Introduction
Renal interstitial fibrosis is the result of
end-stage acute and chronic kidney diseases (1). Renal interstitial fibrosis is
characterized by the activation of interstitial fibroblasts and the
accumulation of extracellular matrix (ECM) components, including
fibronectin (FN) and collagen (2).
Renal interstitial fibroblasts produce ECM during fibrosis, and ECM
is also produced by epithelial cells, endothelial cells, pericytes,
and bone marrow stem cells (3).
Epithelial-mesenchymal transition (EMT) is an important signaling
pathway in the progression of renal interstitial fibrosis (4), which represents the loss of epithelial
cells and their adhesion molecules, including E-cadherin, and an
increase in mesenchymal cells and markers, such as α-smooth muscle
actin (SMA) (5). Transforming growth
factor (TGF)-β1 is an important mediator that promotes EMT via
myofibroblast development by inducing the expression of α-SMA via
various cytokines (6). The
downstream Smad signaling pathway is activated by TGF-β1 during
renal fibrosis, which induces renal scarring (7). Therefore, manipulating downstream
TGF-β1 signaling represents a potent therapeutic target for the
reversal of EMT and renal interstitial fibrosis.
Astragalus membranaceus (AM) is a traditional
Chinese herbal medicine which is used to treat various ailments,
including common colds, fatigue, anorexia, and cardiac diseases
(8,9). Previous studies have demonstrated the
anti-fibrotic effects of AM on pulmonary fibrosis (10), liver fibrosis (11), and nephropathy (12) by inhibiting the activation of the
TGF-β1 signaling pathway. In the present study, the effects of AM
on renal tubular EMT were examined in NRK-52E cells and a mouse
model of unilateral ureteral obstruction (UUO) in order to
elucidate whether AM can ameliorate renal fibrosis via the
inhibition of EMT.
Materials and methods
Animals and experimental design
A total of 30 male C57BL6 mice (age, 8 weeks;
weight, 18–20 g) were purchased from Wuhan University (Wuhan,
China) and housed at the Experimental Animal Center of Wuhan
University. The mice were maintained at 25°C under a 12-h
light/dark cycle, with ad libitum access to food and water.
The purified natural product AM was obtained from Shanghai Yuanye
Biotechnology Co., Ltd. (Shanghai, China). In order to establish a
murine model of UUO, the mice were anesthetized with 1.5 % sodium
pentobarbital (50 mg/kg; Shanghai Haling Biological Technology Co.,
Ltd., Shanghai, China) and a left lateral incision was made on the
back of the mice to expose the kidney. The left ureter was ligated
with 4–0 silk sutures at the junction between the renal pelvis and
ureter and the ligature position was consistent. Following
completion, the incision was closed in layers. Sham-group mice
received the same incision and closure procedures without ligation.
UUO mice were treated by intraperitoneal injection of AM (100, 200,
and 400 mg/kg/day) (n=6 per group). Mice in the sham were
administered equal volumes of sterile saline (n=6 per group). Mice
were sacrificed by cervical dislocation following anesthetization
on day 7 post-surgery, and the obstructed kidneys were harvested.
Kidney samples were fixed in 4% buffered paraformaldehyde (Guge
Biotechnology Co., Ltd., Wuhan, China) and were subsequently
embedded in paraffin (Guge Biotechnology Co., Ltd.) for
histological and immunohistochemical examination. The remaining
kidneys were snap-frozen in liquid nitrogen and stored at −80°C for
protein extraction.
Cell culture and experimental
design
NRK-52E cells were obtained from Boster
Bioengineering Co., Ltd. (Wuhan, China) and cultured in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum
(GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a
humidified atmosphere containing 5% CO2. A total of
1.5×106 cells/ml cells were transferred to 6-well plates
and were divided into the following five treatment groups: Control;
5 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN, USA); 5 ng/ml
TGF-β1 + 10 µg/ml AM; 5 ng/ml TGF-β1 + 20 µg/ml AM; and 5 ng/ml
TGF-β1 + 40 µg/ml AM.
Immunohistochemistry staining
Kidney sections (5-µm) were prepared using a
microtome (RM3225; Leica Geosystems, Milton Keynes, UK). The tissue
sections were placed on slides, deparaffinized in xylene (Boster
Bioengineering Co., Ltd.) and hydrated in graded ethanol prior to
treatment with 3% hydrogen peroxide solution (Guge Biotechnology
Co., Ltd.) for 15 min at room temperature. Following three washes
with phosphate-buffered saline (PBS), the antigens were retrieved
by boiling the tissue sections in Tris-ethylenediaminetetraacetic
acid (EDTA) buffer (12.1 g Tris base; 3.7 g EDTA; 500 ml
H2O; Boster Bioengineering Co., Ltd.) for 15 min in a
microwave oven on high power. Following cooling, the tissue
sections were washed three times with PBS and incubated with the
following primary antibodies at 37°C for 1 h: Mouse anti-α-SMA
monoclonal antibody (1:100; BM0002; Boster Bioengineering Co.,
Ltd.), mouse anti-E-cadherin monoclonal antibody (1:50; 610181; BD
Biosciences, Franklin Lakes, NJ, USA), rabbit anti-collagen I
polyclonal antibody (1:200; BA0326; Boster Bioengineering Co.,
Ltd.), and rabbit anti-FN polyclonal antibody (1:200; ab2413;
Abcam, Cambridge, UK). Subsequently, the tissue sections were
washed three times with PBS and incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit/mouse polyclonal
secondary antibody (1:100; BA1003; Boster Bioengineering Co., Ltd.)
for 10 min at 37°C. The sections were treated with
3,3′-diaminobenzidine (Beyotime Institute of Biotechnology,
Shanghai, China) to develop a color reaction, and the reaction was
subsequently terminated by washing twice with distilled water. The
tissue sections were finally counterstained with hematoxylin (Guge
Biotechnology Co., Ltd.).
Immunofluorescence staining
Cells were fixed in 4% paraformaldehyde for 30 min
at room temperature and subsequently permeabilized with 0.1% Triton
X-100 (Boster Bioengineering Co., Ltd.) in PBS for 5 min prior to
rinsing three times with PBS for 5 min. Following blocking in 5%
bovine serum albumin (BSA; Guge Biotechnology Co., Ltd.) and 1%
Triton X-100/PBS for 30 min at room temperature, the cells were
incubated with primary antibodies targeting α-SMA (1:100),
E-cadherin (1:50) and FN (1:200) overnight at 4°C. The cells were
then cultured with cyanine 3-goat anti-mouse immunoglobulin G (IgG;
1:100; GB21303) and fluorescein isothiocyanate-goat anti-rabbit IgG
(1:100; GB22303; both Boster Bioengineering Co., Ltd.) for 1 h. The
cell nuclei were stained with 4′6′-diamino-2-phenylindole
dihydrochloride (Beyotime Institute of Biotechnology).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from renal cortex samples and
NRK-52E cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. RNA underwent purification with DNase
(Qiagen, Inc., Valencia, CA, USA), after which the concentration of
RNA was measured using a spectrophotometer (Nanodrop™ 2000; Thermo
Fisher Scientific, Inc.) at 260 nm, and the purity was assessed by
determining the absorbance ratio at A260/A280. Subsequently, RNA
(20 µl) was reverse transcribed into cDNA using the RevertAid First
Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher
Scientific, Inc.). qPCR was performed using the iQ SYBR Green
Supermix reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and 7.5 µM each of forward and reverse primers on an T100 thermal
cycler (Bio-Rad Laboratories, Inc.). Primer sequences for the renal
cortex samples were as follows: E-cadherin forward,
5′-GTCAAACGGCATCTAAAGC-3′, and reverse,
5′-CAAAGACCTCCTGGATAAACT-3′; α-SMA, forward
5′-ACTGGGACGACATGGAAAAG-3′, and reverse,
5′-CATCTCCAGAGTCCAGCACA-3′; collagen I forward,
5′-GAGCGGAGAGTACTGGATCG-3′, and reverse,
5′-TACTCGAACGGGAATCCATC-3′; and FN forward,
5′-CCATTGCAAATCGCTGCCAT-3′, and reverse,
5′-AACATTTCTCAGCTATTGGCTT-3′. In the NRK-52E cells, the primer
sequences were as follows: E-cadherin forward,
5′-AACGAGGGCATTCTGAAAACA-3′, and reverse,
5′-CACTGTCACGTGCAGAATGTACTG-3′; α-SMA forward,
5′-GACCCTGAAGTATCCGATAGAACA-3′, and reverse,
5′-CACGCGAAGCTCGTTATAGAAG-3′; and FN forward,
5′-CATGGCTTTAGGCGAACCA-3′, and reverse,
5′-CATCTACATTCGGCAGGTATGG-3′ (Sangon Biotech Co., Ltd., Shanghai,
China). In both cases, GAPDH was used as the reference gene and had
the following primer sequences: Forward 5′-AGTGGCAAAGTGGAGATT-3′
and reverse, 5′-GTGGAGTCATACTGGAACA-3′ (Sangon Biotech Co., Ltd.).
The PCR cycling conditions were as follows: Predenaturation at 95°C
for 10 min, 40 cycles at 95°C for 15 sec, 60°C for 1 min and 72°C
for 20 sec, and a final extension at 60°C for 5 min. The
specificity of the qPCR was confirmed by 1% agarose gel
electrophoresis and melting curve analysis. The relative mRNA
expression levels were determined using the 2−ΔΔCq
method (13).
Western blot analysis
Kidney and cell culture samples were resuspended in
0.4 ml sodium dodecyl sulfate (SDS) lysis buffer containing 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% sodium deoxycholate,
1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA and
5 µg/ml leupeptin (Beyotime Institute of Biotechnology). Protein
concentration was determined using a BCA protein assay kit (cat.
no. p0012s; Beyotime Institute of Biotechnology) and 30 µg total
protein/well was subsequently loaded and separated by 10%
SDS-polyacrylamide gel electrophoresis. The gels were
electroblotted onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA) and blocked with 5% milk for 1 h
prior to incubation with rabbit anti-Smad2/3 (1:1,000; sc-11769)
and anti-TFG-β1 (1:1,000; sc-146) polyclonal antibodies (both Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), and rabbit anti-GAPDH
monoclonal antibody (1:1,000; 5174; Cell Signaling Technology,
Inc., Danvers, MA, USA), overnight at 4°C. Following washing three
times with Tris-buffered saline containing Tween-20, the membranes
were incubated with HRP-conjugated goat anti-rabbit/mouse
polyclonal secondary antibody (1:100; G1201; Guge Biotechnology
Co., Ltd.) for 1 h at room temperature. Bound antibodies were
detected using an enhanced chemiluminescence system (RPN998; GE
Healthcare Life Sciences, Chalfont, UK). Band signals were detected
using an Odyssey Infrared Imaging system Model 9120 (LI-COR
Biotechnology, Lincoln, NE, USA), and band intensities were
analyzed using Quantity-One software (Bio-Rad Laboratories, Inc.).
Relative protein expression levels were normalized to GAPDH and
compared with a control.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Data were analyzed using one-way
analysis of variance and Fisher's least significant difference
t-test was used for multiple comparisons of two sample means. Data
were presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
AM upregulates α-SMA and E-cadherin
expression and decreases ECM in the kidneys of UUO mice
The effects of AM on the expression levels of α-SMA,
E-cadherin, and ECM were investigated during obstructive
nephropathy, which is a well-characterized and widely-used model of
renal interstitial fibrosis. Immunohistochemistry staining
demonstrated that the protein expression of α-SMA, collagen I, and
FN in the renal interstitium were significantly elevated in the
mouse kidney following obstructive injury, whereas E-cadherin
protein expression decreased. AM markedly reversed the changes in
the expression levels of the markers (Fig. 1).
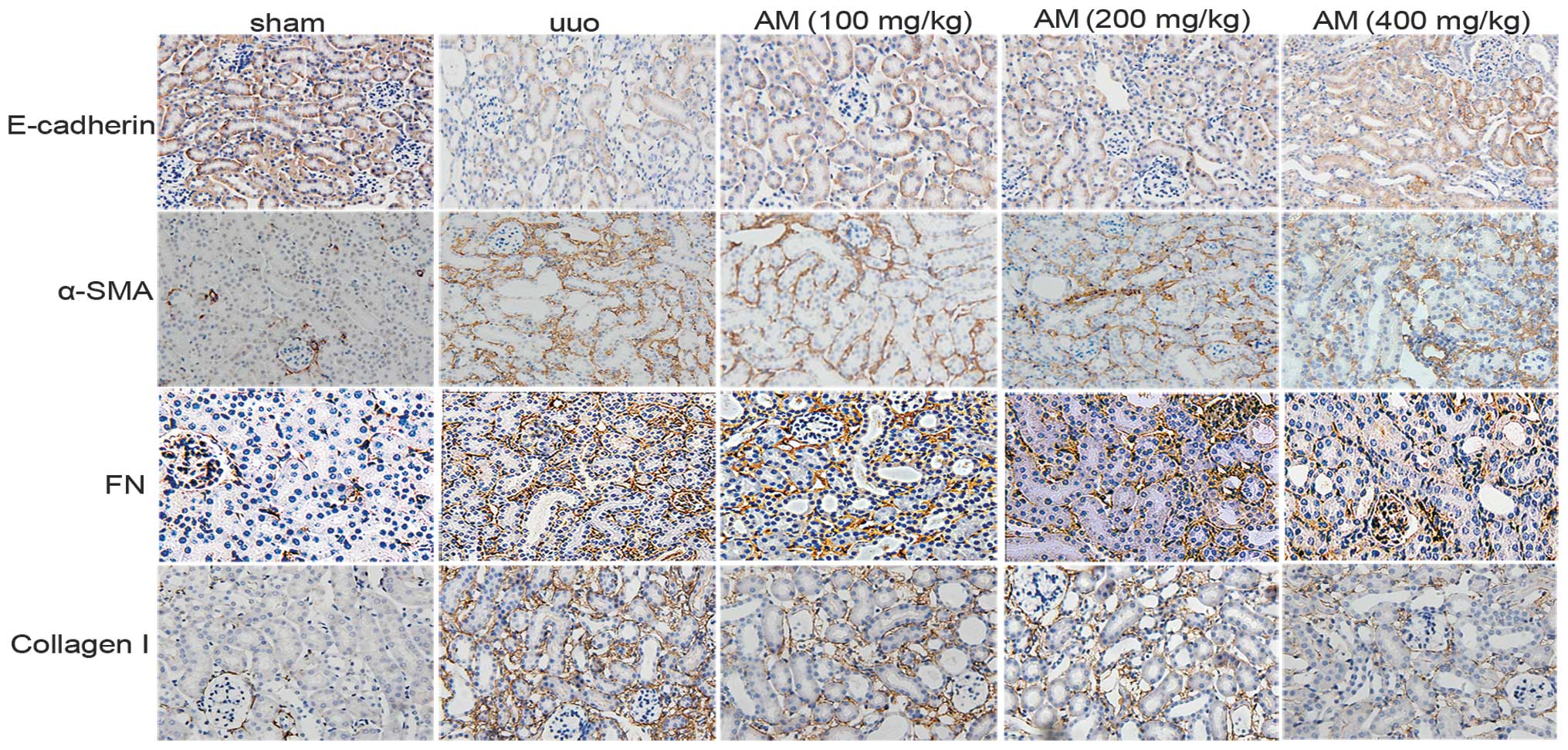 | Figure 1.Renal paraffin sections were
immunostained with antibodies targeting E-cadherin, α-SMA, FN, and
collagen I to determine epithelial-mesenchymal transition
(magnification, ×200). α-SMA, FN, and collagen I were positively
expressed in interstitial cells, whereas E-cadherin was detected in
epithelial cells. In the sham group, no fibrotic septa or other
lesions were detected in α-SMA, FN, and collagen I-positive cells,
whereas numerous stained epithelial cells were detected. In the UUO
model group, numerous interstitial cell fibers were detected, and
epithelial staining decreased. In the AM-treated group, α-SMA, FN
and collagen I expression decreased, whereas epithelial cells
increased. SMA, smooth muscle actin; FN, fibronectin; UUO,
unilateral ureteral obstruction; AM, Astragalus
membranaceus. |
The mRNA expression levels of α-SMA significantly
increased in the UUO group (P<0.05), whereas E-cadherin
expression levels significantly decreased (P<0.05). As compared
with the UUO group, downregulated α-SMA, collagen I, and FN mRNA
expression levels were detected in the AM groups, whereas
E-cadherin mRNA expression levels were upregulated. The difference
between the AM and UUO groups was determined to be significant
(P<0.05), and the three AM treatment groups were significantly
different from the UUO group (P<0.05; Fig. 2). These data suggest that AM may be a
potent agent for the reversal of renal tubular EMT in UUO mice.
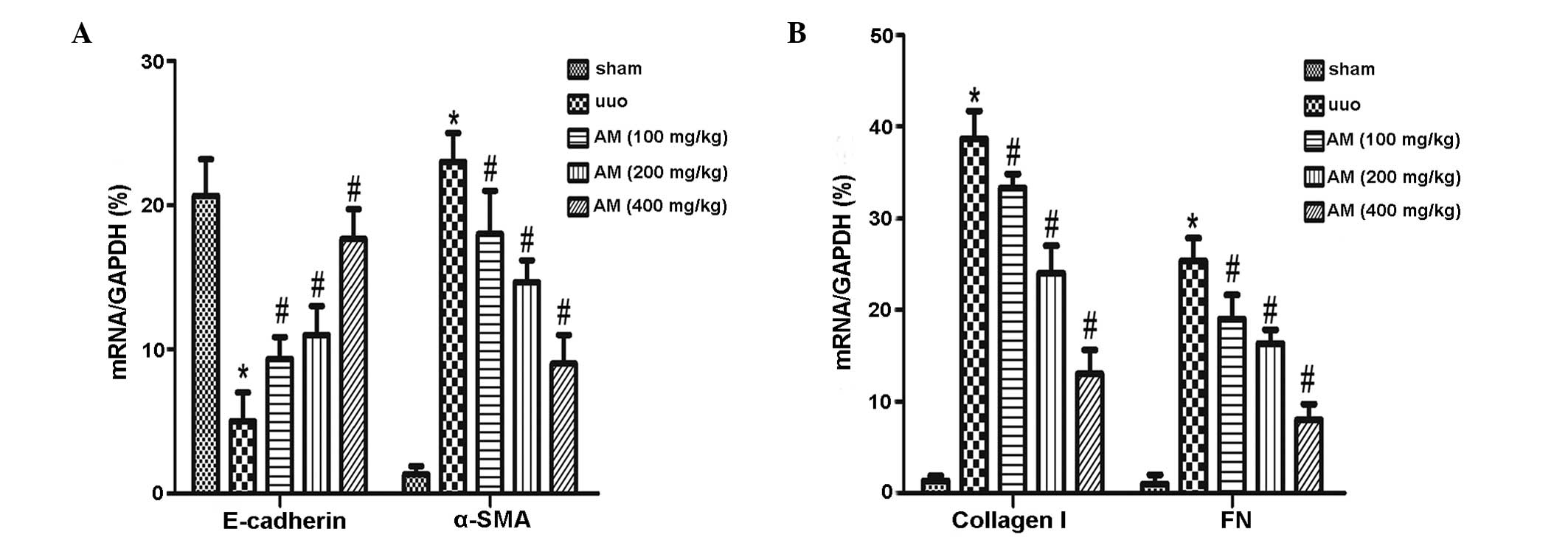 | Figure 2.Analysis of the epithelial-mesenchymal
transition in vitro. Reverse transcription-quantitative
polymerase chain reaction was used to analyze the mRNA expression
levels of (A) E-cadherin and α-SMA, and (B) collagen I and FN in
the sham, UUO, and UUO treated with AM (100, 200 or 400 mg/kg body
weight) groups. Relative mRNA expression levels were quantified by
densitometric analysis. Data are presented as the mean ± standard
deviation. *P<0.05, vs. the sham group and
#P<0.05, vs. the UUO group. SMA, smooth muscle actin;
FN, fibronectin; UUO, unilateral ureteral obstruction; AM,
Astragalus membranaceus. |
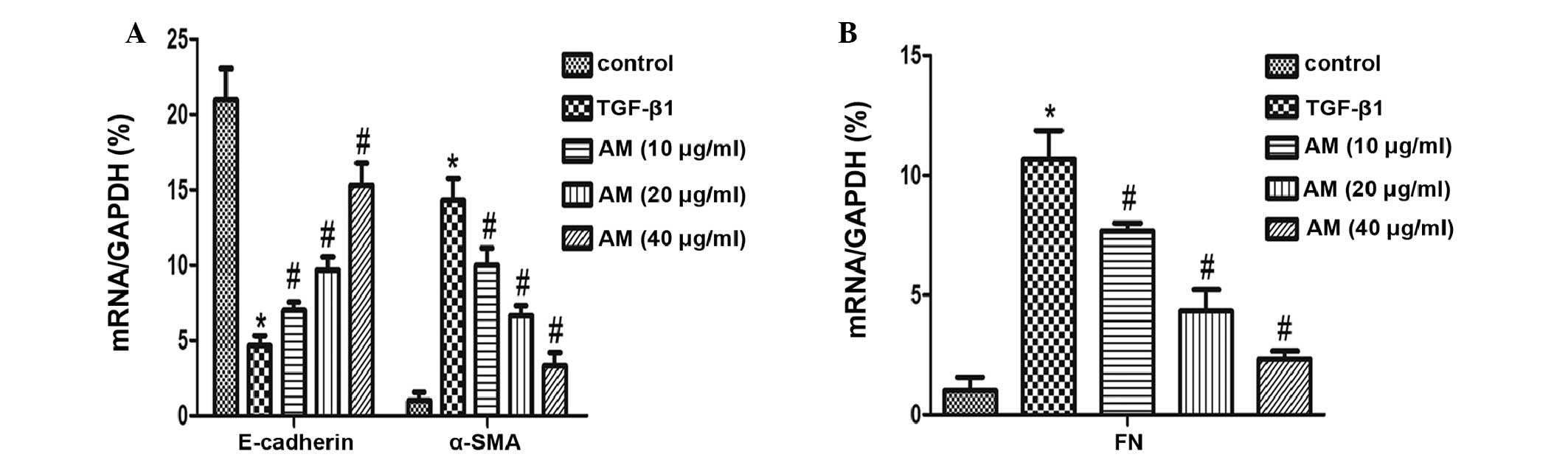
AM upregulates α-SMA and E-cadherin
and decreases ECM in NRK-52E cells stimulated with TGF-β1
Immunofluorescence analysis of the TGF-β1-stimulated
NRK-52E cells demonstrated that the protein expression of α-SMA and
FN increased, whereas E-cadherin protein expression decreased. AM
administration markedly decreased α-SMA protein expression and
increased E-cadherin protein expression (Fig. 3), in a dose-dependent manner;
therefore, the greatest AM concentration (40 µg/ml) markedly
inhibited these changes. These results were concordant with the
RT-qPCR analysis, which demonstrated reduced α-SMA expression
levels and increased E-cadherin expression levels following AM
administration, as compared with the results following TGF-β1
treatment without AM intervention (P<0.05; Fig. 4).
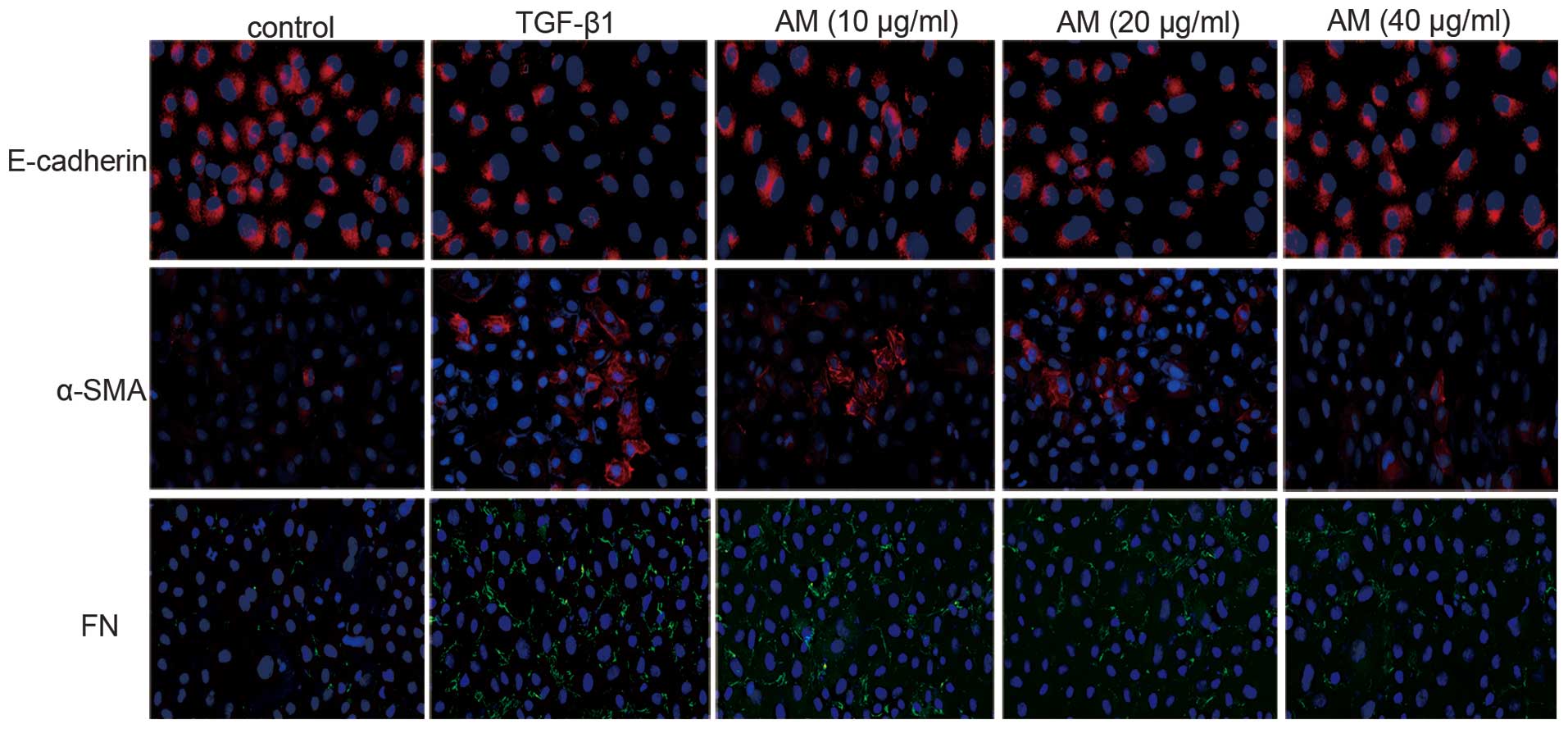 | Figure 3.Murine renal proximal tubule NRK-52E
cells were stained with E-cadherin, α-SMA, and FN to determine
epithelial-mesenchymal transition (magnification, ×200). In the
control group, no α-SMA or FN-positive cells were detected, whereas
numerous E-cadherin-positive cells were detected. E-cadherin
expression decreased in the TGF-β1 group, whereas the number of
α-SMA and FN-positive cells increased, as compared with the control
group. Groups treated with 10, 20, and 40 µg/ml AM exhibited
reverse effects, as compared with the TGF-β1 group. SMA, smooth
muscle actin; FN, fibronectin; TGF, tumour growth factor; AM,
Astragalus membranaceus. |
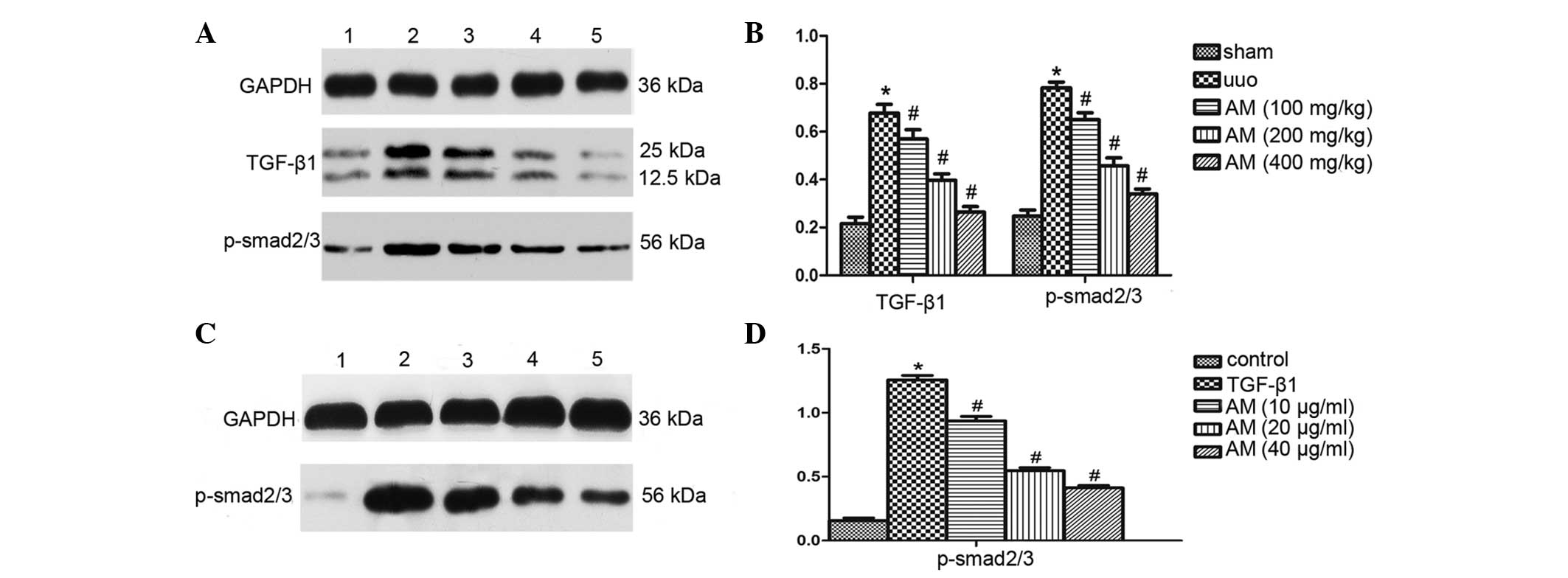
AM inhibits the TGF-β1 signaling
pathway and the phosphorylation of Smad2/3
Western blot analysis demonstrated that ureteral
obstruction markedly increased the protein expression levels of
TGF-β1 and the phosphorylation of Smad2/3 in the UUO group, as
compared with the sham group (P<0.05; Fig. 5A and B). As compared with the UUO
group, the AM treatment groups exhibited significantly reduced
renal TGF-β1 and phosphorylated Smad2/3 expression levels
(P<0.05). Furthermore, western blot analysis demonstrated that
Smad2/3 protein expression levels were significantly increased in
the TGF-β1-stimulated NRK-52E cells, as compared with the control
group (P<0.05). Treatment with AM significantly downregulated
p-Smad2/3 protein expression levels (Fig. 5C and D). These data suggested that AM
may effectively inhibit the expression and phosphorylation of the
Smad2/3 protein in NRK-52E cells.
Discussion
The treatment efficacy of renal fibrosis remains
unclear, however inhibition of EMT may serve as a novel therapeutic
strategy to disrupt the process of fibrosis. The present study
demonstrated that AM is capable of improving fibrosis by inhibiting
expression level alterations associated with EMT in vivo and
in vitro and investigating the underlying mechanisms, which
may involve the TGF-β1/Smad signaling pathway.
Renal interstitial fibrosis is a common pathological
alteration, which is observed in various acute and chronic
end-stage kidney diseases (14). The
pathological basis of fibrosis is the production of ECM from
myofibroblasts and it has been demonstrated that myofibroblasts
predominantly originate through EMT (15). EMT is characterized by the loss of
epithelial cells and the acquisition of mesenchymal markers due to
excessive exposure to profibrotic cytokines (16). TGF-β1 is a well-characterized
fibrogenic cytokine which is associated with renal diseases and has
a key role in EMT (17,18). In the present study, TGF-β1 was used
to induce NRK-52E epithelial cell transformation into myoblasts.
The results demonstrated that TGF-β1 is capable of successfully
inducing EMT, with decreased expression of the epithelial phenotype
detected, via E-cadherin, accompanied by a dose-dependent increase
in α-SMA expression levels.
Due to the important role of EMT in renal fibrosis,
any therapeutic strategy that targets the EMT may improve fibrosis
(19). AM has been used in
traditional Chinese medicine for thousands of years, where it is
used to protect and support the immune system, prevent colds and
upper respiratory infections, lower blood pressure, treat heart
diseases and diabetes, and protect the liver (19). Previous studies have demonstrated the
anti-fibrotic effects of AM by inhibiting the activation of the
TGF-β1 signaling pathway (20,21).
Furthermore, the potential anti-inflammatory effects of AM have
been demonstrated in various in vitro studies (22,23).
Notably, previous studies demonstrated that AM was beneficial in
reducing renal tubulointerstitial fibrosis (24,25);
however, the underlying pharmacological mechanisms have yet to be
elucidated.
The present study demonstrated that treatment with
AM may improve TGF-β1-induced renal fibrosis, and Smad2/3 signaling
pathway activation may be an important factor in this process,
since TGF-β1 signaling pathways are activated by receptor
phosphorylation prior to subsequent phosphorylation. In particular,
R-Smad proteins are phosphorylated by TGF-β type I receptors,
leading to the activation of a series of downstream events
(26,27). The results of the present in
vitro experiment demonstrated that TGF-β1 stimulation of
NRK-52E cells increased the expression levels of the Smad2/3
protein, which were subsequently reduced by co-treatment with AM.
This suggested that AM inhibits the TGF-β1/Smad signaling pathway
via the phosphorylation of Smad proteins, which have an important
role in the activation of the TGF-β1 signaling pathway.
In conclusion, the results of the present study
suggested that AM may inhibit renal fibrosis; as indicated by the
antagonized tubular EMT and ECM accumulation via TGF-β/Smad2/3.
Therefore, AM may be a therapeutic agent for the prevention of
renal fibrosis.
References
|
1
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 18:684–696. 2011. View Article : Google Scholar
|
|
2
|
Liu Y: Renal fibrosis: New insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falke LL, Gholizadeh S, Goldschmeding R,
Kok RJ and Nguyen TQ: Diverse origins of the myofibroblast -
implications for kidney fibrosis. Nat Rev Nephrol. 11:233–244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberg M and Duffield JS: Resolved: EMT
produces fibroblasts in the kidney. J Am Soc Nephrol. 21:1247–1253.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zavadil J, Cermak L, Nieves NS and
Böttinger EP: Integration of TGF-β/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO 10.
23:1155–1165. 2004. View Article : Google Scholar
|
|
7
|
López-Hernández FJ and López-Novoa JM:
Role of TGF-β in chronic kidney disease: An integration of tubular,
glomerular and vascular effects. Cell Tissue Res. 347:141–154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Na D, Liu FN, Miao ZF, Du ZM and Xu HM:
Astragalus extract inhibits destruction of gastric cancer cells to
mesothelial cells by anti-apoptosis. World J Gastroenterol.
15:570–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan C, Pan X, Gong Y, Xia A, Wu G, Tang J
and Han X: Effects of Astragalus polysaccharides (APS) on the
expression of immune response genes in head kidney, gill and spleen
of the common carp, Cyprinus carpio L. Int Immunopharmacol.
8:51–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao J, Feng LJ, Huang Y, Li P, Xu DJ, Li J
and Wu Q: Total glucosides of Danggui Buxue Tang attenuates
bleomycin-induced pulmonary fibrosis via inhibition of
extracellular matrix remodelling. J Pharm Pharmacol. 64:811–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du JX, Sun MY, Du GL, Li FH, Liu C, Mu YP,
Chen GF, Long AH, Bian YQ, Liu J, et al: Ingredients of Huangqi
decoction slow biliary fibrosis progression by inhibiting the
activation of the transforming growth factor-beta signaling
pathway. BMC Complement Altern Med. 12:332012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Zhang L, He W, Zhu C, Yang J and
Sheng M: Astragalus membranaceus inhibits peritoneal fibrosis via
monocyte chemoattractant protein (MCP)-1 and the transforming
growth factor-β1 (TGF-β1) pathway in rats submitted to peritoneal
dialysis. Int J Mol Sci. 22:12959–12971. 2014. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J and Liu Y: Dissection of key events
in tubular epithelial to myofibroblast transition and its
implications in renal interstitial fibrosis. Am J Pathol.
159:1465–1475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan HY: Tubular epithelial-myofibroblast
transdifferentiation mechanisms in proximal tubule cells. Curr Opin
Nephrol Hypertens. 12:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flier SN, Tanjore H, Kokkotou EG, Sugimoto
H, Zeisberg M and Kalluri R: Identification of epithelial to
mesenchymal transition as a novel source of fibroblasts in
intestinal fibrosis. J Biol Chem. 285:20202–20212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuda H, Fukuda N, Ueno T, Katakawa M,
Wang X, Watanabe T, Matsui S, Aoyama T, Saito K, Bando T, et al:
Transcriptional inhibition of progressive renal disease by gene
silencing pyrrole-imidazole polyamide targeting of the transforming
growth factor-β1 promoter. Kidney Int. 79:46–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hills CE and Squires PE: The role of TGF-β
and epithelial-to mesenchymal transition in diabetic nephropathy.
Cytokine Growth Factor Rev. 22:131–139. 2011.PubMed/NCBI
|
|
19
|
Sun WY, Wang L, Liu H, Li X and Wei W: A
standardized extract from Paeonia lactiflora and
Astragalus membranaceus attenuates liver fibrosis induced by
porcine serum in rats. Int J Mol Med. 29:491–498. 2012.PubMed/NCBI
|
|
20
|
Yang Y, Yang S, Chen M and Zhang X, Zou Y
and Zhang X: Compound Astragalus and Salvia
miltiorrhiza extract exerts anti-fibrosis by mediating
TGF-beta/Smad signaling in myofibroblasts. J Ethnopharmacol.
118:264–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen B, Li R, Yan N, Chen G, Qian W, Jiang
HL, Ji C and Bi ZG: Astragaloside IV controls collagen reduction in
photoaging skin by improving transforming growth factor-β/Smad
signaling suppression and inhibiting matrix metalloproteinase-1.
Mol Med Rep. 11:3344–3348. 2015.PubMed/NCBI
|
|
22
|
Huang WM, Liang YQ, Tang LJ, Ding Y and
Wang XH: Antioxidant and anti-inflammatory effects of Astragalus
polysaccharide on EA.hy926 cells. Exp Ther Med. 6:199–203.
2013.PubMed/NCBI
|
|
23
|
Lai PK, Chan JY, Wu SB, Cheng L, Ho GK,
Lau CP, Kennelly EJ, Leung PC, Fung KP and Lau CB:
Anti-inflammatory activities of an active fraction isolated from
the root of Astragalus membranaceus in RAW 264.7 macrophages.
Phytother Res. 28:395–404. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Che X, Wang Q, Xie Y, Xu W, Shao X, Mou S
and Ni Z: Astragaloside IV suppresses transforming growth factor-β1
induced fibrosis of cultured mouse renal fibroblasts via inhibition
of the MAPK and NF-κB signaling pathways. Biochem Biophys Res
Commun. 464:1260–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuo C, Xie XS, Qiu HY, Deng Y, Zhu D and
Fan JM: Astragalus mongholicus ameliorates renal fibrosis by
modulating HGF and TGF-beta in rats with unilateral ureteral
obstruction. J Zhejiang Univ Sci B. 10:380–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Böttinger EP and Bitzer M: TGF-beta
signaling in renal disease. J Am Soc Nephrol. 13:2600–2610. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta signaling through the Smad pathway: Role in
extracellular matrix gene expression and regulation. J Invest
Dermatol. 118:211–215. 2002. View Article : Google Scholar : PubMed/NCBI
|