Introduction
Collapsing glomerulopathy (CG) is a distinct
clinical and pathological entity of idiopathic nephrotic syndrome,
and its incidence in Asia has increased in recent years (1). The Columbia classification defines CG
as the presence of glomerular capillary collapse in ≥1 glomerulus
(2). It exhibits marked hypertrophy
and hyperplasia of the visceral epithelial cells, which is
accompanied by tubular-interstitial injury and high resistance to
immunosuppressive therapy (3).
The key epidemiological factors associated with CG
include recreational drug use, hematolymphoid malignancies,
autoimmune diseases and genetic mutations. CG has also been linked
to infection with several types of viruses, including human
immunodeficiency virus (HIV), Epstein-Barr virus (also called human
herpesvirus-4), cytomegalovirus (CMV) and parvovirus B19 (4,5). The
present study reports a case of relapsing CG unusually associated
with a coxsackie virus infection.
Case report
A 38-year-old Chinese man was admitted to the Second
Xiangya Hospital of the Central South University (Changsha, China)
on 22nd January 2008 presenting with eyelid edema and
anuria for 10 days. A 24-h urine protein test (cat. no. 11877801
190; Roche Diagnostics GmbH, Mannheim, Germany) result was 5.3 g.
No serum HIV-1 (Intec Products, Inc., Anaheim, CA, USA), nor plasma
CMV (cat. no. 3400637; Beijing Beier Biological Engineering, Co.,
Ltd., Beijing, China) or hepatitis B and C antibodies (Shanghai
Kehua Biotech Co., Ltd., Shanghai, China), were detected, although
high-titer serum coxsackie virus-specific immunoglobulin M (IgM;
cat. no. 3400604; Beijing Beier Biological Engineering, Co., Ltd.)
was observed. Serum creatinine levels were markedly increased (cat
no. 77789; KANTO-PPC Inc., Shanghai, China), reaching 20.33 mg/dl
and requiring hemodialysis.
Routine blood tests (Siemens Healthcare GmbH,
Erlangen, Germany) detected the following: Hemoglobin, 97 g/l;
total white blood cell count, 14.3×109 cells/l;
neutrophils, 79.2%; and lymphocytes, 6%. Serum creatinine and
albumin levels (cat no. 811RCM; Sekisui Medical, Co., Ltd., Tokyo,
Japan) were 18.38 mg/dl and 23.4 g/l, respectively. A peripheral
blood smear examination by light microscopy (Olympus BX51; Olympus
Corporation, Tokyo, Japan) detected reactive lymphocytes. An
abdominal ultrasound (SIEMENS CV70; Siemens Healthcare GmbH)
revealed that the right kidney measured 135×66 mm and the left
122×68 mm. Coagulation function tests detected the following:
Activated partial thromboplastin time (cat. no. 557155; Siemens
Healthcare GmbH), 39.8 sec; fibrinogen degradation product (cat.
no. 852RCM; Sekisui Medical, Co., Ltd.), 94.9 µg/ml; D-dimer (cat.
no. OPBP07; Siemens Healthcare GmbH), 46.34 µg/ml; and fibrinogen
(cat. no. B4233-27; Siemens Healthcare GmbH), 5.36 g/l. With regard
to immunological parameters, the levels of complement C3 and C4
were 0.6 and 0.13 g/l, respectively (cat. nos. 446450 and 446490,
respectively; Beckman Coulter, Inc., Brea, CA, USA). Antinuclear
and antineutrophil cytoplasmic antibodies were negative (cat. no.
708297; Inova Diagnostics Inc., San Diego, CA, USA). No serum
paraprotein, nor urinary Bence-Jones protein κ or λ (cat. nos.
446440 and 446470, respectively; Beckman Coulter, Inc.), were
detected. Serum angiotensin-converting enzyme activity was shown to
be within the normal range using a commercially available kit (cat.
no. MA125290; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
The patient underwent an initial renal biopsy 5 days
following admission. The fresh renal tissue was fixed in 10%
formaldehyde solution (Changsha Antai Fine Chemical Co., Ltd.,
Changsha, China), dehydrated using ethanol and turpentine oil
(Hengyang Kaixin Chemical Reagent Co., Ltd., Hengyang, China) and
paraffin-embedded (Leica Microsystems GmbH, Wetzlar, Germany),
after which 2-µm sections were cut for staining with haematoxylin
& eosin (Shanghai Lanji Science and Technology Development Co.,
Ltd., Shanghai, China), Periodic Schiff-Methenamine Silver
(Sigma-Aldrich, St. Louis, MO, USA), Masson's trichrome (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) and Periodic
acid-Schiff (Sigma-Aldrich) solutions. Light microscopy (Olympus
BX51) revealed that 6/9 glomeruli exhibited segmental collapse of
the tuft with marked hypertrophy and hyperplasia of the visceral
epithelial cells (Fig. 1A).
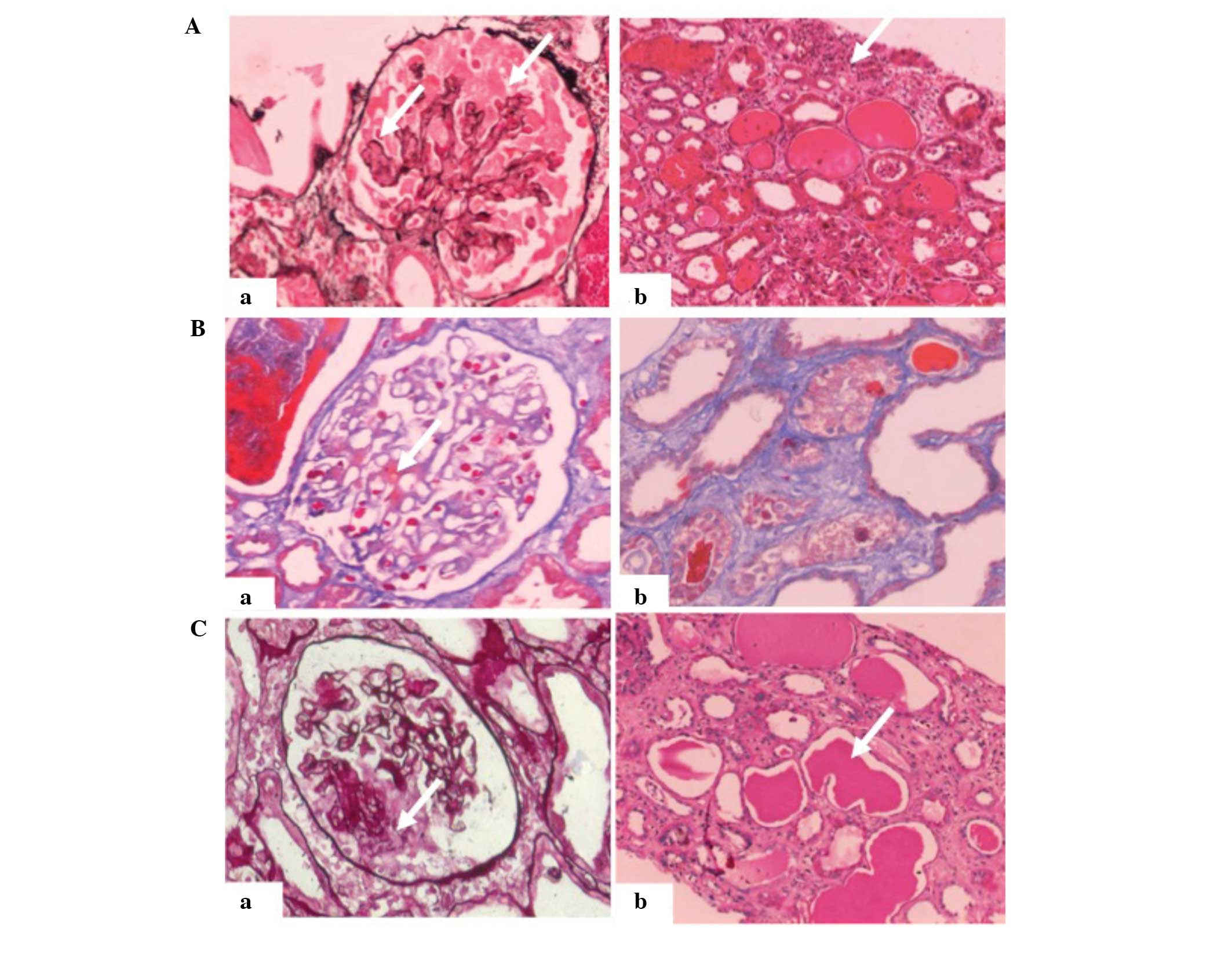 | Figure 1.Renal biopsies of a 38-year-old man
with collapsing glomerulopathy. (A) First renal biopsy. Light
microscopy showed collapsing lesions and visceral epithelial cell
proliferation with (a) vacuolar changes (PASM staining;
magnification, ×200; white arrow) and (b) proteinaceous casts and
interstitial inflammation (hematoxylin and eosin staining;
magnification, ×200; white arrow). (B) Second renal biopsy, 41 days
after the first. Light microscopy showed (a) mild mesangial
proliferation in the glomerulus and immune deposits in the area of
the mesothelium (Masson's trichrome staining; magnification, ×200;
white arrow), as well as (b) localized tubular atrophy and
interstitial fibrosis (Masson's trichrome staining; magnification,
×200). (C) Third renal biopsy, ~3 months after the first. Light
microscopy showed (a) segmental capillary collapse with
hyperplastic visceral epithelial cells with vacuoles (PASM
staining; magnification, ×200, white arrow) and (b) tubular cell
flattening with massive proteinaceous casts (periodic acid-Schiff
staining; magnification, ×200, white arrow). PASM, periodic
Schiff-methenamine. |
For immunostaining, renal tissue sections were
incubated with fluorescein isothiocyanate (FITC)-conjugated rabbit
anti-human complement C3 polyclonal antibody (1:40; F0201; Dako
North America, Inc., Carpinteria, CA, USA). Immunofluorescence (IF;
Olympus BX51) showed C3-positive staining in the mesangial area of
glomeruli. Kidney tissue sections for electron microscopy were
fixed in 2–5% glutaraldehyde (Ted Pella, Inc., Redding, CA, USA) at
4°C for 1 h and post-fixed in 1% osmic acid (Ted Pella, Inc.) for 2
h. The tissues were then dehydrated through graded ethanol and
acetone (Tianjin Fuyu Jingxihuagong, Tianjin, China) and embedded
in Epon 812 (Structure Probe Inc., West Chester, PA, USA). Electron
microscopy (EM; Hitachi H7500, Hitachi, Ltd., Tokyo, Japan)
revealed thickening of the capillary wall and extensive effacement
of podocyte foot processes (Fig. 2).
No immune deposits were found by EM. The pathological diagnosis of
CG was made.
The patient was intravenously administered a high
dose of anti-inflammatory therapy, including 0.25 g Acyclovir
(Yabao Pharmaceutical Group Co., Ltd., Yuncheng, China) in 250 ml
saline, together with pulse steroids (500 mg/day for 3 days; Paiang
Pharmaceuticals Co., Ltd., Xi'an, China) and rapamycin (2 mg once
daily; Pfizer, Inc., New York, NY, USA), after which hemodialysis
(SWS-4000A Hemodialysis Equipment; SWS Medical, Chongqing, China)
was performed. The urinary protein test yielded a negative result
and serum creatinine returned to its normal levels.
The second renal biopsy was performed 41 days after
the first. This time, only 1 in 16 glomeruli exhibited glomerular
sclerosis (Fig. 1B). Mild
proliferation of mesangial cells and focal segmental thickening
were observed in the capillary walls. Capillary loops were dilated
without collapse; however, IF using FITC-conjugated rabbit
anti-human IgM (1:40; F0203; Dako North America, Inc.) and rabbit
anti-human C1q (1:40; F0254; Dako North America, Inc.) polyclonal
antibodies showed a small amount of nonspecific mesangial trapping
of IgM and complement C1q trapping within the glomeruli. Serum
coxsackie virus-specific IgM testing was negative. EM revealed
well-formed podocyte foot processes (not shown).
On April 15, 2008, the patient presented again with
fever. The 24-h urine protein test result was 5.6 g and the serum
creatinine level had increased to 11.00 mg/dl. High-level serum
coxsackie virus-specific IgM was detected. A third renal biopsy was
performed on April 20, 2008. The results showed pathological
changes similar to those shown by the initial biopsy (Fig. 1C). Segmental or global capillary
collapse and hyperplastic epithelial cells were found in all
glomeruli. The tubules showed evident tubular cell flattening with
massive proteinaceous casts. Immunohistochemistry was negative. The
diagnosis made was that of recurrent CG. The renal function of the
patient showed no sign of improvement following treatment with
Acyclovir (0.25 g in 250 ml saline), pulse steroids (500 mg/day for
3 days) and rapamycin (2 mg once daily). Therefore, the patient was
diagnosed with end-stage renal disease (ESRD) and is currently
receiving hemodialysis three times a week. Written informed consent
was obtained from the patient for publication of the present
study.
Discussion
The majority of coxsackie virus infections are
asymptomatic; however, in a few cases such infections can be
life-threatening (6). There are a
limited number of case reports of any type of renal involvement in
patients with coxsackie virus infection, including severe
rhabdomyolysis and acute renal failure (7–9). To the
best of our knowledge, this is the first case report of CG
associated with coxsackie virus infection.
In the present study, coxsackie virus infection was
detected in the first biopsy, but became undetectable following
treatment, and was followed by recovery. However, ~3 months later,
serological testing for coxsackie virus antibody was positive once
again, and was accompanied by the recurrence of the disease. This
indicates that there is a strong association between the
development of CG and coxsackie virus infection.
The pathogenesis of CG is not fully understood.
Several studies have indicated that dysregulation of the podocyte
phenotype is involved in the pathogenesis of CG (10,11).
Furthermore, proximal tubular epithelial cells and glomerular
podocytes have been found to be highly susceptible to coxsackie
virus, which thus leads to cytolysis, suggesting that coxsackie
virus infections may be associated with acute and progressive renal
injury (12). This supports the
hypothesis that coxsackie virus infection may have been the main
precipitating factor for the development of CG in the present
patient.
CG is considered to be an aggressive renal disease,
which usually follows a rapid course to ESRD. In a study by Conaldi
et al (12), the response of
CG to corticosteroids and cyclophosphamide treatment was poor. In
the present case, it was demonstrated that this severe subset of
FSGS could be relieved by early intervention and effective
treatment with antiviral drugs, such as pulse steroid therapy and
rapamycin. Following these treatments, the coxsackie virus antibody
became undetectable. In addition, the second renal biopsy revealed
showed relatively mild injury of glomeruli in the renal tissue of
the patient, which suggests that HIV-negative CG could be less
pernicious than it is considered to be, and could even be recovered
under the appropriate therapy.
In conclusion, the present case of recurrent CG was
found to be closely associated with relapsed coxsackie virus
infection. Despite the recurrence of the disease, however, it is
noteworthy that the patient temporarily recovered under antiviral
treatment, using pulse steroids and rapamycin.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant. no. 81300600) and the
Free Explore Plan of Central South University (grant no.
2012QNZT146).
Glossary
Abbreviations
Abbreviations:
|
CG
|
collapsing glomerulopathy
|
|
EM
|
electron microscopy
|
|
CMV
|
cytomegalovirus
|
References
|
1
|
Albaqumi M and Barisoni L: Current views
on collapsing glomerulopathy. J Am Soc Nephrol. 19:1276–1281. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Agati VD, Fogo AB, Bruijn JA and
Jennette JC: Pathologic classification of focal segmental
glomerulosclerosis: A working proposal. Am J Kidney Dis.
43:368–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mubarak M and Kazi JI: Collapsing FSGS: A
clinicopathologic study of 10 cases from Pakistan. Clin Exp
Nephrol. 14:222–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura T, Yasuda K, Obi Y, Satoh T, Namba
T, Sasaki K, Muramoto N, Wada A, Rakugi H, Isaka Y and Hayashi T:
Case of HIV-associated nephropathy accompanied by nephrotic
syndrome and acute worsening of kidney function. Nihon Jinzo Gakkai
Shi. 54:94–98. 2012.(In Japanese). PubMed/NCBI
|
|
5
|
Presne C, Cordonnier C, Makdassi R, Pruna
A and Fournier A: Collapsing glomerulopathy and cytomegalovirus,
what are the links? Presse Med. 29:1815–1817. 2000.(In French).
PubMed/NCBI
|
|
6
|
Sin J, Mangale V, Thienphrapa W, Gottlieb
RA and Feuer R: Recent progress in understanding coxsackievirus
replication, dissemination, and pathogenesis. Virology.
484:288–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fodili Fl and van Bommel EF: Severe
rhabdomyolysis and acute renal failure following recent Coxsackie B
virus infection. Neth J Med. 61:177–179. 2003.PubMed/NCBI
|
|
8
|
Yen MH, Lee JJ, Yeh CF, Wang KC, Chiang
YW, Chiang LC and Chang JS: Yakammaoto inhibited human
coxsackievirus B4 (CVB4)-induced airway and renal tubular injuries
by preventing viral attachment, internalization, and replication. J
Ethnopharmacol. 151:1056–1063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kollios KD, Skiadopoulou E, Siondi I and
Papadopoulou ZL: Coxsackie virus B1 infection in a child,
complicated by severe oliguric renal failure. Clin Nephrol.
67:260–262. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhdanova O, Srivastava S, Di L, Li Z,
Tchelebi L, Dworkin S, Johnstone DB, Zavadil J, Chong MM, Littman
DR, et al: The inducible deletion of Drosha and microRNAs in mature
podocytes results in a collapsing glomerulopathy. Kidney Int.
80:719–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barisoni L and Nelson PJ: Collapsing
glomerulopathy: An inflammatory podocytopathy? Curr Opin Nephrol
Hypertens. 16:192–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conaldi PG, Biancone L, Bottelli A, De
Martino A, Camussi G and Toniolo A: Distinct pathogenic effects of
group B coxsackieviruses on human glomerular and tubular kidney
cells. J Virol. 71:9180–9187. 1997.PubMed/NCBI
|
















