Introduction
In order to effectively control bleeding during
hepatobiliary surgery, hepatic portal occlusion is often used, as
it can temporarily interrupt the portal vein and hepatic artery
inflow. This method is widely accepted since it is clinically
simple, practical and effective; however, there is an inherent risk
of subsequent liver ischemia-reperfusion injury (IRI). Therefore,
in recent years, clinical research in the field of hepatic surgery
has focused on how to reduce blood loss and improve the safety of
hepatic portal occlusion, whilst simultaneously reducing the risk
of liver IRI.
Previous studies investigating tissues and organs
such as the brain, heart, kidney and retina have demonstrated that
numerous free radicals are produced during IRI, when the endogenous
radical scavengers cannot function appropriately, leading to cell
damage (1,2). Therefore, investigating effective drugs
that alleviate IRI-induced functional liver damage, and improve the
postoperative survival rate, has become a research hotspot in the
field of hepatic surgery. Melatonin (MT) is a neural endocrine
hormone that is secreted by the pineal gland (PG), which exerts
known antioxidant, antitoxic, anti-stress and anti-inflammatory
effects (3,4). Previous studies have demonstrated that
MT has a protective role in IRI of the brain, heart, kidney and
retina (5); however, its protective
effects in liver IRI and the underlying mechanism require further
research. An animal model of hepatic portal occlusion was used in
the present study. MT was administered in advance, and the
influence and significance of MT on antioxidant capacity, lipid
peroxidation damage and liver function in liver IRI was
investigated.
Materials and methods
Experimental animals
A total of 66 male Sprague-Dawley rats were used in
the present study, and were purchased from the Laboratory Animal
Department of Shanghai Jiaotong University School of Medicine
(Shanghai, China). The rats were aged 3–6 months, and weighed
220±30 g. The experimental procedures of the present study
conformed to the guidelines outlined in the guide for the Care and
Use of Laboratory Animals and were approved by the Research Ethics
Committee of Renji Hospital [Shanghai, China; approval no.
SYXY(hu)2011-0121]. The rats were randomly assigned into three
groups: i) The normal control group (N group; n=6), ii) the
ischemia-reperfusion (IR) group (n=30); iii) and the MT-treated
group (n=30). The IR and MT groups were divided into five subgroups
(n=6), according to the following time points: 35 min of ischemia,
and 2, 4, 8 or 24 h of reperfusion.
Animal model and sample
collection
The rats were fasted overnight prior to surgery, and
were subsequently anesthetized with 3% pentobarbital sodium (1
ml/kg; Sigma-Aldrich, St. Louis, MO, USA) via intraperitoneal
injection. Following this, the rats were fixed in the supine
position on a regularly disinfected and surgically draped operating
table. A straight ~2 cm incision was made in the middle of the
upper abdomen, along which the abdomen was cut step-by-step. The
peri-hepatic ligament was carefully dissected to expose the
hepatoduodenal ligament. In order to completely block the portal
vein, hepatic artery and bile duct, an atraumatic hemostatic clamp
was used to block the first porta hepatis, according to the Pringle
manoeuver (6). The clamp was
released after 35 min to recover the inflow of hepatic blood, and
the abdomen was subsequently sutured. In the MT group,
intraperitoneal injections of 10 mg/kg MT [1ml (ip)] were
administered 35 and 70 min prior to ischemia, as well as at the
early stage of reperfusion, and at 1 and 2 h post-reperfusion
(7–12).
Portal vein blood samples (1 ml) were obtained from
the rats in each group at various time points (n=6 at each time
point). The time points were as follows: Prior to ischemia, 35 min
of ischemia, and 2, 4, 8 and 24 h post-reperfusion. The rats were
sacrificed immediately after each time point. Blood samples were
placed in a sterile Eppendorf tube without pyrogen for 20 min prior
to centrifugation at 4,000 × g for 10 min. The serum was
subsequently separated and stored at −30°C. A total of 1 g incised
liver tissue was rinsed with normal saline (NS) at 0°C, and stored
in a liquid nitrogen tank for inspection; whereas another 1 g of
incised liver tissue was fixed with 10% formaldehyde solution for
inspection.
Instruments
The experimental table, atraumatic vascular clamp,
scissors, forceps, and hemostatic forceps were purchased from
Shanghai Medical Equipment Works Co. (Shanghai, China). A high
speed centrifuge and an ultraviolet (UV) spectrophotometer were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA);
an enzyme-linked immunosorbent assay plate reader was obtained from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA); and a CL-7150
automatic biochemical analyzer (ACA) was purchased from Shimadzu
Corporation (Kyoto, Japan).
Reagents
Malondialdehyde (MDA), superoxide dismutase (SOD)
and glutathione (GSH) peroxidase assay kits and the reagents for
total protein quantification, including biuret, were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Reagent configurations were prepared according to the
manufacturer's protocol. MT was purchased from Sigma-Aldrich.
Determination of liver function
Alanine aminotransferase (ALT), aspartate
aminotransferase (AST) and lactate dehydrogenase (LDH) levels were
measured using a CL-7150 ACA according to the manufacturer's
protocol. These experiments were performed as previously described
(13).
Determination of MDA using
thiobabituric acid
Thiobabituric acid was used to detect MDA levels as
the two compounds form a red product through condensation, with a
maximum absorption peak of 532 nm. The tissue homogenate was
prepared as follows: 100 mg liver tissue was harvested and
supplemented with 9X physiological saline prior to ultrasonic
homogenization for 20 sec and subsequent centrifugation at 300 × g
at 4°C for 15 min. The resultant supernatant was tested using a
standard, and reagents 1–3. According to the manufacturer's
protocol, the standard, anhydrous ethanol and sample (0.1 ml each)
were added into the standard tube, test tube and test control tube,
respectively; and 0.1 ml reagent 1 was subsequently added into each
tube. Following this, the tube rack was agitated in order to mix
the reagents, and 3 ml reagent 2 and 1 ml reagent 3 were added to
the standard control and test tubes, respectively; whereas 3 ml
reagent 2 and 50% glacial acetic acid (1 ml) were added into the
test control tube, and mixed in a swirl mixer, with the tube mouth
tightly covered with plastic wrap with a small hole. The optical
density (OD) values of each tube were measured at 532 nm using a UV
spectrophotometer. The MDA content was subsequently calculated
according to the formula outlined in the manufacturer's
protocols.
Determination of SOD levels, using the
xanthine oxidase method
Utilizing the specific inhibition of SOD on
superoxide anion free radicals, the xanthine oxidase method was
used to determine the levels of SOD. The tissue homogenate was
prepared as follows: 100 mg liver tissue was harvested and
supplemented with 9X physiological saline prior to ultrasonic
homogenization for 20 sec and subsequent centrifugation at 300 × g
at 4°C for 15 min. The resultant supernatant was tested according
to the manufacturer's protocols. The following was added to the
test tube: 1 ml reagent 1, 0.03 ml sample, 0.5 ml distilled water,
0.1 ml reagent 2, 0.1ml reagent 3 and 0.1 ml reagent 4; whereas in
the control tube, the sample was substituted with 0.03 ml distilled
water. The solutions were subsequently mixed in a swirl mixer, and
incubated in a thermostatic water bath at 37°C for 40 min. Once the
tubes had cooled, 2 ml chromogenic agent was added to each tube,
and the solution was agitated for 10 min. The OD values were
subsequently measured at 550 nm using a UV spectrophotometer. SOD
activity was calculated according to the formula outlined in the
manufacturer's protocol.
Determination of GSH by
colorimetry
GSH was detected as the yellow product formed in the
reaction between sulfhydryl compounds and
dithiobisnitrobenzoicacid. Briefly, the tissue homogenate was
prepared as follows: 100 mg liver tissue was harvested and
supplemented with 9X physiological saline prior to ultrasonic
homogenization for 20 sec and subsequent centrifugation at 300 × g
at 4°C for 15 min. The resultant supernatant was tested according
to the manufacturer's protocol. A total of 0.03 ml standard, 0.03
ml distilled water, 0.03 ml sample and 0.03 ml sample were added to
the standard, control, test and test control tubes, respectively;
and each tube was supplemented with 0.03 ml reagent 1, 1 ml reagent
2 and 0.5 ml reagent 3 (excluding the test control tube). Following
this, the solution was agitated and incubated at room temperature
for 10 min, prior to the determination of the OD values at 412 nm
using a UV spectrophotometer. GSH activity was calculated according
to the formula outlined in the manufacturer's protocol.
Determination of protein content via
the biuret method
The tissue homogenate was prepared as follows: 100
mg liver tissue was harvested and supplemented with 9X
physiological saline prior to ultrasonic homogenization for 20 sec
and subsequent centrifugation at 300 × g at 4°C for 15 min. The
resultant supernatant was tested using a stock solution of
Coomassie Brilliant Blue (CBBG250; Sigma-Aldrich), prepared for
standard protein solution. A total of 0.05 ml distilled water+3 ml
CBBG250, 0.05 ml standard + 3 ml CBBG250, and 0.05 ml sample + 3 ml
CBBG250 were added into the control, standard tube and test tubes,
respectively. The solution was agitated and incubated at room
temperature for 10 min, and the OD values were measured at 495 nm
using a UV spectrophotometer in order to calculate the protein
content, based on the following formula: Protein content = (OD
value of test tube / OD value of standard tube) × standard protein
content.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to determine statistical differences. Quantitative data
are expressed as the mean ± standard deviation. One-way analysis of
variance and a Student Newman-Keuls post-hoc test were performed to
analyze variance between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ischemia significantly increases MDA
levels
At each time point, the MDA levels in the IR and MT
groups were significantly increased following ischemia (P<0.05),
as compared with pre-ischemic levels. MDA levels in the two groups
peaked 2 h after reperfusion, and subsequently declined. At all
time points, the levels of MDA were significantly decreased
(P<0.05; Table I) in the MT group
following liver ischemia, as compared with those of the IR
group.
 | Table I.Malondialdehyde activity in rats with
ischemia-reperfusion injury (nmol/mg prot). |
Table I.
Malondialdehyde activity in rats with
ischemia-reperfusion injury (nmol/mg prot).
|
| Time after
reperfusion (hours) |
|---|
|
|
|
|---|
| Group | 0 | 2 | 4 | 8 | 24 |
|---|
| N | 28.02±0.88 | – | – | – | – |
| IR |
43.40±0.97a |
58.07±0.95a |
53.38±0.67a |
45.17±0.4a |
44.73±0.75a |
| MT |
41.47±0.84a,b |
56.65±0.13a,b |
46.73±1.10a,b |
43.08±0.48a,b |
43.08±0.48a,b |
MT administration increases SOD
activity following ischemia
SOD values were lower at each time point after
ischemia in the IR and MT groups, as compared with the N group
(P<0.05). SOD levels of the two groups were similar to those of
Group N at 24 h post-reperfusion. Following liver ischemia, the SOD
activities in the MT group were significantly increased (P<0.05;
Table II) at all time points, as
compared with those in the IR group.
 | Table II.Superoxide dismutase activity in rats
with ischemia-reperfusion injury (U/mg prot). |
Table II.
Superoxide dismutase activity in rats
with ischemia-reperfusion injury (U/mg prot).
|
| Time after
reperfusion (hours) |
|---|
|
|
|
|---|
| Group | 0 | 2 | 4 | 8 | 24 |
|---|
| N | 91.33±0.60 | – | – | – | – |
| IR |
35.98±1.18a |
47.17±1.78a |
50.50±1.81a |
60.10±0.70a |
84.97±0.61a |
| MT |
41.63±0.85a,b |
53.35±3.98a,b |
52.94±1.72a,b |
63.62±1.97a,b |
86.45±0.65a,b |
MT administration increases GSH levels
following ischemia
At each post-ischemic time point, the GSH values of
the IR and MT groups were significantly reduced (P<0.05), as
compared with those of group N; however, at 8 h post-reperfusion,
the GSH levels of the two groups were similar to that of group N.
Furthermore, the GSH levels in group MT were significantly higher
than those of the IR group at 2, 4, and 8 h post-reperfusion
(P<0.05; Table III).
 | Table III.Glutathione activity changes of rats
with ischemia-reperfusion injury (U/mgprot). |
Table III.
Glutathione activity changes of rats
with ischemia-reperfusion injury (U/mgprot).
|
| Time after
reperfusion (hours) |
|---|
|
|
|
|---|
| Group | 0 | 2 | 4 | 8 | 24 |
|---|
| N | 97.17±0.73 | – | – | – | – |
| IR |
77.90±1.85a |
75.93±1.50a |
76.50±2.02a |
84.75±0.87a |
83.07±0.25a |
| MT |
76.63±1.46a,b |
80.28±2.59a,b |
83.35±0.29a,b |
86.77±0.59a,b |
82.98±1.00a,b |
MT administration significantly
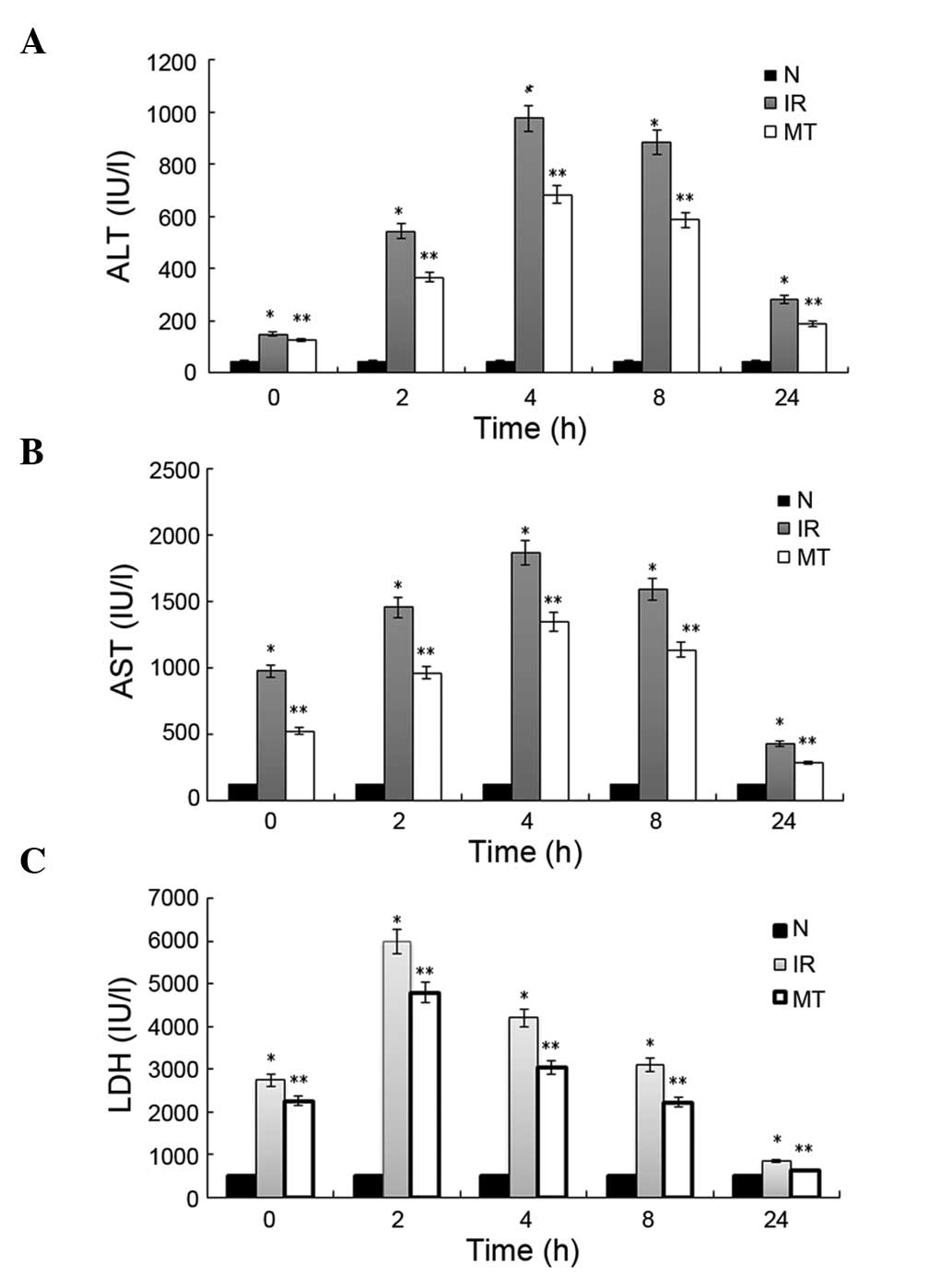
decreases ALT levels following ischemia
The ALT values corresponding to 35 min of hepatic
portal occlusion, and 2, 4, 8 and 24 h post-reperfusion, were
150.17±13.85, 543.93±50.50, 976.50±32.92, 884.75±21.78 and
281.87±10.75 IU/l, respectively, in the IR group; as compared with
127.63±19.46, 367.28±12.53, 683.35±20.21, 586.77±29.57 and
188.08±11.09 IU/l in the MT treatment group. All of the ALT values
were significantly higher than those determined in the N group
(46.22±8.28, P<0.05). Furthermore, in the MT group, the ALT
levels were significantly lower (P<0.05) than those determined
in the IR group, at each time point (Fig. 1A).
MT administration significantly
decreases AST levels following ischemia
The AST values corresponding to 35 min of hepatic
portal occlusion, and 2, 4, 8 and 24 h post-reperfusion, were
974.70±53.65, 1453.73±56.70, 1866.53±156.46, 1588.56±141.89 and
428.76±35.23 IU/l, respectively, in the IR group; as compared with
521.83±29.46, 961.24±62.45, 1345.98±167.78, 1134.45±129.68 and
283.79±21.43 IU/l in the MT group. All of these AST values were
significantly higher than those determined in the N group
(115.22±15.08; P<0.05). Furthermore, in the MT group, the AST
levels were significantly lower (P<0.05) than those of the IR
group, at each time point (Fig.
1B).
MT administration significantly
decreases LDH levels following ischemia
The LDH values corresponding to 35 min of hepatic
portal occlusion, and 2, 4, 8 and 24 h post-reperfusion were
2749.67±278.23, 5987.32±956.77, 4207.65±715.54, 3098.11±556.90 and
856.11±130.42 IU/l, respectively, in the IR group, as compared with
2251.23±329.61, 4789.12±1053.12, 3045.45±677.21, 2229.05±629.53 and
637.31±91.02 in the MT group. All of the LDH values were
significantly higher than those determined in the N group
(509.56±100.67; P<0.05). Furthermore, in the MT group, the LDH
activities were significantly lower (P<0.05) than that of the IR
group, at each time point (Fig.
1C).
Discussion
Hepatectomy is the surgical resection of the liver
and is considered the main therapeutic strategy for the treatment
of numerous liver diseases. Since the liver has an abundant blood
supply, hepatic portal occlusion is often adopted in order to
reduce hemorrhage during the operation; however, this method may
lead to hepatic IRI (14).
In the present study, in order to generate a rat
model of hepatic acute IRI, an atraumatic vascular clamp was used
to completely block the first porta hepatis, which was reopened
following 35 min in order to recover the hepatic blood inflow. The
present study demonstrated that, at the following time points: 35
min of hepatic portal occlusion, and 2, 4, 8 and 24 h
post-reperfusion, MDA levels were significantly increased
(P<0.05); whereas the levels of SOD and GSH were significantly
decreased (P<0.05) in the liver tissue, as compared with the
normal group. SOD and GSH are endogenous antioxidant enzymes which
clear oxygen free radicals and protect cells from oxidative damage
(15). As a product of lipid
peroxidation, MDA reflects the state of oxidative damage. Large
quantities of oxygen free radicals may cause serious damage to the
body, resulting in: DNA degradation, protein oxidation, lipid
peroxidation and cell death. In the present study, SOD and GSH
activities were decreased in response to IR, whereas MDA content
increased, which indirectly demonstrated that oxygen free radicals
were predominantly produced in this process, the endogenous
antioxidant was consumed, and cells were exposed to lipid
peroxidation damage.
Hepatic portal occlusion can cause hepatic ischemia
and hypoxia, therefore it may affect the synthesis of mitochondrial
energy and electron transport in the respiratory chain (16,17),
producing numerous oxygen free radicals, which cause cell damage
via lipid peroxidation of biological membranes (18). Lipid peroxidation itself produces
toxic substances, including MDA. Furthermore, in addition to
directly damaging the structure of cells (19), hypoxia can induce disturbances in
hepatic microcirculation, leading to further ischemia and anoxic
liver damage (20). MT consists of
both hydrophilic and hydrophobic moieties, and is synthesized by
the PG, which exists in all vertebrates. In addition to regulating
biological rhythms, anti-inflammatory actions and sleep onset
latency, previous studies have demonstrated MT is a powerful free
radical scavenger. MT is capable of removing various oxygen free
radicals, particularly hydroxyl free radicals (21,22). Its
ability to clear hydroxyl radicals is 500 times stronger than
benzoate, a conventional hydroxyl radical scavenger (23). A previous study (24) demonstrated that MT is able to inhibit
the production of excessive free radicals in myocardial IR, and can
directly eliminate the formed free radicals and the activities of
its related reactants, demonstrating that MT has a protective
effect in the myocardium following IR.
Under normal physiological conditions, only a small
amount of oxygen free radicals are produced during normal metabolic
processes. In this event, the radical is immediately cleared by the
in vivo defense mechanisms in order to minimize further
damage. A large quantity of oxygen free radicals are generated in
the liver during IRI and, due to ischemia, the intrahepatic defense
mechanisms are disordered, therefore the radical cannot be cleared
effectively, thus leading to the characteristic cellular damage.
The damage induced by oxygen free radicals can be summarized as
follows: Peroxidation of polyunsaturated fatty acids (PUFA) in cell
membranes induces crosslinking in biological macromolecules such as
DNA, RNA and proteins, in addition to an oxidation reaction, which
prompts the polymerization and depolymerization of polysaccharide
molecules (25,26). Therefore, ischemic injury is
characterized by a reduction in selective PUFA and is marked by
lipid peroxidation of the biological membrane, with MDA as the
metabolic product of membrane lipid peroxidation. By inducing a
cross-linking reaction between proteins and their respective
primary amino groups, MDA leads to the destruction of cell
function. Therefore, by measuring the serum content of MDA, the
degree of cell membrane damage can be indirectly determined
(27). In the present study, the MDA
increase was significantly lower in the MT group, as compared with
that of the IRI group, thus suggesting that MT may promote the
degradation of oxygen free radicals into non-toxic or low toxic
substances, and thus enhance antioxidant capacity.
SOD is an enzyme that contains metal cofactors,
which are capable of catalyzing the dismutation of superoxide
anions, thus removing oxygen free radicals and protecting cells
(18). The results of the present
study demonstrated that, at each time point of rat liver IRI, SOD
activity in the IR group was significantly decreased, as compared
with the MT group; whereas the MDA content was significantly
increased, as compared with the MT group. These results
demonstrated that the administration of MT may partially reverse
the decline of SOD activity following ischemia, whilst
simultaneously maintaining the scavenging ability of ischemic
tissue to oxygen free radicals, thus reducing damage associated
with lipid peroxidation. Parlaktas et al (28) and Kerem et al (29) have also shown that MT can promote the
activity of SOD.
In the present study, in the early stages of IR, SOD
levels were significantly reduced in the two groups, as compared
with the normal group; and SOD rose gradually as time progressed.
The SOD levels were increasing continuously from 8 h
post-reperfusion, while MDA dropped to the relatively low degree
from the highest point following reperfusion. The changes in SOD
and MDA reflect the respective protective and destructive effects
induced by IRI. SOD activity was increased in the groups without MT
following reperfusion in a time-dependent manner; this may be a
compensatory response to the huge increase in oxygen free radicals
and MDA during reperfusion. In addition, SOD activity was increased
in the MT group, as compared with the IR group, and the serum
content of MDA was significantly decreased, suggesting that oxygen
radical scavenging in the MT group was associated with MT, although
it does not entirely depend on the effects of SOD. Therefore,
protecting SOD activity and reducing the content of oxygen free
radicals may be one of the mechanisms associated with MT and its
protective role in IRI.
In the present study, the mitochondrial respiration
rate significantly increased following IR, and a large number of
free radicals were produced. Free radicals produced in vivo
can easily damage unsaturated fatty acid of lipids in cells,
leading to lipid peroxidation. In addition to stabilizing the cell
membrane and various other of membranous organelles, MT can protect
the mitochondria (30). Mitochondria
maintain the physiological function of cells; however they are also
the most sensitive organelles to reperfusion injury. This damage
presents as transmembrane potential dissipation and disorder of
cell energy and metabolism; eventually leading to irreversible cell
death. Since mitochondria have a key role in the occurrence and
development of IRI, reducing or preventing mitochondrial damage may
protect reperfused liver cells. Mitochondrial damage is mainly
caused by oxygen free radicals and calcium overload (31) in the process of IR, which mainly
presents as a decrease in GSH, which is a mitochondrial
antioxidant.
Mitochondrial damage may induce obstacles to
oxidative phosphorylation, thus damaging the cells' ability to
synthesize ATP. Following reperfusion, the synthesis of ATP remains
low, which is not conducive to the recovery of cell function. The
present study demonstrated that administration of MT decreased the
MDA levels, which may indicate damage to the mitochondrial
membrane. This suggests that MT may reduce damage caused by oxygen
free radicals and reduce calcium overload. Furthermore, the present
study demonstrated that MT increased mitochondrial GSH levels, thus
increasing its ability to resist damage. These results suggested
that MT may: Maintain the structural integrity of mitochondria,
maintain normal cell oxidative phosphorylation function, promote
the formation of ATP in reperfused liver cells and reduce the
IRI-induced damage in liver cells.
In conclusion, MT may clear oxygen free radicals in
three ways: i) Directly combine with free radicals to prevent the
chain reaction of free radical oxidation; ii) maintain and protect
activity of key enzymes in the antioxidant system, such as
glutathione peroxidase and glucose-6-phosphate dehydrogenase, and
limit the production of oxygen free radicals (32); and iii) reduce electron leakage and
free radical generation in the mitochondrial respiratory chain
(33). In particular, the latter two
indirect antioxidant effects amplify the protection against damage
caused by free radicals.
Serum ALT is highest in the liver. ALT is one of the
most sensitive liver function indices (34), as long as 1% of liver cells undergo
necrosis, the serum ALT can be increased by 1 over time. In the
present study, as compared with the N group, ALT levels were
significantly increased in the MT and IR groups following IRI, and
this increase continued for 24 h post-reperfusion. As compared with
the N group, the levels of AST and LDH, which can reflect acute
liver damage, were also raised, demonstrating the destructive
effect of IRI on liver function. Notably, these three indices for
liver function were significantly reduced in the MT group at all
time points following reperfusion in the present study, as compared
with the IR group. These results suggested that rat liver IRI was
significantly reduced following MT treatment. Therefore, exogenous
MT may improve liver function following IRI, and may have a
protective role in reperfusion injury caused by hepatic portal
occlusion.
In conclusion, the present study demonstrated that
MT treatment may effectively remove oxygen free radicals, thus
reducing liver function damage; attenuate the increased levels of
MDA, and decrease the levels of SOD and GSH caused by hepatic IR.
Therefore the administration of MT may create favorable conditions
for the recovery of liver function following IRI, confirming MT has
strong antioxidant properties. Furthermore, no serious side effects
of MT have been demonstrated yet; therefore, MT may be a promising
therapeutic agent that offers clinical protection from reperfusion
injury in the liver.
Acknowledgements
This study was supported by a grant from the
Scientific Research Innovation Project of Shanghai Education
Committee, China (no. 13yz040) and by the Shanghai Municipal
Commission of Health and Family Planning Fund (award no.
20154Y0207).
References
|
1
|
Ebrahimkhani MR, Daneshmand A, Mazumder A,
Allocca M, Calvo JA, Abolhassani N, Jhun I, Muthupalani S, Ayata C
and Samson LD: Aag-initiated base excision repair promotes ischemia
reperfusion injury in liver, brain, and kidney. Proc Natl Acad Sci
USA. 111:E4878–E4886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rao J, Qin J, Qian X, Lu L, Wang P, Wu Z,
Zhai Y, Zhang F, Li G and Wang X: Lipopolysaccharide
preconditioning protects hepatocytes from ischemia/reperfusion
injury (IRI) through inhibiting ATF4-CHOP pathway in mice. PLoS
One. 8:e655682013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García JJ, López-Pingarrón L,
Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter
RJ, Ramírez JM and Bernal-Pérez M: Protective effects of melatonin
in reducing oxidative stress and in preserving the fluidity of
biological membranes: A review. J Pineal Res. 56:225–237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Acuña-Castroviejo D, Escames G, Venegas C,
Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martins PN and Markmann JF: Age-related
differences in hepatic ischemia/reperfusion: Gene activation, liver
injury, and protective effect of melatonin. J Surg Res. 85:e19–e21.
2013. View Article : Google Scholar
|
|
6
|
Frich L, Mala T and Gladhaug IP: Hepatic
radiofrequency ablation using perfusion electrodes in a pig model:
Effect of the Pringle manoeuvre. Eur J Surg Oncol. 32:527–532.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh PO: Melatonin prevents hepatic
injury-induced decrease in Akt downstream targets phosphorylations.
J Pineal Res. 51:214–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aydogan MS, Erdogan MA, Polat A, Yücel A,
Ozgül U, Parlakpinar H, Duran ZR, Yildiz A and Durmus M: Protective
effects of melatonin and β-d-glucan against liver injury in rats -
a comparative study. Adv Clin Exp Med. 22:621–627. 2012.
|
|
9
|
Kireev R, Bitoun S, Cuesta S, Tejerina A,
Ibarrola C, Moreno E, Vara E and Tresguerres JA: Melatonin
treatment protects liver of Zucker rats after ischemia/reperfusion
by diminishing oxidative stress and apoptosis. Eur J Pharmacol.
701:185–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kireev RA, Cuesta S, Ibarrola C, Bela T,
Gonzalez EM, Vara E and Tresguerres JA: Age-related differences in
hepatic ischemia/reperfusion: Gene activation, liver injury, and
protective effect of melatonin. J Surg Res. 178:922–934. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang JW and Lee SM: Melatonin inhibits
type 1 interferon signaling of toll-like receptor 4 via heme
oxygenase-1 induction in hepatic ischemia/reperfusion. J Pineal
Res. 53:67–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang JW, Koh EJ and Lee SM: Melatonin
protects liver against ischemia and reperfusion injury through
inhibition of toll-like receptor signaling pathway. J Pineal Res.
50:403–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Selvam NT, Venkatakrishnan V, Dhamodharan
R, Murugesan S and Kumar SD: Hepatoprotective activity of
methanolic extract of Syzygium jambos (Linn.) leaf against
paracetamol intoxicated Wistar albino rats. Ayu. 34:305–308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin LM, Jin SF, Liu YX, Zhou L, Xie HY,
Yan S, Xu X and Zheng SS: Ischemic preconditioning enhances
hepatocyte proliferation in the early phase after ischemia under
hemi-hepatectomy in rats. Hepatobiliary Pancreat Dis Int.
11:521–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pires KM, Lanzetti M, Rueff-Barroso CR,
Castro P, Abrahão A, Koatz VL, Valença SS and Porto LC: Oxidative
damage in alveolar macrophages exposed to cigarette smoke extract
and participation of nitric oxide in redox balance. Toxicol In
Vitro. 26:791–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papadopoulos D, Siempis T, Theodorakou E
and Tsoulfas G: Hepatic ischemia and reperfusion injury and trauma:
Current concepts. Arch Trauma Res. 2:63–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peralta C, Jiménez-Castro MB and
Gracia-Sancho J: Hepatic ischemia and reperfusion injury: Effects
on the liver sinusoidal milieu. J Hepatol. 59:1094–1106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaeschke H and Woolbright BL: Current
strategies to minimize hepatic ischemia-reperfusion injury by
targeting reactive oxygen species. Transplant Rev (Orlando).
26:103–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaeschke H: Molecular mechanisms of
hepatic ischemia-reperfusion injury and preconditioning. Am J
Physiol Gastrointest Liver Physiol. 284:G15–G26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mendes-Braz M, Elias-Miró M,
Jiménez-Castro MB, Casillas-Ramírez A, Ramalho FS and Peralta C:
The current state of knowledge of hepatic ischemia-reperfusion
injury based on its study in experimental models. J Biomed
Biotechnol. 2012:2986572012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai J, Dong L, Song Z, Ge H, Cai X, Wang G
and Liu P: The role of melatonin as an antioxidant in human lens
epithelial cells. Free Radic Res. 47:635–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vishwas DK, Mukherjee A, Haldar C, Dash D
and Nayak MK: Improvement of oxidative stress and immunity by
melatonin: An age dependent study in golden hamster. Exp Gerontol.
48:168–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bandyopadhyay D, Biswas K, Bandyopadhyay
U, Reiter RJ and Banerjee RK: Melatonin protects against
stress-induced gastric lesions by scavenging the hydroxyl radical.
J Pineal Res. 29:143–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dominguez-Rodriguez A and Abreu-Gonzalez
P: Myocardial ischemia-reperfusion injury: Possible role of
melatonin. World J Cardiol. 2:233–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elias-Miró M, Jiménez-Castro MB, Rodés J
and Peralta C: Current knowledge on oxidative stress in hepatic
ischemia/reperfusion. Free Radic Res. 47:555–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Golen RF, van Gulik TM and Heger M:
Mechanistic overview of reactive species-induced degradation of the
endothelial glycocalyx during hepatic ischemia/reperfusion injury.
Free Radic Biol Med. 52:1382–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palladini G, Ferrigno A, Rizzo V,
Tarantola E, Bertone V, Freitas I, Perlini S, Richelmi P and
Vairetti M: Lung matrix metalloproteinase activation following
partial hepatic ischemia/reperfusion injury in rats.
ScientificWorldJournal. 2014:8675482014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parlaktas BS, Atilgan D, Ozyurt H, Gencten
Y, Akbas A, Erdemir F and Uluocak N: The biochemical effects of
ischemia-reperfusion injury in the ipsilateral and contralateral
testes of rats and the protective role of melatonin. Asian J
Androl. 16:314–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kerem H, Akdemir O, Ates U, Uyanıkgıl Y,
Sezer Demırel E, Bılkay U, Turgut M, Sozmen E and Songur E: The
effect of melatonin on a dorsal skin flap model. J Invest Surg.
27:57–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ortiz F, García J A, Acuña-Castroviejo D,
Doerrier C, López A, Venegas C, Volt H, Luna-Sánchez M, López LC
and Escames G: The beneficial effects of melatonin against heart
mitochondrial impairment during sepsis: Inhibition of iNOS and
preservation of nNOS. J Pineal Res. 56:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hernández-Esquivel L, Pavón N,
Buelna-Chontal M, González-Pacheco H, Belmont J and Chávez E:
Citicoline (CDP-choline) protects myocardium from
ischemia/reperfusion injury via inhibiting mitochondrial
permeability transition. Life Sci. 96:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang P, Yin L, Liang D, Li C, Ma F and Yue
Z: Delayed senescence of apple leaves by exogenous melatonin
treatment: Toward regulating the ascorbate-glutathione cycle. J
Pineal Res. 53:11–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reiter RJ, Tan DX, Manchester LC and
El-Sawi MR: Melatonin reduces oxidant damage and promotes
mitochondrial respiration: Implications for aging. Ann NY Acad Sci.
959:238–250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lhuillier F, Parmantier P, Goudable J,
Crova P, Delafosse B, Annat G, Cespuglio R and Viale JP: Hepatic
ischemia is associated with an increase in liver parenchyma nitric
oxide that is in part enzyme-independent. Anesthesiology.
98:373–378. 2003. View Article : Google Scholar : PubMed/NCBI
|