Introduction
Anti-glomerular basement membrane (anti-GBM) disease
is a classic organ-specific autoimmune disease, mainly involving
the lungs and kidneys. It is characterized by circulating anti-GBM
antibodies and deposition of these antibodies in the renal GBM
(1,2). The incidence of anti-GBM disease is
estimated to be 1 case per million per year, but it accounts for
~20% of all cases of rapidly progressive or crescentic
glomerulonephritis, and the age distribution is bimodal, 20–30
years old and 60–70 years old (3,4). Renal
pathological analysis in anti-GBM patients has previously
demonstrated that immunoglobulin (Ig) G with or without C3 was
linearly deposited in the basement membrane, while light microscopy
mainly identified crescentic nephritis (5). Renal involvement in anti-GBM is more
severe when compared with other types of immune-mediated
glomerulonephritis. The majority of patients present progressive
renal failure, resulting in end-stage renal disease, and require
long-term treatment to improve the prognosis (3). According to the Kidney Disease -
Improving Global Outcomes Clinical Practice Guideline for
glomerulonephritis, corticosteroids and cyclophosphamide in
combination with plasmapheresis are recommended as the initial
immunosuppressive treatment for anti-GBM disease (6). The association between anti-GBM disease
and antineutrophil cytoplasmic antibody-associated vasculitis has
been well established in an increased number of studies (7–9).
However, only a limited number of studies have reported the
association between anti-GBM disease and IgA nephropathy or other
immune complex glomerulonephritis, such as Schönlein-Henoch purpura
and membranous glomerulonephritis (10–13).
The present study reports the case of a patient who
was diagnosed with anti-GBM disease with IgA nephropathy, and was
successfully treated with corticosteroids in combination with
mycophenolate mofetil (MMF).
Case report
A 50-year-old female was admitted to the Kidney
Institute of PLA (Changzheng Hospital, Shanghai, China) in June
2014 with complaints of gross hematuria and rapidly decreasing
kidney function for the past 2 weeks. The patient did not
experience any fever, skin rash and hemoptysis. Gross hematuria was
presented 2 weeks prior to admission, and laboratory tests
performed in a local hospital reported hematuria with a red blood
cell (RBC) count of 336 cells/µl (the normal level is <25
cells/µl), as well as decreased kidney function with a serum
creatinine (Scr) level of 157 µmol/l (the normal level is 61–116
μmol/l) and blood urea nitrogen level of 11.29 mmol/l (the normal
level is 2.9–7.2 mmol/l). Ultrasonography reveal that the kidneys
were normal in size and shape, with the exception of several small
kidney stones. The diagnosis of renal lithiasis was established at
the local hospital, without administration of any specific
treatment. However, the patient was advised to drink more water and
was prescribed a traditional Chinese medicine called Loosestrife
(Guangxi Wantong Pharmaceutical Co., Ltd., Nanning, China).
However, gross hematuria did not show any improvement 10 days
later, and further laboratory tests suggested a rapidly decreasing
kidney function with an Scr level of 220 µmol/l.
Subsequently, the patient was transferred to
Changzheng Hospital for further diagnosis and treatment. Physical
examination was not remarkable. Laboratory examination upon
admission included counting the number of RBCs per HP by observing
the urine under a DM2500 HP microscope (Leica, Heidelberg,
Germany). The results showed heavy hematuria [10–15 RBCs per
high-power field (HP); the normal level is <3 RBCs per HP], with
90% dysmorphic RBCs, mild urinary protein excretion (0.41 g/24 h;
the normal level is <0.15 g/24 h), and decreased kidney function
with an Scr level of 232 µmol/l. In addition, the hemoglobin (Hb)
level was 99 g/l (the normal level is 110–150 g/l) and the
erythrocyte sedimentation rate (ESR) was 141 mm/h (the normal level
is 0–20 mm/h), while a positive concentration serum monoclonal
anti-GBM antibody (isolated from bovine kidney as an antigen) was
reported (258.3 EU/ml; cat no. EA 1251–9601 G, EUROIMMUN Medical
Laboratory Diagnostics Co., Ltd, Luebeck, Germany). Serologic tests
for antinuclear, anti-double-strand DNA and anti-neutrophil
cytoplasmic antibodies were all negative. In addition, the serum
levels of IgA, IgG, IgM, complement C3 and C4, and total hemolytic
complement (CH50) were within the normal ranges. Chest X-ray
radiography (Ysio 50019, Siemens, Munich, Germany) was also normal,
while ultrasonography (ACUSON S2000 Ultrasound system, Siemens,
Chicago, IL, USA) showed normal shape of kidneys without kidney
stones, and the sizes of the right and left kidneys were 102×51 and
101×51 mm (the normal level is 90–110×40–60 mm), respectively. This
can be due to the previous diagnosis being wrong or by the removal
of the kidney stones with the previous treatment. Previous medical
history of the patient revealed that the Scr was 57 µmol/l at 6
months prior to admission, while the patient had a 20-year history
of recurrent tonsillitis. Based on the aforementioned findings,
rapidly progressive glomerulonephritis was diagnosed and renal
biopsy was immediately performed.
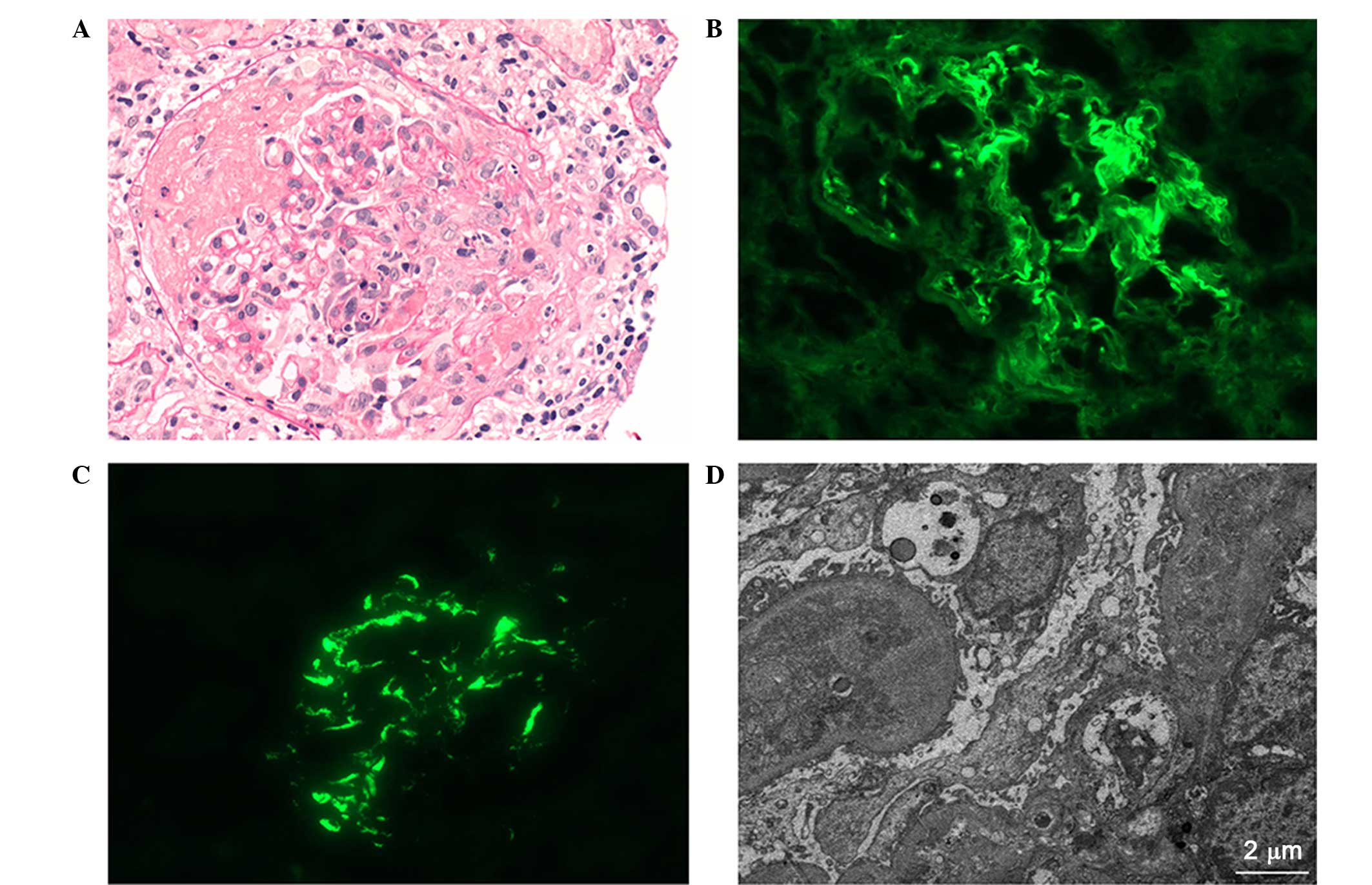
Light microscopy (DM2500, Leica) identified 18
glomeruli with 12 cellular crescents and 4 cellulofibrous
crescents, and the glomeruli presented capillary occlusion with
segmental fibrinoid necrosis (Fig.
1A). Numerous necrotic tubular epithelial cells and diffused
infiltration of inflammatory cells indicated severe injury to the
tubulointerstitium. The small arterial walls were thickened without
significant evidence of necrosis or hyaline degeneration.
Immunofluorescent examination (DM2500, Leica) revealed that strong
IgG (3+) linear deposition in the capillary loop (Fig. 1B), along with mesangial staining for
IgA (4+; Fig. 1C), IgM (1+) and C3
(2+). The staining intensity was scored as follows: 0+, negative;
1+, mild; 2+, moderate or 3+, strong (8). Furthermore, electron microscopy
(Hitachi 7700, Tokyo, Japan) demonstrated electron-dense mass
deposition in the mesangium (Fig.
1D). Based on the aforementioned findings, the diagnosis of
anti-GBM disease with IgA nephropathy was confirmed.
The patient was immediately administered 500 mg/day
intravenous methylprednisolone (Pfizer Ltd., Kent, UK) for 3 days,
which was then changed to 60 mg/day oral prednisone (Shanghai Xinyi
Pharmaceutical Co., Ltd, Shanghai, China); however, the dosage was
quickly decreased to 20 mg/day two weeks later due to the side
effect of hyperglycemia. Treatment with cyclophosphamide and
plasmapheresis were then recommended, however this treatment was
not accepted by the patient due to the possibility of side effects.
Therefore, MMF (0.5 g, twice a day; Roche Pharmaceutical Co., Ltd,
Shanghai, China) in combination with prednisone (20 mg/day) were
administered to the patient and the dose of prednisone was
decreased by 5 mg every month. Four months later the prednisone
treatment stopped. One month later gross hematuria was gradually
relieved following MMF treatment. Further laboratory examinations
after 3 months of MMF treatment showed the following results:
Hematuria with 5–6 RBCs/HP; Scr level, 102 µmol/l; Hb, 112 g/l;
ESR, 22 mm/h; positivity for anti-GBM antibody (176.0 EU/ml). After
6 months, the Scr level was found to be 100 µmol/l and the ESR was
23 mm/h, showing no significant difference as compared with these
levels 3 months before, whereas the Hb level was increased to 129
g/l and the test for anti-GBM antibody was negative. The total MMF
treatment was 15 months in total. Patient informed consent was
obtained prior to the study.
In the last observation in February 2016 the MMF
treatment had ceased and the laboratory examinations demonstrated
the following results: Urine test showed 4 5 RBCs/HP; Scr level, 74
μmol/l; Hb, 132 g/l; ESR, 4 mm/h and the test for anti GBM antibody
was negative.
Discussion
In the present study, a rare case of anti-GBM
disease with IgA nephropathy was reported. Previous animal studies
have demonstrated that complement activation through the classical
complement pathway is one of the major mechanisms underlying
glomerular injury in anti-GBM disease (14,15).
According to this proposed theory, complement C1q may serve an
important role in the underlying mechanism of anti-GBM disease.
However, a number of studies have reported that complement C1q
deposition was seldom present along the GBM in the renal tissue of
patients, and circulating and urinary levels of C1q were not
significantly correlated with the severity of kidney injury
(8,16). Hu et al (9) further found that, even in patients with
C1q deposition, no association of C1q with the disease activity and
severity was identified, suggesting that the classical complement
pathway may not serve a pathogenic role in the development of
kidney injury in human anti-GBM disease. In the present case,
immunofluorescence examination did not identify any C1q deposition,
which is consistent with previous findings (9). However, the diagnosis of anti-GBM
disease remains appropriate in the present case.
As the progression of anti-GBM disease can be rapid
and the outcome is greatly associated with the severity at
presentation, treatment must be initiated immediately. It is widely
recognized that corticosteroids and cyclophosphamide in combination
with plasmapheresis is the preferred standard treatment
administered as the initial immunosuppressive therapy for anti-GBM
disease (6). Notably, in the present
study, the patient declined the standard treatment for anti-GBM
disease, however a relatively improved therapeutic outcome with
less side-effects was achieved compared with treatment with
corticosteroids in combination with MMF. To date, there is little
clinical evidence to recommend MMF for the anti-GBM disease
therapy, although previous animal experiments in a rat model of
anti-GBM disease have suggested the preventive effect of MMF
against glomerular crescent formation (17). The case reported in the present study
may provide useful evidence concerning the effect of MMF for the
clinical treatment of anti-GBM disease.
As mentioned earlier, strong IgA deposition in the
renal tissue may have a profound impact on the pathogenesis of this
rare disease (11,18). The findings from randomized
controlled trials (RCTs) investigating MMF administration in IgA
nephropathy are conflicting, since favorable outcomes of MMF have
only been demonstrated in studies conducted in China (19–22).
This is due to the fact that Asian IgG nephropathy patients may be
more sensitive to MMF treatment, and the variation of ethnicity may
be partly the reason for this observation. Based on the updated
treatment options, the use of MMF is recommended for selected
patients in whom steroid therapy has failed or who are intolerant
to steroid therapy in IgA nephropathy (23). Nevertheless, MMF may prove to be a
novel treatment for this rare disease, although the exact
underlying mechanism requires further in-depth investigation.
In conclusion, the present study reported a case
with anti-GBM disease and IgA nephropathy, in which the patient
responded well to corticosteroids plus MMF treatment. The
underlying mechanism renders further in-depth examination.
References
|
1
|
Lahmer T and Heemann U: Anti-glomerular
basement membrane antibody disease: a rare autoimmune disorder
affecting the kidney and the lung. Autoimmun Rev. 12:169–173. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pedchenko V, Bondar O, Fogo AB, Vanacore
R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB,
Neilson EG, et al: Molecular architecture of the Goodpasture
autoantigen in anti-GBM nephritis. N Engl J Med. 363:343–354. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang W, McDonald SP, Hawley CM, Badve SV,
Boudville NC, Brown FG, Clayton PA, Campbell SB, de Zoysa JR and
Johnson DW: Anti-glomerular basement membrane antibody disease is
an uncommon cause of end-stage renal disease. Kidney Int.
83:503–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui Z and Zhao MH: Advances in human
antiglomerular basement membrane disease. Nat Rev Nephrol.
7:697–705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jennette JC and Nickeleit V:
Anti-glomerular basement membrane glomerulonephritis and
Goodpasture's syndrome. Heptinstall's Pathology of the Kidney.
Jennette JC, Olson JL, Schwartz MM and Silva FG: (6th). Lippincott
Williams and Wilkins. (Philadelphia, PA). 615–641. 2007.
|
|
6
|
Kidney Disease - Improving Global Outcomes
Glomerulonephritis Work Group: KDIGO Clinical Practice Guideline
for Glomerulonephritis. Kidney Inter Suppl 2. 139–274. 2012.
|
|
7
|
Hellmark T, Niles JL, Collins AB,
McCluskey RT and Brunmark C: Comparison of anti-GBM antibodies in
sera with or without ANCA. J Am Soc Nephrol. 8:376–385.
1997.PubMed/NCBI
|
|
8
|
Fischer EG and Lager DJ: Anti-glomerular
basement membrane glomerulonephritis: A morphologic study of 80
cases. Am J Clin Pathol. 125:445–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu SY, Jia XY, Yang XW, Yu F, Cui Z and
Zhao MH: Glomerular C1q deposition and serum anti-C1q antibodies in
anti-glomerular basement membrane disease. BMC Immunol. 14:422013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao B, Li M, Xia W, Wen Y and Qu Z:
Rapidly progressive glomerulonephritis due to anti-glomerular
basement membrane disease accompanied by IgA nephropathy: A case
report. Clin Nephrol. 81:138–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang A, Wang Y, Wang G, Zhou Z, Xun Z and
Tan X: Mesangial IgA deposits indicate pathogenesis of
anti-glomerular basement membrane disease. Mol Med Rep.
5:1212–1214. 2012.PubMed/NCBI
|
|
12
|
Carreras L, Poveda R, Bas J, Mestre M,
Rama I and Carrera M: Goodpasture syndrome during the course of a
Schönlein-Henoch purpura. Am J Kidney Dis. 39:E212002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kielstein JT, Helmchen U, Netzer KO, Weber
M, Haller H and Floege J: Conversion of Goodpasture's syndrome into
membranous glomerulonephritis. Nephrol Dial Transplant.
16:2082–2085. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otten MA, Groeneveld TW, Flierman R,
Rastaldi MP, Trouw LA, Faber-Krol MC, Visser A, Essers MC,
Claassens J, Verbeek JS, et al: Both complement and IgG fc
receptors are required for development of attenuated antiglomerular
basement membrane nephritis in mice. J Immunol. 183:3980–3988.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheerin NS, Springall T, Carroll MC,
Hartley B and Sacks SH: Protection against anti-glomerular basement
membrane (GBM)-mediated nephritis in C3-and C4-deficient mice. Clin
Exp Immunol. 110:403–409. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma R, Cui Z, Liao YH and Zhao MH:
Complement activation contributes to the injury and outcome of
kidney in human anti-glomerular basement membrane disease. J Clin
Immunol. 33:172–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeda S, Takahashi M, Sado Y, Takeuchi K,
Hakamata Y, Shimizu H, Kaneko T, Yamamoto H, Ito C, Ookawara S, et
al: Prevention of glomerular crescent formation in
glomerulonephritis by mycophenolate mofetil in rats. Nephrol Dial
Transplant. 19:2228–2236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trpkov K, Abdulkareem F, Jim K and Solez
K: Recurrence of anti-GBM antibody disease twelve years after
transplantation associated with de novo IgA nephropathy. Clin
Nephrol. 49:124–128. 1998.PubMed/NCBI
|
|
19
|
Maes BD, Oyen R, Claes K, Evenepoel P,
Kuypers D, Vanwalleghem J, Van Damme B and Vanrenterghem YF:
Mycophenolate mofetil in IgA nephropathy: Results of a 3-year
prospective placebo-controlled randomized study. Kidney Int.
65:1842–1849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frisch G, Lin J, Rosenstock J, Markowitz
G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A and
Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with
moderately advanced IgA nephropathy: A double-blind randomized
controlled trial. Nephrol Dial Transplant. 20:2139–2145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang S, Leung JC, Chan LY, Lui YH, Tang
CS, Kan CH, Ho YW and Lai KN: Mycophenolate mofetil alleviates
persistent proteinuria in IgA nephropathy. Kidney Int. 68:802–812.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang SC, Tang AW, Wong SS, Leung JC, Ho YW
and Lai KN: Long-term study of mycophenolate mofetil treatment in
IgA nephropathy. Kidney Int. 77:543–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hogan J, Mohan P and Appel GB: Diagnostic
tests and treatment options in glomerular disease: 2014 update. Am
J Kidney Dis. 63:656–666. 2014. View Article : Google Scholar : PubMed/NCBI
|