold change for each gene following DAPT treatment
was determined from 2−∆∆Cq.
Statistical analysis
All experiments were repeated at least twice, and
the results are expressed as the mean ± standard deviation. The
differences between control and experimental groups were evaluated
using one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cellular morphology
Primary NMMEE cells demonstrated a flat monolayer of
culture with a polygonal cobblestone-like appearance at 5–7 days of
culturing, a typical morphology for epithelial cells (Fig. 1A, left panel). The cells exhibited
stable growth and maintained the cobblestone-like morphology for up
to 10 days. However, the cells showed deterioration of their
morphology and significantly swelled and flattened after 10
days.
Cultures of epithelial cells were subjected to
immunocytochemistry for pan-CK. The majority of the cells cultured
as epithelial cells were pan-CK positive (Fig. 1A, right panel). Thus, it was
concluded that epithelial cells were cultured as pure cell
populations.
Cellular localization of Notch1,
Notch2 and Jagged1 in NMMEE cells
Expression patterns of Notch receptors and their
ligands in NMME cells were identified using immunofluorescent
staining. As shown in Fig. 1B,
marked cell membrane staining of Notch1, Notch2 and Jagged1 was
observed, which was co-localized in the majority of cells within
the mucosa. These images indicate that Notch receptors and their
ligands are expressed in the majority of NMMEE cells.
Expression of Notch receptors and
ligands in NMMEE cells
RT-PCR assay was used to detect the expression of
Notch receptors and ligands in cultured NMMEE cells. The results
indicate that all the receptors (Notch1, Notch2, Notch3 and Notch4)
and the majority of the ligands (with the exception of Dll3) were
expressed in the cultured NMMEE cells. These results suggest that
Notch signaling-associated proteins are present and functional in
the mucosa of the mouse middle ear (Fig.
1C).
Inhibition of Notch signaling
decreases the proliferation of epithelial cells
The enhanced proliferation of middle ear epithelial
cells, particularly mucous cells, is a crucial underlying process
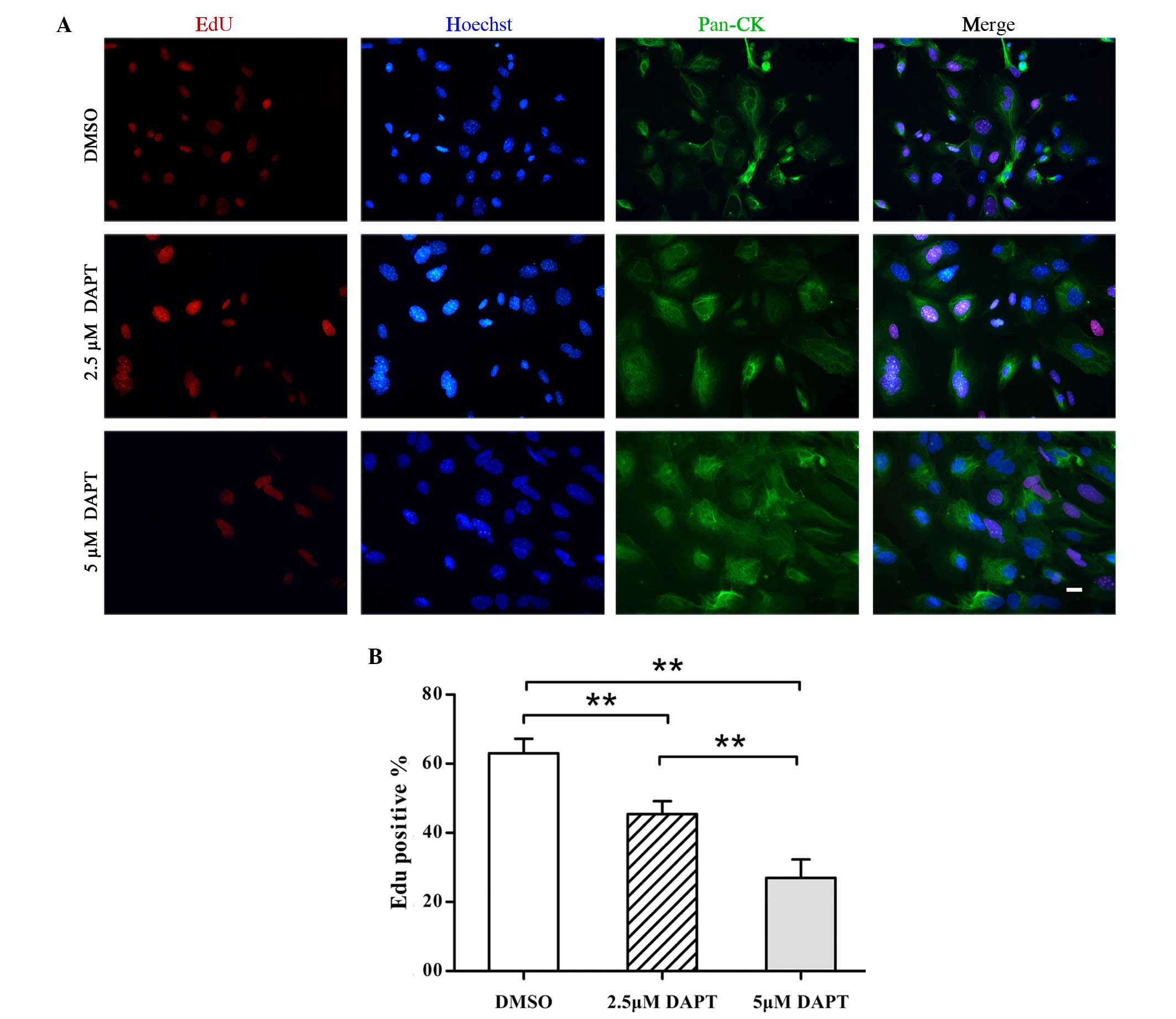
in the pathogenesis of OM. Therefore, EdU detection was performed
in conjunction with immunofluorescence staining for the epithelial
cell proliferation marker pan-CK and the apoptosis marker caspase-3
to evaluate the effect of DAPT on NMMEE cell proliferation and
apoptosis in vitro. The results of this experiment were
quantitated using image analysis software. Fewer EdU-positive cells
were detected among the DAPT-treated NMEE cell samples indicating a
reduction in cellular proliferation (Fig. 2A). All of the cultured cells were
identified to be pan-CK-positive epithelial cells. The expression
of EdU was significantly decreased in DAPT-treated NMMEE cells
compared with a DMSO-treated control. Furthermore, the alterations
in cellular proliferation appeared to be dose-dependent, since the
levels of EdU expression differed significantly between the cells
treated with 2.5 DAPT and those treated with 5 µM DAPT (P<0.01;
Fig. 2A). In addition, the
inhibition of γ-secretase/Notch signaling by DAPT resulted in a
dose-dependent reduction in the percentage of cells with positive
cell nuclei-associated EdU immunoreactivity (Fig. 2B). The percentages of EdU-positive
cells were 63.0±4.2, 45.4±3.7 and 27.0±5.4% in the cells treated
with 0, 2.5 and 5 µM DAPT, respectively (P<0.01). By contrast,
the expression of caspase-3 was not found to be increased in the
DAPT-treated NMMEE cells compared with the DMSO-treated control
cells (data not shown). These data indicate that DAPT inhibited the
proliferation of NMMEE cells; however, DAPT is indicated to have no
apoptosis-inducing effect on the basis of the immunochemical
analysis of caspase-3 expression.
Collectively, these results indicate that DAPT may
exert a therapeutic effect on OM, potentially involving
anti-proliferative effects on NMMEE cells.
DAPT activates mucous cell-associated
genes in cultured NMMEE cells
Notch signaling triggers mucous cell differentiation
in intestinal tissue (9). In colon
cancer cells, Notch signal inhibitors are able to activate mucous
cell genes (11). In the present
study, the effect of the γ-secretase inhibitor DAPT on NMMEE cell
differentiation was investigated. A total of five mucous cell genes
(Arg2, Muc2, Spdef, Spink4 and Tff1) were selected due to their
effect on intestinal, cancer and airway epithelial cells.
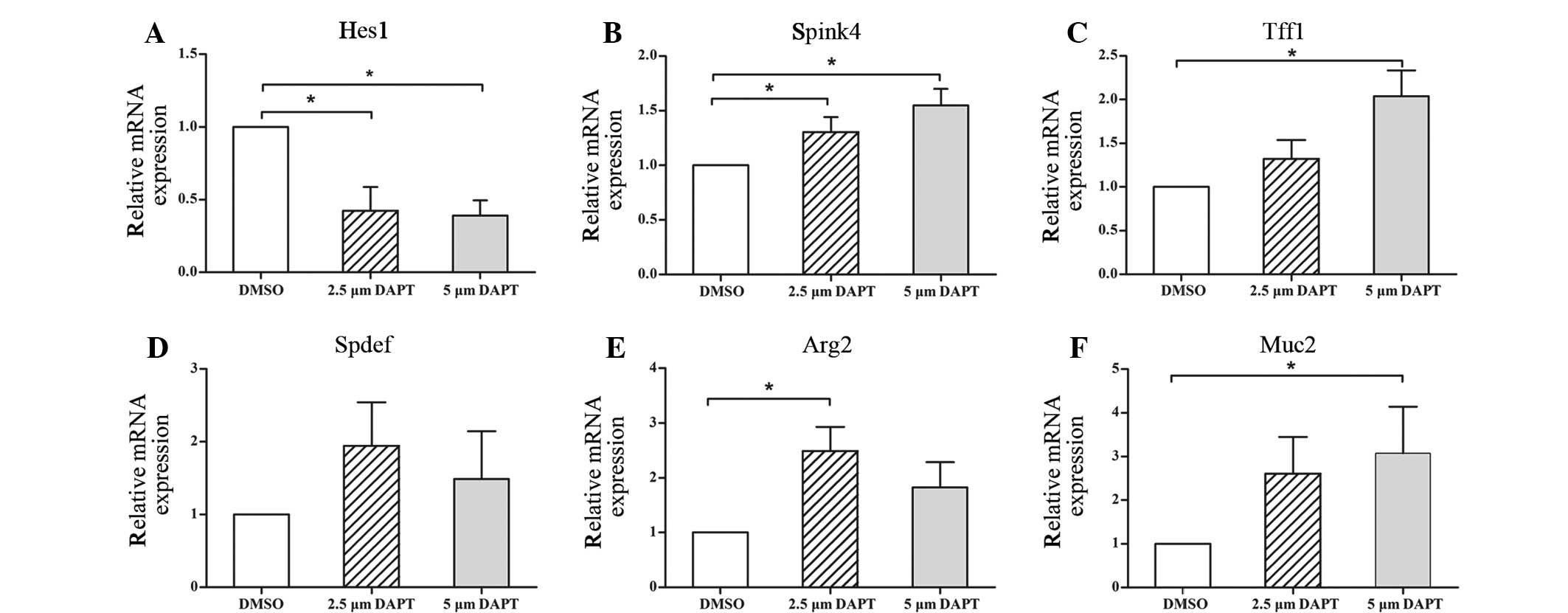
Following subconfluence, NMMEE cells were treated
with 2.5 or 5 µM DAPT or DMSO (control) for 24 h. To determine the
capacity of the treatment to inhibit Notch signaling, Hes1 gene
levels were evaluated with and without DAPT treatment. The results
indicate that in the 2.5 and 5 µM DAPT-treated cells the relative
mRNA expression levels of Hes1 were significantly reduced compared
with those in the control group 24 h after treatment with DAPT
(Fig. 3A). No statistically
significant differences were detected between the two DAPT groups.
These observations suggested that 2.5 and 5 µM DAPT was able to
block Notch signaling within 24 h of treatment.
To examine the effects that the inhibition of Notch
signaling had on the differentiation of NMMEE cells, the expression
of Spink4, Tff1, Spdef, Arg2 and Muc2 in NMMEE cells with and
without DAPT was investigated. The expression levels of all five
mucous cell-associated genes were increased compared with those in
the control group at 24 h (Fig.
3B–F). Among the five mucous cell-associated genes, the
expression levels of Muc2 and Arg2 increased the most markedly. The
expression levels of all the genes increased in a dose-dependent
manner along with the concentration of DAPT, with the exceptions of
Spdef and Arg2. The expression levels of Spdef and Arg2 declined in
the 5 µM DAPT-treated group compared with the 2.5 µM DAPT-treated
group, although the reduction was not statistically significant.
These results suggest that the disruption of Notch signaling leads
to a reduction in cell proliferation, with a concomitant increase
in mucous cell differentiation.
Discussion
The mammalian middle ear is an air-filled cavity
within the auditory bulla, which has three ossicles suspended
within it connecting the eardrum to the inner ear. The cavity is
lined by an epithelium, and the auditory tube and eardrum are
surrounded with epithelium, which is derived from the first
pharyngeal pouch endodermal cells (15,17,18).
These cells develop ciliated cells and mucous cells, forming a
pseudostratified ciliated columnar epithelium, similar to airway
epithelial cells (15). Middle ear
epithelial cells are in the frontline against the environment.
Mucous cells secrete mucus, and with the cilia constitute a
mucociliary system for clearing effusion and debris. The epithelium
thickens due to mucous cell metaplasia/hyperplasia in response to
disease and inflammation (4,5). In the present study, Notch signaling
was inhibited in NMMME cells in order to investigate the mechanism
by which middle ear cells proliferate and differentiate, with the
aim of elucidating the pathogenesis of mucous cell
metaplasia/hyperplasia in OM.
Notch signaling has been well demonstrated in the
intestines (9–11). The differentiation of intestinal
cells to either an absorptive or secretory cell linage is
controlled by the Notch signaling system via lateral inhibition.
However, few studies have been published investigating Notch
signaling in the middle ear epithelium (19,20). To
the best of our knowledge, the present study is the first to reveal
the presence of Notch ligands and receptors in NMMEE cells. The
mRNA expression of all receptors and ligands investigated was
detected in the primary cultured NMMEE cells, with the exception of
Dll3, suggesting that Notch signaling may perform certain functions
in the epithelium.
DAPT, a Notch/γ-secretase inhibitor, is able to
inhibit the NICD cleaved by γ-secretase, which is subsequently
translocated into the nucleus and activates downstream genes
(9,11). The results of the present study
indicate that the mechanisms underlying the DAPT-mediated
suppression of NMMEE cell proliferation involve repressing the
activity of the Notch signaling pathway, thereby increasing the
expression levels of genes associated with mucous cell
differentiation. The expression of the Hes1 gene in cultured NMMEE
cells was significantly decreased following treatment with 2.5 or 5
µM DAPT for 24 h. The number of EdU positive cells, which
represents proliferating cells, decreased as the concentration of
DAPT increased. To the best of our knowledge, the present study is
the first to demonstrate that Notch signaling is repressed, leading
to the suppression of NMMEE cell proliferation by the
administration of DAPT. However, no significant differences were
detected in the frequency of apoptosis in the DAPT-treated NMMEE
cells. These results indicate that DAPT is not able to induce the
apoptosis of NMMEE cells in vitro. By contrast, previous
studies have clearly demonstrated that γ-secretase inhibition can
enhance the apoptosis of intestinal epithelial cells in vivo
(9,11). Therefore, further studies are
required to clarify the impact of DAPT within the middle ear
epithelium in murine models with OM effusion. In addition to
decreased cellular proliferation, DAPT appeared to promote the
expression of genes associated with differentiation towards the
mucous cell lineage. Further investigation is required to determine
by what mechanism these genes influence cell differentiation. On
the basis of the present results and the aforementioned previous
studies, it is possible that DAPT may exert a therapeutic effect on
OM as a result of its suppressive effect on proliferation.
In summary, Notch signaling ligands and receptors
are active in the mouse middle ear epithelium. In NMMEE cells
primarily cultured in vitro, DAPT was able to inhibit Notch
signaling and to thereby suppress the proliferation of the NMMEE
cells. Furthermore, DAPT may be able to facilitate the
differentiation of epithelial cells into mucous cells. Therefore,
the γ-secretase inhibitor DAPT may provide a specific therapeutic
agent for the reversal of multiple pathological processes that are
associated with epithelium thickening.
Acknowledgements
This study was supported by the Major State Basic
Research Development Program of China (973 Program; grant no.
2011CB504500 and 2011CB504506), the National Natural Science
Foundation of China (grant no. 81420108010, 81000413, 81370022,
81570920 and 81200740), the Training Program of the Excellent Young
Talents of the Shanghai Municipal Health System (grant no.
XYQ2013084) and the Innovation Project of Shanghai Municipal
Science and Technology Commission (grant no. 11411952300).
Glossary
Abbreviations
Abbreviations:
|
NMMEE
|
normal mouse middle ear epithelium
|
References
|
1
|
Teele DW, Klein JO and Rosner B:
Epidemiology of otitis media during the first seven years of life
in children in greater Boston: A prospective, cohort study. J
Infect Dis. 160:83–94. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daly KA, Hoffman HJ, Kvaerner KJ, Kvestad
E, Casselbrant ML, Homoe P and Rovers MM: Epidemiology, natural
history, and risk factors: Panel report from the Ninth
International Research Conference on Otitis Media. Int J Pediatr
Otorhinolaryngol. 74:231–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ting PJ, Lin CH, Huang FL, Lin MC, Hwang
KP, Huang YC, Chiu CH, Lin TY and Chen PY: Epidemiology of acute
otitis media among young children: A multiple database study in
Taiwan. J Microbiol Immunol Infect. 45:453–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cayé-Thomasen Hermansson A, Bakaletz L,
Hellstrøm S, Kanzaki S, Kerschner J, Lim D, Lin J, Mason K and
Spratley J: Panel 3: Recent advances in anatomy, pathology, and
cell biology in relation to otitis media pathogenesis. Otolaryngol
Head Neck Surg. 148(4 Suppl): E37–E51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kerschner JE, Li J, Tsushiya K and
Khampang P: Mucin gene expression and mouse middle ear epithelium.
Int J Pediatr Otorhinolaryngol. 74:864–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Movahedan A, Majdi M, Afsharkhamseh N,
Sagha HM, Saadat NS, Shalileh K, Milani BY, Ying H and Djalilian
AR: Notch inhibition during corneal epithelial wound healing
promotes migration. Invest Ophthalmol Vis Sci. 53:7476–7483. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma XB, Jia XS, Liu YL, Wang LL, Sun SL,
Song N, Wang EH and Li F: Expression and role of Notch signalling
in the regeneration of rat tracheal epithelium. Cell Prolif.
42:15–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kazanjian A, Noah T, Brown D, Burkart J
and Shroyer NF: Atonal homolog 1 is required for growth and
differentiation effects of notch/γ-secretase inhibitors on normal
and cancerous intestinal epithelial cells. Gastroenterology.
139:918–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fre S, Huyghe M, Mourikis P, Robine S,
Louvard D and Artavanis-Tsakonas S: Notch signals control the fate
of immature progenitor cells in the intestine. Nature. 435:964–968.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Es JH, van Gijn ME, Riccio O, van den
Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ,
Radtke F and Clevers H: Notch/gamma-secretase inhibition turns
proliferative cells in intestinal crypts and adenomas into goblet
cells. Nature. 435:959–963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma A, Boulton M, Zhao B, Connon C, Cai J
and Albon J: A role for notch signaling in human corneal epithelial
cell differentiation and proliferation. Invest Ophthalmol Vis Sci.
48:3576–3585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blanpain C, Lowry WE, Pasolli HA and Fuchs
E: Canonical notch signaling functions as a commitment switch in
the epidermal lineage. Genes Dev. 20:3022–3035. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whitsett JA and Kalinichenko VV: Notch and
basal cells take center stage during airway epithelial
regeneration. Cell Stem Cell. 8:597–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouras T, Pal B, Vaillant F, Harburg G,
Asselin-Labat ML, Oakes SR, Lindeman GJ and Visvader JE: Notch
signaling regulates mammary stem cell function and luminal
cell-fate commitment. Cell Stem Cell. 3:429–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thompson H and Tucker AS: Dual origin of
the epithelium of the mammalian middle ear. Science. 339:1453–1456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuchiya K, Kim Y, Ondrey FG and Lin J:
Characterization of a temperature-sensitive mouse middle ear
epithelial cell line. Acta Otolaryngol. 125:823–829. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura Y, Hamajima Y, Komori M, Yokota
M, Suzuki M and Lin J: The role of atoh1 in mucous cell metaplasia.
Int J Otolaryngol. 438609:2012.
|
|
20
|
Nakamura Y, Komori M, Yamakawa K, Hamajima
Y, Suzuki M, Kim Y and Lin J: Math1, retinoic acid, and TNF-α
synergistically promote the differentiation of mucous cells in
mouse middle ear epithelial cells in vitro. Pediatr Res.
74:259–265. 2013. View Article : Google Scholar : PubMed/NCBI
|