Introduction
Banana peel has been widely used in traditional
Chinese medicine for the treatment of inflammation of the oral
cavity and to promote bowel movements (1). Furthermore, as banana peel contains
large amounts of vitamins and minerals, it may be used to produce
cosmetics as industrial products. In addition to vitamins and
minerals, banana peel contains a certain quantity of polyphenols
(2). Polyphenols, which are also
present in tea and pomegranate, have been demonstrated to protect
the liver against alcohol-associated damages (3). Polyphenols exert a marked antioxidative
effect, and the most evident manifestation of liver damage is
oxidative damage of liver cells (4).
As a result, polyphenols may be able to protect liver cells against
oxidative damage and promote the repair of oxidatively-damaged
cells, in order to maintain health (5). Polyphenols present in banana peel may
function in a similar manner.
Oxidative stress and inflammation may result in
liver disease, cardiovascular disease and cancer (6). CCl4 produces reactive-free
radicals if metabolized, and thus had been used to induce hepatic
damage in animal models (7).
Furthermore, CCl4 is able to increase lipid peroxidation
on the cell membrane and alter enzyme activity, thereby inducing
hepatic injury and necrosis (8).
In the present study, the preventive activity of
banana peel polyphenols (BPPs) on CCl4-induced
experimental hepatic damage was determined. Serum levels of
aspartate aminotransferase (AST), alanine aminotransferase (ALT),
lactate dehydrogenase (LDH), malondialdehyde (MDA), glutathione
(GSH) and triglyceride (TG) were evaluated, and in addition tissue
levels of MDA, GSH and TG were determined. Cytokine levels of
interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α and
interferon (IFN)-γ in the serum were also measured. In addition,
liver tissue was subjected to histological examination and the
expression levels of inflammation-associated genes were determined
in Kunming mice.
Materials and methods
Preparation of BPPs
Bananas were purchased from a local market
(Chongqing, China), and were native to Hainan, China. The banana
pulp was removed and the peel was washed and stored at 20°C.
Subsequently, 1 kg banana peel was blanched at 95°C for 3 min to
remove the polyphenol oxidase. The banana peel was incubated in 40%
ethanol solution (5 liters), and then ultrasonic-assisted
extraction (Ultrasonic extractor, THC 300, Jining Tianhua
Ultrasonic Electronic Instrument Co. Ltd., Shandong, China) was
performed at 50°C for 1 h. The extract was passed through an AB-8
macroporous resin (Donghong Chemical Co., Ltd., Zhongshan,
Guangdong, China) and the BPPs were adsorbed. The adsorbed BPPs
were washed with 80% ethanol solution, and the eluent was
evaporated and concentrated using an N-1100 rotary evaporator
(Eyela; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) (9).
Inducing hepatic injury
Male ICR mice (n=50; age, 7 weeks) were purchased
from the Experimental Animal Center of Chongqing University of
Education (Chongqing, China). The mice were allocated at random
into five groups (n=10 mice per group): Normal; control; 100 mg/kg
banana peel polyphenol; 200 mg/kg banana peel polyphenol; and
silymarin (Shanghai Yuanye Bio Technology Co., Ltd., Shanghai,
China) groups. The normal and control group mice were administered
0.2 ml physiological saline everyday for 14 days. The two banana
peel polyphenol group mice received 100 or 200 mg/kg banana peel
polyphenol solution in 0.2 ml doses by oral gavage for 14 days.
Silymarin was used for drug control (10), at a concentration of 100 mg/kg, and
the mice were administered 0.2 ml silymarin solution for 14 days.
At day 14, the control, 100 and 200 mg/kg banana peel polyphenol
and silymarin group mice received abdominal subcutaneous injections
of CCl4 solution (0.2 ml/kg dissolved in olive oil, 1:1
v/v) to induce hepatic damage. After 24 h, the mice were sacrificed
using CO2. Blood and liver tissues were collected and
preserved at −70°C until required for the biological assays.
Experimental protocols were approved by the Animal Ethics Committee
of Chongqing University of Education (11).
Levels of AST, ALT, LDH, MDA, GSH and
TG
The blood of the mice was centrifugalized at 1,795 ×
g for 10 min. After centrifugation, the serum supernatant was
collected, the serum (0.1 ml) and kit reagents were mixed and then
the levels determined according to the instructions described in
the kits at 532 nm using a UV-2600 spectrophotometer (Shimadzu,
Tokyo, Japan). A total of 0.1 g mice tissues were added into 0.5 ml
sucrose buffer (0.25 mol/l sucrose, 10 mmol/l HEPES, 1 mmol/l EDTA
at pH 7.4) and then this mixture was homogenized by a High speed
tissue homogenate machine (T10, IKA, Staufenim Breisgau, Germany).
The homogenized tissues were centrifuged at 1,795 × g, 10 min, the
supernatant fluid was collected and was determined as the serum
test method.
Serum levels of AST, ALT, LDH, MDA, GSH and TG were
determined using AST (no. C010), ALT (no. C009), LDH (no. A020),
MDA (no. A003), reduced GSH (no. A006) and TG (no. F001) assay
kits, respectively (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China). In addition, the tissue levels of MDA, GSH and TG
were determined using the kits from Nanjing Jiancheng
Bioengineering Institute.
Serum cytokine levels determined using
an enzyme-linked immunosorbent assay (ELISA)
Mouse blood from the inferior vena cava was
collected in a tube and centrifuged at 1,795 × g for 10 min at 4°C.
The serum (0.1 ml) and kit reagents were mixed and the
concentrations of the proinflammatory-associated cytokines, IL-6,
IL-12, TNF-α and IFN-γ, were determined using ELISA, according to
the manufacturer's instructions (BioLegend ELISA MAX™ Deluxe kit;
BioLegend, San Diego, CA, USA) (11)
and using a UV-2600 spectrophotometer at a wavelength of 450 nm
(Shimadzu, Tokyo, Japan).
Histological examination of
hematoxylin and eosin (H&E) stained sections
The liver tissues of mice were collected and washed.
Then the liver tissues were fixed in 10% (v/v) buffered formalin
for 24 h and they were score cut and embedded into paraffin. The
paraffin was cut 4-µm thick and stained by a H&E kit (Beijing
Nobleryder Technology Co., Ltd., Biejing, China). The stained
sections were then observed by a microscope (BX41, Olympus, Tokyo,
Japan) (12).
Western blot analysis of protein
expression levels in liver tissue
Total protein was obtained from the mice liver
tissue samples using a radioimmunoprecipitation assay buffer as
previously described (12). Protein
concentrations were determined using the RC DC protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Nitrocellulose
membranes (Schleicher and Schuell BioScience, Inc., Keene, NH, USA)
were then subjected to immunoblot analysis and proteins were
visualized using an enhanced chemiluminescence (ECL) method (GE
Healthcare Life Sciences) (7). Liver
tissue cell lysates were separated using 12% SDS-PAGE, transferred
onto a polyvinylidene fluoride membrane (GE Healthcare Life
Sciences), blocked with 5% skimmed milk and hybridized with primary
antibodies (dilution, 1:1,000). The following primary antibodies
were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA): monoclonal mouse anti-human cyclooxygenase-2 (COX-2; cat. no.
sc-514489), monoclonal mouse anti-human nitric oxide synthase
(iNOS; cat. no. sc-7271), monoclonal mouse anti-human TNF-α (cat.
no. sc-48418) and monoclonal mouse anti-human IL-1β (cat. no.
sc-52013). The membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Blots were washed
three times with phosphate-buffered saline with Tween-20 and
developed using an ECL reagent (Amersham Life Science, Arlington
Heights, IL, USA), and the protein expressions were also
quantitative analyzed using ImageJ software (version 1.44).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between the mean values for individual groups were
assessed using one-way analysis of variance with Duncan's multiple
range test. P<0.05 was considered to indicate a statistically
significant difference. SAS software, version 9.1 (SAS Institute
Inc., Cary, NC, USA) was used for statistical analyses.
Results
Serum levels of AST, ALT and LDH
The levels of AST, ALT and LDH in the serum of the
normal group mice were reduced compared with the other groups
(Table II). Control group mice were
subjected to hepatic injury but received no further treatment, and
thus exhibited markedly increased serum levels of AST, ALT and LDH.
Treatment with silymarin (100 mg/kg) appeared to significantly
(P<0.05) reduce the serum levels of these proteins compared with
the control mice, as the serum levels of AST, ALT and LDH in the
silymarin-treated mice were the most comparable to the normal group
mice. Treatment with BPPs appeared to induce a statistically
significant reduction in the serum levels of AST, ALT and LDH
compared with the control group mice (P<0.05). These levels were
increased in the 100 mg/kg banana peel polyphenol group mice
compared with the normal and silymarin group mice, but reduced
compared with control mice. Furthermore, the 200 mg/kg BPP group
mice exhibited lower levels of AST, ALT and LDH compared with the
100 mg/kg dose group. In addition, the AST/ALT ratio was used as a
key index of hepatic injury. The high AST/ALT ratio meant severe
hepatic injury (13). The AST/ALT
ratio in the normal group mice was ~1, and the control mice had the
highest ratio. The 100 and 200 mg/kg BPP and silymarin groups
(1.55, 1.23 and 1.18, respectively) had reduced ratios compared
with the control mice, but remained >1. Therefore, the results
of the present study indicated that BPPs are able to significantly
reduce the levels of a number of key markers of hepatic injury, and
these effects were comparable to those of the drug of
silymarin.
 | Table II.Serum levels of AST, ALT and LDH in
mice following CCl4-induced hepatic damage. |
Table II.
Serum levels of AST, ALT and LDH in
mice following CCl4-induced hepatic damage.
| Group | AST (IU/l) | ALT (IU/l) | AST/ALT | LDH (IU/l) |
|---|
| Normal |
207.3±23.6a |
209.3±25.6a |
0.99±0.02a |
1243.6±178.2b |
| Control |
2571.6±97.6c |
1511.3±122.9c |
1.70±0.11c |
6512.3±202.6c |
| Banana peel
polyphenols (mg/kg) |
|
|
|
|
|
100 |
1874.3±65.9d |
1207.1±112.3d |
1.55±0.08d |
4876.3±152.4d |
|
200 |
746.2±62.3e |
605.3±58.9e |
1.23±0.09e |
3103.2±121.5e |
| Silymarin |
712.0±68.3e |
602.3±49.8e |
1.18±0.10e |
2801.3±107.6a |
MDA, GSH and TG levels in serum and
liver tissues
The MDA, GSH and TG levels in the serum and liver
tissues of the mice were determined using a variety of testing
kits. The MDA and TG levels in the serum and liver tissues
significantly decreased (P<0.05) as a result of the
CCl4-induced hepatic injury. Control mice exhibited the
highest levels of MDA and TG, while the banana peel polyphenol and
silymarin groups exhibited reduced levels of these analytes
(P<0.05). The levels of MDA and TG in the 200 mg/kg banana peel
polyphenol and silymarin group mice were comparable to those in the
normal group mice (Tables III and
IV; P<0.05). However, the
differences (P<0.05) between groups in GSH levels followed a
different trend from that of MDA and TG. The control mice exhibited
the lowest levels of GSH, while the BPPs and silymarin group mice
expressed increased levels of GSH compared with the control group
(P<0.05). However, no statistically significant difference was
identified in the levels of GSH between the 200 mg/kg BPP and
silymarin group mice (P>0.05), and these levels were moderately
reduced compared with the normal group mice (P<0.05).
 | Table III.Serum levels of MDA, GSH and TG in
mice following CCl4-induced hepatic damage. |
Table III.
Serum levels of MDA, GSH and TG in
mice following CCl4-induced hepatic damage.
| Group | MDA (nmol/ml) | GSH (mg/l) | TG (mmol/l) |
|---|
| Normal |
5.48±0.62a |
339.45±42.18b |
0.92±0.10c |
| Control |
14.05±0.82b |
141.08±20.36d |
1.32±0.11b |
| Banana peel
polyphenols (mg/kg) |
|
|
|
|
100 |
10.12±0.59e |
207.36±16.87c |
1.19±0.08e |
|
200 |
7.31±0.41c |
255.21±19.87e |
0.95±0.06c |
| Silymarin |
6.65±0.32d |
265.32±18.33e |
0.94±0.08c |
 | Table IV.Hepatic tissues of MDA, GSH and TG in
mice following CCl4-induced hepatic damage. |
Table IV.
Hepatic tissues of MDA, GSH and TG in
mice following CCl4-induced hepatic damage.
| Group | MDA (nmol/ml) | GSH (mg/l) | TG (mmol/l) |
|---|
| Normal |
2.10±0.31a |
25.32±2.69b |
0.022±0.003c |
| Control |
7.87±0.71b |
5.56±0.42a |
0.047±0.004b |
| Banana peel
polyphenols (mg/kg) |
|
|
|
|
100 |
5.97±0.62d |
13.58±1.69e |
0.040±0.002d |
|
200 |
3.32±0.30e |
17.69±1.48d |
0.031±0.004e |
| Silymarin |
3.23±0.32e |
18.21±1.71d |
0.026±0.002a |
Serum cytokine levels
IL-6, IL-12, TNF-α and IFN-γ are proinflammatory
cytokines. The control group mice exhibited the highest cytokine
levels (P<0.05), while normal group mice had the lowest cytokine
levels (Table V). BPPs and silymarin
group mice presented with significantly decreased cytokine levels
(P<0.05) compared with the control group mice. The levels of
IL-6, IL-12 and TNF-α in the 200 mg/kg BPP group mice were
moderately increased compared with the silymarin group mice;
however, no statistically significant difference was detected in
IFN-γ levels between the 200 mg/kg BPP and silymarin group mice
(P<0.05).
 | Table V.Cytokine levels of IL-6, IL-12, TNF-α
and IFN-γ in mice following following CCl4 induced
hepatic damage. |
Table V.
Cytokine levels of IL-6, IL-12, TNF-α
and IFN-γ in mice following following CCl4 induced
hepatic damage.
| Group | IL-6 (pg/ml) | IL-12 (pg/ml) | TNF-α (pg/ml) | IFN-γ (pg/ml) |
|---|
| Normal |
43.6±4.9a |
211.3±15.3a |
22.6±4.8a |
19.6±2.5b |
| Control |
285.2±26.5c |
795.3±26.9c |
89.9±6.8c |
75.3±4.9c |
| Banana peel
polyphenols (mg/kg) |
|
|
|
|
100 |
212.6±22.9d |
572.6±29.3d |
68.1±4.2d |
55.1±2.6d |
|
200 |
152.6±19.2e |
415.8±21.2e |
47.2±3.2e |
32.9±1.8e |
| Silymarin |
125.3±12.3b |
390.6±24.6b |
39.2±2.6b |
31.9±3.7e |
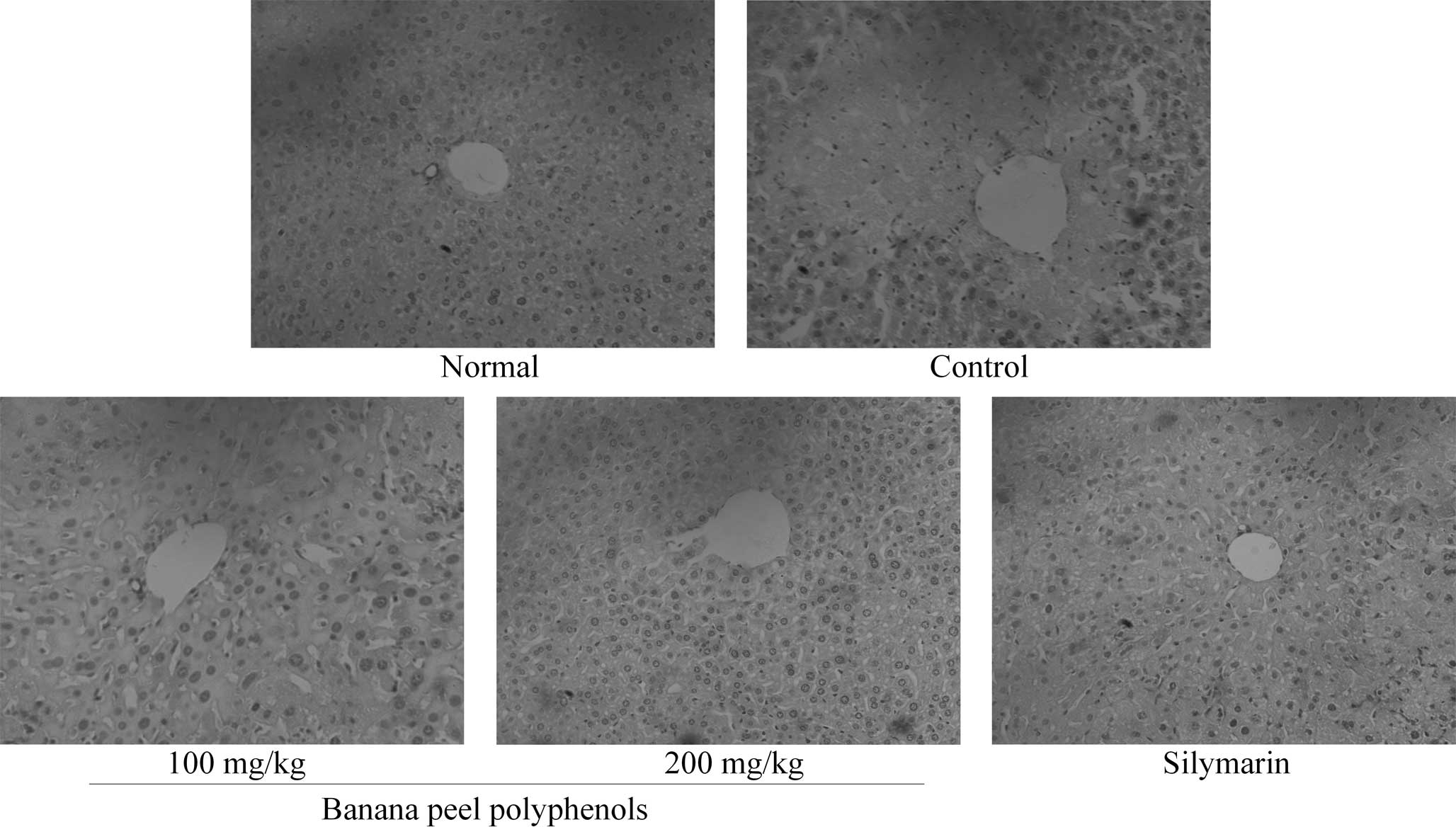
Histopathological examination of liver
tissues
Observation of the H&E-stained sections revealed
that the structure of hepatic lobules in the tissues of the normal
group mice was complete with a clear boundary (Fig. 1). Normal group liver cells exhibited
funicular radiation, central veins showed no expansion, and the
size of liver cells was normal. Control group liver cells exhibited
widespread CCl4-induced liver cell necrosis and tissue
damage, including collapse of the hepatic lobule mesh stent and a
disorderly tissue structure. In the 100 mg/kg BPP group, the
hepatic lobules were damaged and the central areas were partially
necrotic; however, these necrotic regions were reduced in size
compared with those observed in the control group mice. The 200
mg/kg BPP group exhibited a markedly reduced degree of liver
damage, with complete hepatic lobules and no necrosis in the
central region of the liver. The condition of the silymarin group
livers was comparable to that of the 200 mg/kg BPP group, with no
large areas of necrosis, and complete and normal liver tissue
structure.
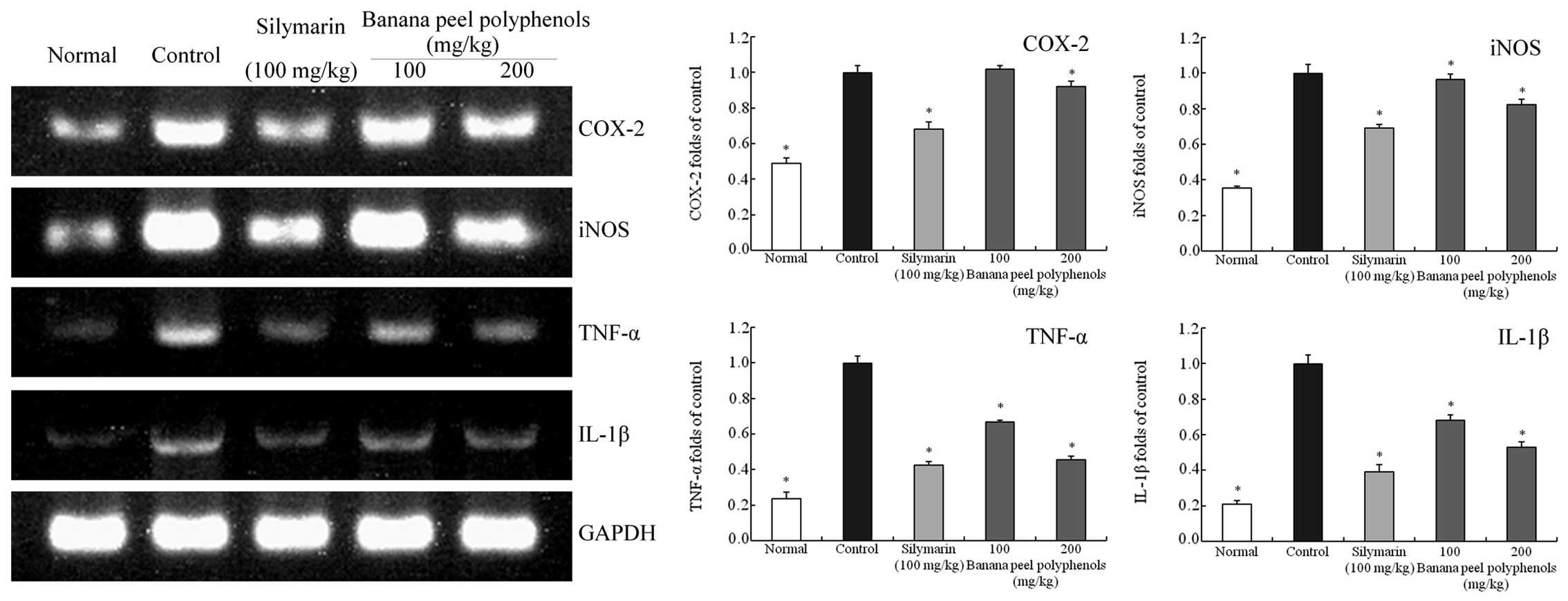
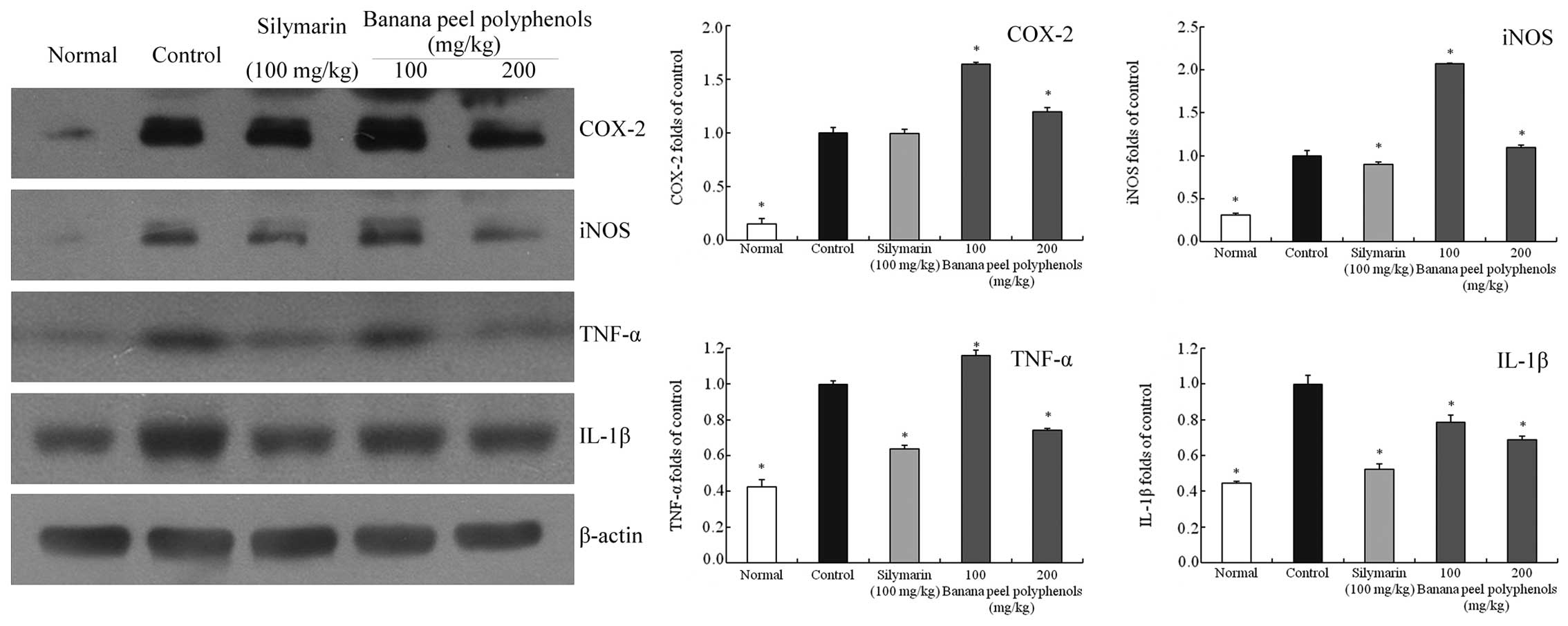
Inflammation-associated gene
expression in the liver tissues
The mRNA and protein expression levels of COX-2,
iNOS, TNF-α and IL-1β in mice liver tissues were determined using
RT-qPCR and western blot assays (Figs.
2 and 3). The liver tissue mRNA
levels of COX-2 (0.49-fold of control), iNOS (0.35-fold), TNF-α
(0.23-fold) and IL-1β (0.21-fold) in the normal group mice were at
reduced levels compared with the control group, as the
CCl4-induced hepatic injury caused these expression
levels to increase in the control group mice. The protein
expression levels of COX-2 (0.15-fold of control), iNOS
(0.31-fold), TNF-α (0.43-fold) and IL-1β (0.45-fold) in liver
tissues were also reduced compared with the control. Banana peel
polyphenol treatment significantly reduced the expression levels of
these factors compared with those of the control mice (P<0.05).
The mRNA expression levels of COX-2, iNOS, TNF-α and IL-1β in the
silymarin group mice (0.68-, 0.69-, 0.42- and 0.39-fold of control
levels, respectively) were comparable to those in the 200 mg/kg BPP
group mice (0.92-, 0.82-, 0.46- and 0.53-fold of control levels,
respectively), and lower compared with those of the 100 mg/kg BPP
group mice (1.02-, 0.97-, 0.67- and 0.68-fold of control levels,
respectively). However, the 200 mg/kg BPP group mice also showed
reduced protein expression levels of TNF-α (0.74-fold of control)
and IL-1β (0.69-fold of control) compared with the 100 mg/kg BPP
group and control mice, however, the expression of COX-2 (1.20-fold
of control) and iNOS (1.10-fold of control) in the 200 mg/kg BPP
group mice was not significantly reduced compared with the control
group mice (P<0.05).
Discussion
ALT and AST are primarily expressed in liver cells,
and liver cell necrosis will elevate the content of ALT and AST.
This elevation is consistent with the degree of liver cell damage,
and is thus the most commonly-used indicator of liver function
(14). The distribution of the two
enzymes in liver cells differs: ALT is predominantly distributed in
the cytoplasm, while AST is located in the cytoplasm and the
mitochondria (15). In liver
function examinations, ALT levels indicate liver cell damage and
AST is a marker of liver cell necrosis; thus, the two enzymes are
accurate indicators of liver cirrhosis, fibrosis and cancer
(16). The elevation of ALT and AST
expression levels and the AST/ALT ratio may differ between patients
with various different types of hepatitis. In cases of mild
hepatitis, although liver cells may be damaged, the mitochondria in
the liver cells remain intact. As a result, only the ALT from the
cytoplasm of liver cells is released into the blood, so liver
function examinations indicate elevated levels of ALT and an
AST/ALT ratio of <1. However, in cases of fulminant hepatitis
and severe liver damage, including cirrhosis and liver cancer,
mitochondria in liver cells may be severely damaged (17). As a result, AST is released from the
mitochondria and cytoplasm and AST levels are evidently increased,
resulting in an AST/ALT ratio of >1 (18). LDH, a glycolytic enzyme, is a key
analyte in liver function examinations, which indicates the degree
of liver damage. If the liver is damaged, the activity of LDH is
expected to increase significantly (19).
CCl4 is generated through hepatic P-450
enzyme metabolism. CCl4 is able to induce lipid
peroxidation of the liver cell membrane, which is a crucial factor
in liver cell damage (20). MDA is a
key index of lipid peroxidation. By detecting the activity of MDA,
lipid peroxidation of liver tissues may be detected, indicating the
influence of CCl4 on liver health (21). GSH is a crucial antioxidant and free
radical scavenger, which is able to combine with free radicals and
heavy metals to transform harmful toxins into harmless substances
for excretion (22). Furthermore,
GSH combines with harmful substances produced by CCl4 in
the liver and reduces liver damage in order to protect the liver.
Previous studies have indicated that GSH may be used to detect the
liver damage caused by CCl4 (23,24).
Elevation of TG levels indicated higher fatty acid content, while
in a clinical context very high levels of TG are usually associated
with liver disease (25).
Experimental results have indicated that liver damage induced by
CCl4 may result in increased levels of MDA and TG and
reduced levels of GSH (26,27). In the present study, BPPs appeared to
mitigate these changes, reducing MDA and TG levels, and elevating
GSH levels.
Cytokines are a class of micromolecule polypeptide
secreted by various cells, which are able to regulate cell growth
and differentiation, immune function, inflammation and wound
healing (28). Confirmed
proinflammatory cytokines include TNF-α, IL-1β and IL-6, which
serve key functions in the pathogenesis and development of
biological damage (29). As key
inflammatory mediators, cytokines such as IL-6 and TNF-α are
crucially involved in the inflammatory response. Under pathological
conditions, the serum levels of TNF-α and IL-6 may increase,
resulting in the release of marked quantities of various
inflammatory factors, which may in turn lead to inflammation and
damages of liver tissue (30). IL-6
may induce increased levels of INF-γ, the primary function of which
is to activate non-specific effector cells and mediate the cellular
immune process (31). Under
laboratory and clinical conditions, the levels of TNF-α, IL-1β,
IL-6 and INF-γ may be used as markers of the degree of liver
damage. The higher the levels of these markers, the faster the
liver cell injury will be and the more severe the resulting liver
injury. COX-2 is an inducible enzyme that is expressed more
markedly in liver cells that are stimulated to exhibit
inflammation. The expression of COX-2 increases significantly in
liver tissue that has undergone various types of damage, so COX-2
has been regarded as a therapeutic target for the treatment of
liver damage (32). In a number of
previous studies, the expression levels of iNOS and COX-2 were
found to be comparable and positively correlated, and thus the
expression of iNOS is increased in liver tissue that exhibits
lesions (33–35).
In the present study, BPPs appeared to significantly
reduce the serum levels of AST, ALT and LDH in a
CCl4-induced mouse model of hepatic damage. Furthermore,
BPPs were able to regulate proinflammatory cytokine levels,
resulting in the CCl4-induced alterations in serum
cytokine levels being comparable to those of normal mice. By
detecting liver tissues, it was observed that BPPs were able to
reduce liver damage. By evaluating the levels of MDA, GSH and TG in
the mouse serum and tissues, BPPs were found to reduce the
CCl4-induced lipid peroxidation of the liver by
increasing the levels of GSH and reducing the levels of MDA and TG.
Furthermore, molecular experiments demonstrated that BPPs were able
to reduce the mRNA and protein expression levels of COX-2, iNOS,
TNF-α and IL-1β in the hepatic injury model mice, thus mitigating
liver damage. Thus, BPPs may serve a preventive role in hepatic
injury by reducing the expression levels of COX-2, iNOS, TNF-α and
IL-1β. In addition, the examination of H&E liver tissue
sections indicated that BPPs were able to reduce the manifestations
of liver injury.
In conclusion, the results of the present study
suggest that BPPs are able to significantly improve a number of the
symptoms of CCl4-induced liver damage in mice, and that
the effect is more marked with an increased treatment dose. In
future, banana peel could be used in waste utilization or may be
used as medicine or a functional compound.
Acknowledgements
This study was supported by the Scientific and
Technological Research Program of Chongqing Municipal Education
Commission (grant no. KJ1501415) and the Program for Innovative
Research Team in Chongqing University of Education (grant no.
KYC-cxtd03-20141002).
References
|
1
|
Sang LW, Li L and Zheng FC: Study on the
comprehensive utilization of banana by-product. Hei Long Jiang Nong
Ye Ke Xue. 4:96–98. 2006.(In Chinese).
|
|
2
|
Liu S, Tang YF, Zhao QL, Li Y, Chen J, Li
Ml, Zhao H and Zhou Y: Extraction of polyphenols from banana peel
and anti-fungal effect research. Hu Nan Sheng Chang Sha Shi Xian
Jia Hu Hu Nan Shi Fan Da Xue Yi Xue. 6:12–14. 2009.(In
Chinese).
|
|
3
|
Kaviarasan S and Anuradha CV: Fenugreek
(Trigonella foenum-graecum) seed polyphenols protect liver
from alcohol toxicity: a role on hepatic detoxification system and
apoptosis. Pharmazie. 62:299–304. 2007.PubMed/NCBI
|
|
4
|
Kang MC, Kang SM, Ahn G, Kim KN, Kang N,
Samarakoon KW, Oh MC, Lee JS and Jeon YJ: Protective effect of a
marine polyphenol, dieckol against carbon tetrachloride-induced
acute liver damage in mouse. Environ Toxicol Pharmacol. 35:517–523.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sebai H, Sani M, Yacoubi MT, Aouani E,
Ghanem-Boughanmi N and Ben-Attia M: Resveratrol, a red wine
polyphenol, attenuates lipopolysaccharide-induced oxidative stress
in rat liver. Ecotoxicol Environ Saf. 73:1078–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cauwels A and Brouckaert P: Survival of
TNF toxicity: dependence on caspases and NO. Arch Biochem Biophys.
462:132–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao X: Hawk tea (Litsea coreana
Levl. var. lanuginose) attenuates CCl4-induced
hepatic damage in Sprague-Dawley rats. Exp Ther Med. 5:555–560.
2013.PubMed/NCBI
|
|
8
|
Weber LW, Boll M and Stampfl A:
Hepatotoxicity and mechanism of action of haloalkanes: Carbon
tetrachloride as a toxicological model. Crit Rev Toxicol.
33:105–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang D, Xu XQ and Wu WJ: Study of
extraction of polyphenols from different states banana peels. Food
Sci Technol. 35:204–208. 2010.(In Chinese).
|
|
10
|
Jahan S, Khan M, Imran S and Sair M: The
hepatoprotective role of Silymarin in isoniazid induced liver
damage of rabbits. J Pak Med Assoc. 65:620–622. 2015.PubMed/NCBI
|
|
11
|
Li GJ, Sun P, Wang Q, Qian Y, Zhu K and
Zhao X: Dendrobium candidum Wall. ex Lindl. attenuates
CCl4-induced hepatic damage in imprinting control region
mice. Exp Ther Med. 8:1015–1021. 2014.PubMed/NCBI
|
|
12
|
Melgar S, Karlsson L, Rehnström E,
Karlsson A, Utkovic H, Jansson L and Michaëlsson E: Validation of
murine dextran sulfate sodium-induced colitis using four
therapeutic agents for human inflammatory bowel disease. Int
Immunopharmacol. 8:836–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen SS, Zhu K, Wang R and Zhao X:
Preventive effect of polysaccharides from the large yellow croaker
swim bladder on HCl/ethanol induced gastric injury in mice. Exp
Ther Med. 8:316–322. 2014.PubMed/NCBI
|
|
14
|
Liu D, He H, Yin D, Que A, Tang L, Liao Z,
Huang Q and He M: Mechanism of chronic dietary iron
overload-induced liver damage in mice. Mol Med Rep. 7:1173–1179.
2013.PubMed/NCBI
|
|
15
|
Liu H, Qi X, Cao S and Li P: Protective
effect of flavonoid extract from Chinese bayberry (Myrica
rubra Sieb. et Zucc.) fruit on alcoholic liver oxidative injury
in mice. J Nat Med. 68:521–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poortahmasebi V, Alavian SM, Keyvani H,
Norouzi M, Mahmoodi M and Jazayeri SM: Hepatic steatosis:
prevalence and host/viral risk factors in Iranian patients with
chronic hepatitis B infection. Asian Pac J Cancer Prev.
15:3879–3884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nyblom H, Berggren U, Balldin J and Olsson
R: High AST/ALT ratio may indicate advanced alcoholic liver disease
rather than heavy drinking. Alcohol Alcohol. 39:336–9. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mabrouk M, El-Raziky M, Zayed N, Salama R,
El-Akel W, Awad T, El Beshlawy M and Esmat G: Clinical, biochemical
and pathological profiles of 5464 Egyptian chronic hepatitis
C-infected patients. Hepatogastroenterology. 60:1731–1735.
2013.PubMed/NCBI
|
|
19
|
Dickens H, Ullrich A, Runge D, Mueller B,
Olszewski U and Hamilton G: Anticancer drug
cis-4-hydroxy-L-proline: Correlation of preclinical toxicology with
clinical parameters of liver function. Mol Med Rep. 1:459–464.
2008.PubMed/NCBI
|
|
20
|
Srivastava SP, Chen NQ and Holtzman JL:
The in vitro NADPH-dependent inhibition by CCl4 of the
ATP-dependent calcium uptake of hepatic microsomes from male rats.
Studies on the mechanism of the inactivation of the hepatic
microsomal calcium pump by the CCl3 radical. J Biol
Chem. 265:8392–8399. 1990.PubMed/NCBI
|
|
21
|
Liu L, Fan H, Qi P, Mei Y, Zhou L, Cai L,
Lin X and Lin J: Synthesis and hepatoprotective properties of
Acanthus ilicifolius alkaloid A and its derivatives. Exp
Ther Med. 6:796–802. 2013.PubMed/NCBI
|
|
22
|
Karabulut AB, Kafkas ME, Kafkas AS, Onal Y
and Kiran TR: The effect of regular exercise and massage on oxidant
and antioxidant parameters. Indian J Physiol Pharmacol. 57:378–383.
2013.PubMed/NCBI
|
|
23
|
Yin L, Wei L, Fu R, Ding L, Guo Y, Tang L
and Chen F: Antioxidant and hepatoprotective activity of
Veronica ciliata Fisch. extracts against carbon
tetrachloride-induced liver injury in mice. Molecules.
19:7223–7236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ranjbar A, Sharifzadeh M, Karimi J,
Tavilani H, Baeeri M, Shayesteh Heidary T and Abdollahi M: Propofol
attenuates toxic oxidative stress by CCl4 in liver
mitochondria and blood in rat. Iran J Pharm Res. 13:253–262.
2014.PubMed/NCBI
|
|
25
|
Tomizawa M, Kawanabe Y, Shinozaki F, Sato
S, Motoyoshi Y, Sugiyama T, Yamamoto S and Sueishi M: Elevated
levels of alanine transaminase and triglycerides within normal
limits are associated with fatty liver. Exp Ther Med. 8:759–762.
2014.PubMed/NCBI
|
|
26
|
Song HY, Mao ZM, Yang LL, Liu T, Li DF,
Zhang L, Ge YL, Zheng PY, Liu P, Zhang XQ and Ji G: Dangfei
liganning capsules attenuate the susceptibility of rat nonalcoholic
fatty liver to carbon tetrachloride toxicity. J Tradit Chin Med.
31:327–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Yang X, Lu X, Wang D and Zhao Y:
Protective effects of Keemun black tea polysaccharides on acute
carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food
Chem Toxicol. 58:184–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schett G, Elewaut D, McInnes IB, Dayer JM
and Neurath MF: How cytokine networks fuel inflammation: Toward a
cytokine-based disease taxonomy. Nat Med. 19:822–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bae UJ, Yang JD, Ka SO, Koo JH, Woo SJ,
Lee YR, Yu HC, Cho BH, Zhao HY, Ryu JH, Lee SM, et al: SPA0355
attenuates ischemia/reperfusion-induced liver injury in mice. Exp
Mol Med. 46:e1092014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Yang J and Jiang Q: The protective
effect of huperzine A against hepatic ischemia reperfusion injury
in mice. Transplant Proc. 46:1573–1577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bodienkova GM, Alekseev R, Boklazhenko E
and Kurchevenko S: Inflammation mediators in employees in chronic
exposure to neurotoxicants. Int J Occup Med Environ Health.
27:619–626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malekinejad H, Rezabakhsh A, Rahmani F and
Razi M: Paraquat exposure up-regulates cyclooxygenase-2 in the
lungs, liver and kidneys in rats. Iran J Pharm Res. 12:887–896.
2013.PubMed/NCBI
|
|
33
|
Ibrahim MA, Abdel-Gaber SA, Amin EF,
Ibrahim SA, Mohammed RK and Abdelrahman AM: Molecular mechanisms
contributing to the protective effect of levosimendan in liver
ischemia-reperfusion injury. Eur J Pharmacol. 741:64–73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong M, Hong T, Liu S, Zhao J, Meng Y and
Mu J: Hepatoprotective effect of the flavonoid fraction isolated
from the flower of Inula britannica against
D-Galactosamine-induced hepatic injury. Mol Med Rep. 7:1919–1923.
2013.PubMed/NCBI
|
|
35
|
Huang GJ, Deng JS, Huang SS, Lee CY, Hou
WC, Wang SY, Sung PJ and Kuo YH: Hepatoprotective effects of
eburicoic acid and dehydroeburicoic acid from Antrodia
camphorata in a mouse model of acute hepatic injury. Food Chem.
141:3020–3027. 2013. View Article : Google Scholar : PubMed/NCBI
|