Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare
clonal hematopoietic disorder arising from a mutation in the X
chromosome-linked phosphatidylinositol glycan anchor biosynthesis,
class A (PIGA), leading to a deficiency of proteins linked to the
cell membrane via glycophosphatidylinositol (GPI) anchors. Abnormal
and normal clones co-exist in the bone marrow of patients and the
disease is characterized by bone marrow failure, intravascular
hemolysis and thrombosis (1,2). The clinical association between PNH and
aplastic anemia (AA) has long been recognized. PNH frequently
occurs in association with suppressed hematopoiesis, including bone
marrow failure syndrome. Hemolytic PNH can occur in the setting of
AA, and conversely AA can be a late complication of PNH (3).
In a previous study, we confirmed that the
expression levels of the erythropoietin receptor (EPOR) and
thrombopoietin receptor (TPOR) on bone marrow (BM) GPI−
hematopoietic stem cells [HSCs; cluster of differentiation
(CD)34+CD59−] in patients with PNH are
significantly lower than those on the BM GPI+ HSCs
(CD34+CD59+) in these patients (4). Furthermore, in vitro, following
stimulation with erythropoietin (EPO) and thrombopoietin (TPO), the
signal transducer and activator of transcription 5 (STAT5)
phosphorylation levels of the EPOR and TPOR in the GPI+
clone of patients with PNH were clearly superior to those in the
GPI− clone (5).
The aim of the present study was to further
investigate the GPI− HSC response to granulocyte
colony-stimulating factor (G-CSF) and stem cell factor (SCF) in
PNH/AA syndrome. The expression of their respective receptors,
CD114 and CD117, on GPI− and GPI+ HSCs were
explored, and the mean fluorescence intensity (MFI) of the
intracellular signaling pathway proteins STAT5 and phosphorylated
(P)-STAT5 were measured before and after stimulation with G-CSF or
SCF.
Materials and methods
Patients
A total of 23 patients with PNH/AA syndrome were
enrolled in the study. These patients were hospitalized in the
Department of Hematology of Tianjin Medical University General
Hospital (Tianjin, China) from March 2012 to May 2013 and diagnosed
according to international criteria (6). Characteristics of the patients are
listed in Table I. In addition, 15
healthy volunteers with a median age of 36 years (range, 22 to 65
years) were included as healthy controls. The study was approved by
the Ethics Committee of Tianjin Medical University. Informed
written consent was obtained from all patients or their parents in
accordance with Declaration of Helsinki.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Patient no. | Age (years) | Gender | Disease
phenotype | PNH clone (%) |
|---|
| 1 | 33 | F | PNH/AA | 31.2 |
| 2 | 24 | M | PNH/AA | 44.4 |
| 3 | 29 | F | PNH/AA | 41.4 |
| 4 | 77 | M | PNH/AA | 37.7 |
| 5 | 25 | M | PNH/AA | 39.6 |
| 6 | 72 | F | PNH/AA | 20.5 |
| 7 | 56 | F | PNH/AA | 45.3 |
| 8 | 40 | F | PNH/AA | 50.5 |
| 9 | 63 | F | PNH/AA | 26.8 |
| 10 | 28 | M | PNH/AA | 32.2 |
| 11 | 32 | M | PNH/AA | 49.7 |
| 12 | 60 | F | PNH/AA | 42.0 |
| 13 | 55 | F | PNH/AA | 37.9 |
| 14 | 30 | M | PNH/AA | 35.0 |
| 15 | 14 | F | PNH/AA | 32.1 |
| 16 | 39 | F | PNH/AA | 22.9 |
| 17 | 11 | M | PNH/AA | 47.8 |
| 18 | 42 | M | PNH/AA | 61.9 |
| 19 | 51 | F | PNH/AA | 29.8 |
| 20 | 36 | M | PNH/AA | 44.7 |
| 21 | 18 | F | PNH/AA | 31.0 |
| 22 | 44 | M | PNH/AA | 40.1 |
| 23 | 35 | M | PNH/AA | 28.4 |
Determination of CD114 and CD117
expression by flow cytometry (FCM)
Samples (2 ml) of fresh bone marrow (BM) were
obtained from the patients and healthy controls. After filtration,
300 μl BM samples were divided into one control and two test tubes.
An antibody against monoclonal mouse IgG1-PE (cat no. 555749; 1:5)
was added as a negative control and the mouse monoclonal antibodies
CD114-PE (cat no., 554538; 1:5) and CD117-PE (cat no., 340529; 1:5)
were added to each test tube, respectively. Mouse monoclonal
antibodies CD34-PerCP (cat no., 340430; 1:5) and CD59-FITC (cat
no., 555763; 1:5) were stained in all tubes as markers of stem
cells and the PNH clone. All antibodies were purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Following incubation at 4°C
for 30 min, red blood cells (RBCs) in the sample were lysed with 2
ml RBC lysing solution (BD Biosciences). Next, the bone marrow
hemopoietic cells were washed twice with phosphate-buffered saline
(PBS) and analyzed using a BD FACSCalibur flow cytometer (BD
Biosciences) with CellQuest software, version 3.1. At least 100,000
cells were acquired for each sample.
STAT5 and P-STAT5 analysis
BM mononuclear cells (BMMNCs) were isolated by
Ficoll-Hypaque density gradient centrifugation (750 g × for 5 min
at 20°C; density, 1.077; Tianjin Hao Yang Biological Products
Technology Co., Ltd., Tianjin, China). The samples were stimulated
with BMMNCs (2–3×106cells) G-CSF (100 ng/ml) or SCF (100
ng/ml) to induce the phosphorylation of STAT5 and fixed with
crosslinking reagent (2% paraformaldehyde; Sigma-Aldrich, St.
Louis, MO, USA). To stabilize the phosphorylation, the cells were
fixed using 2% paraformaldehyde in PBS for 10 min at 4°C. The
monoclonal antibodies CD34-PerCP and CD59-FITC were then added to
the samples. After incubating at 4°C for 30 min, RBCs in the
samples were lysed with 2 ml RBC lysing solution. The cells were
mixed with 1.0 ml of FACS™ permeabilizing solution (BD Biosciences)
for 10 min in the dark, and then incubated with STAT5-PE (cat no.,
562077; 1:100 dilution) and p-STAT5-PE (cat no., 129010; 1:100
dilution) mouse monoclonal antibodies (BD Biosciences) at 4°C for
30 min. The cells were then washed twice with PBS. Information
about ≥100,000 cells was acquired for each sample by FCM.
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used. Measurement data are displayed as the mean ±
standard deviation. Analysis of variance was used to evaluate
differences between groups, followed by Student-Newman-Keuls test
for multiple comparisons. A value of P<0.05 was considered
statistically significant.
Results
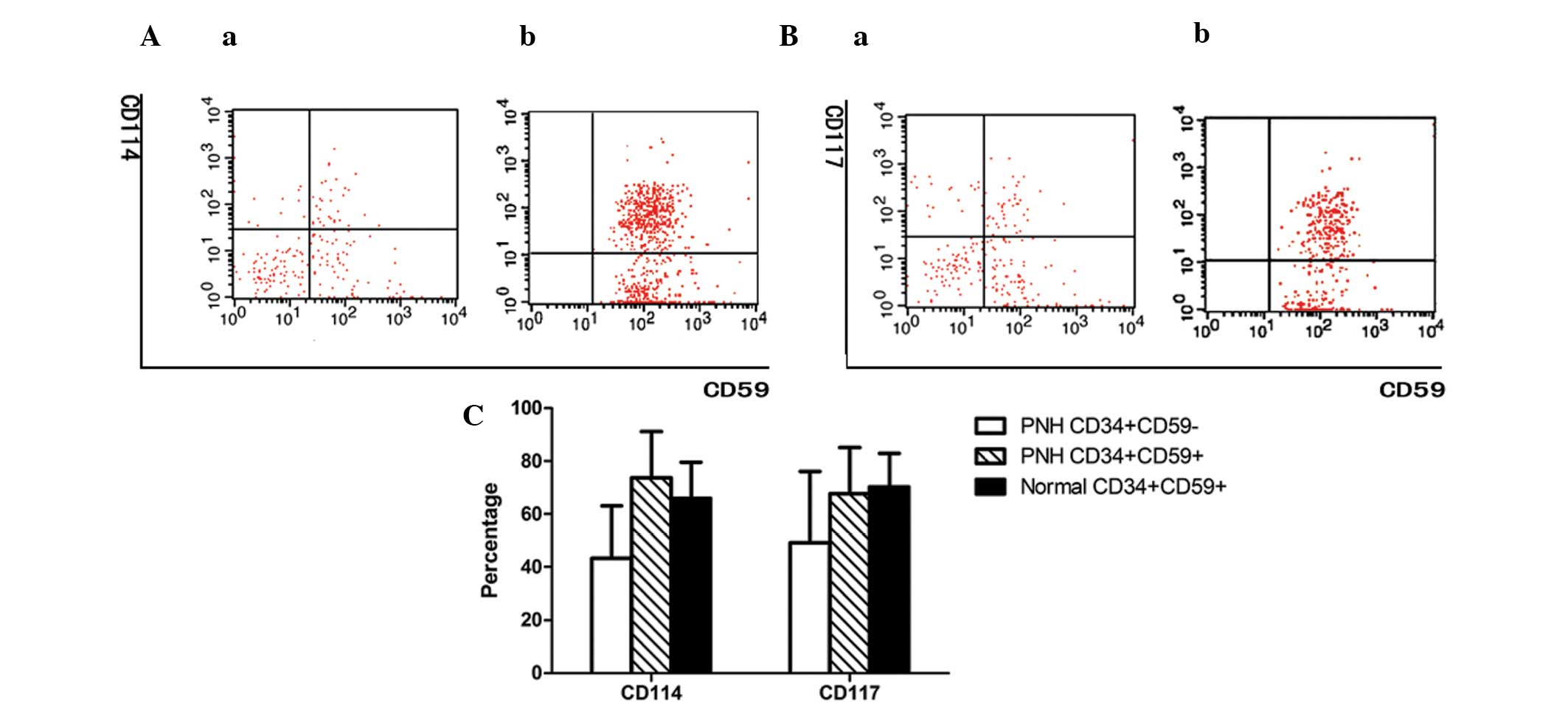
Expression of CD114 and C117 on
GPI+ and GPI− HSCs isolated from BM
The expression levels of CD114 and CD117 on the
GPI− HSCs of patients with PNH/AA (44.23±19.77 and
49.20±26.80%, respectively) were significantly lower than those on
the GPI+ HSCs of patients with PNH/AA (73.72±17.42 and
67.62±17.41%, respectively; P<0.01) or normal controls
(65.91±13.70 and 70.21±12.68%, respectively; P<0.01). No
significant difference was observed between the latter two groups
(P>0.05; Fig. 1).
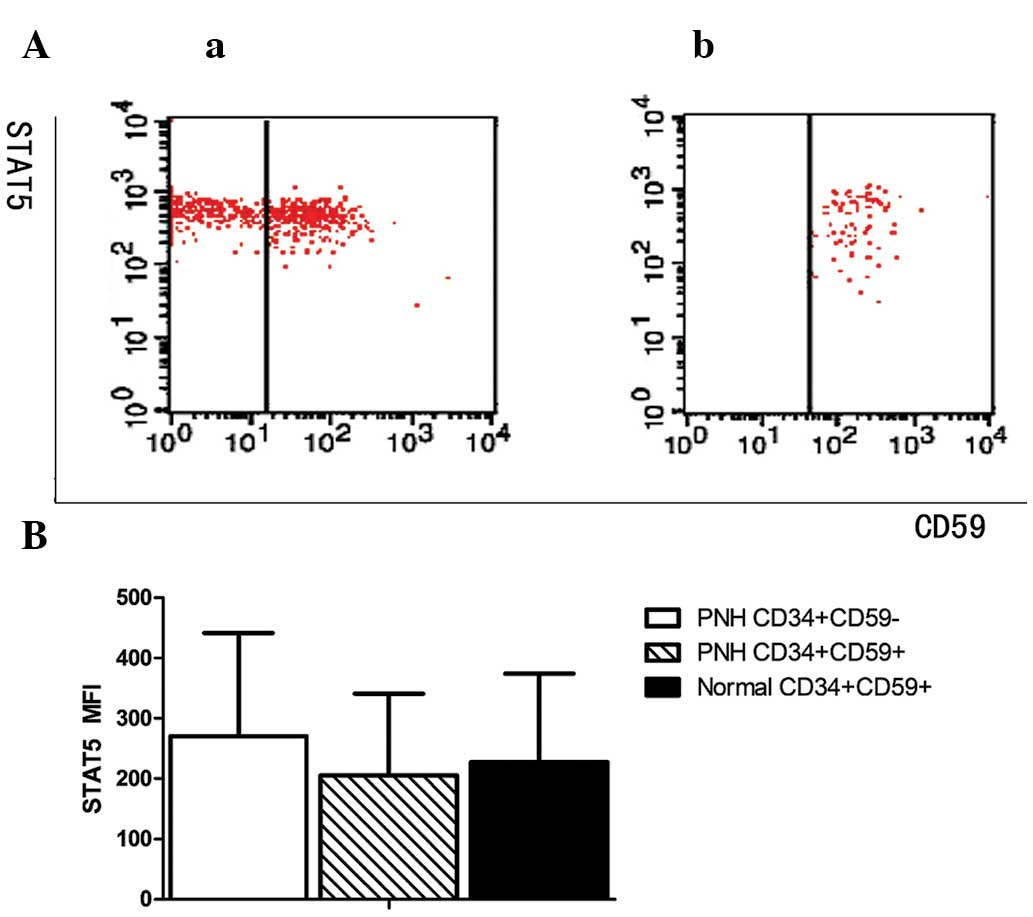
Expression of STAT5 on GPI+
and GPI− HSCs isolated from BM
The MFI values for STAT5 in the GPI− and
GPI+ HSCs of patients with PNH/AA and the
GPI+ HSCs of the normal controls were 270.01±181.26,
205.05±146.16 and 227.39±156.65, respectively. No significant
difference was observed among the three groups (P>0.05; Fig. 2).
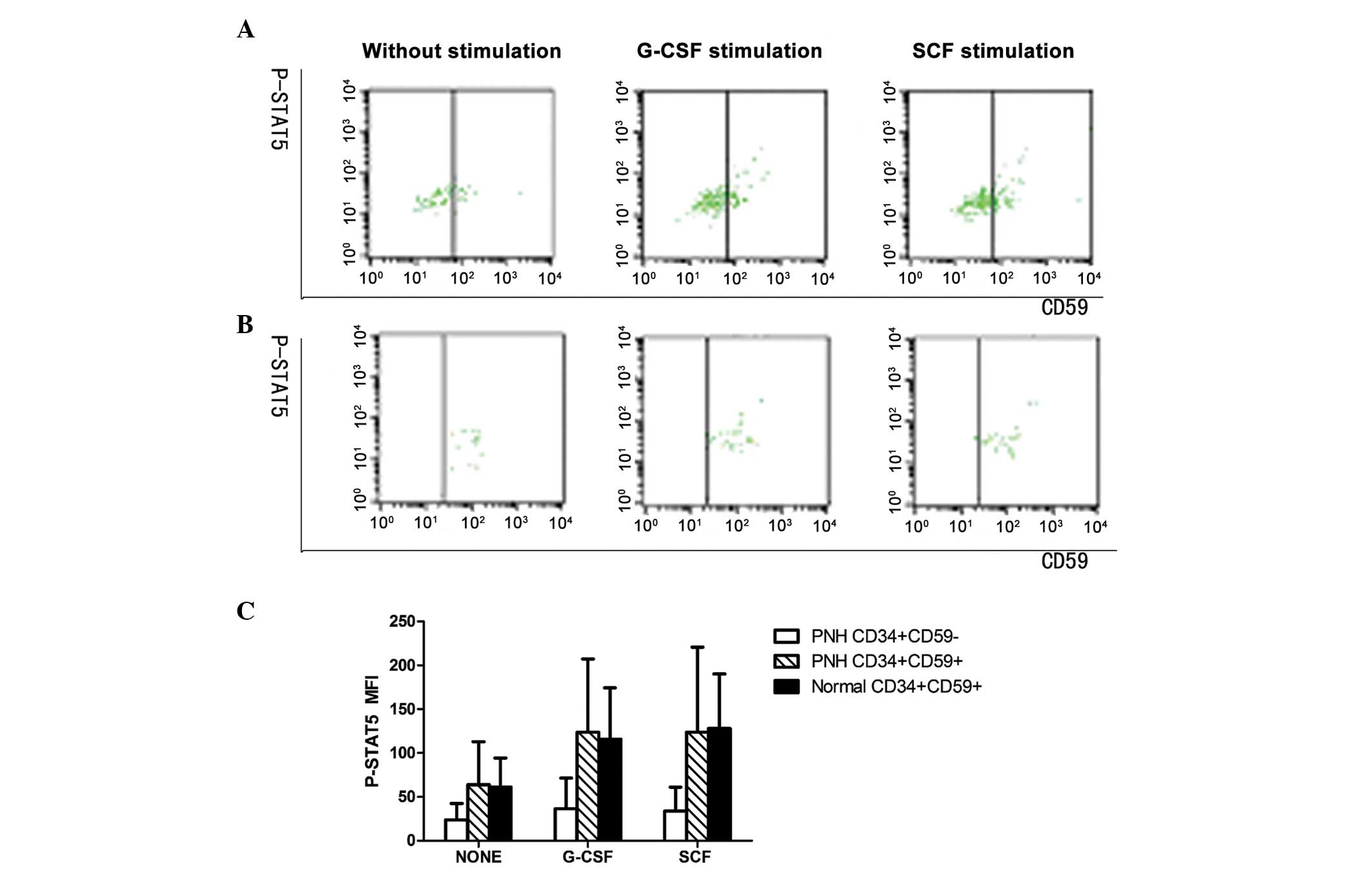
Expression of P-STAT5 on
GPI+ and GPI− HSCs isolated from BM
Prior to stimulation and following stimulation with
G-CSF or SCF, the MFI values for P-STAT5 in the GPI−
HSCs of PNH/AA patients were 23.44±17.90, 35.73±33.93 and
33.19±26.45, respectively, which were significantly lower than
those in the GPI+ HSCs of PNH/AA patients (63.42±47.63,
123.45±80.40 and 123.41±93.40, respectively; P<0.01) or normal
controls (61.30±32.13, 116.00±56.52 and 126.86±60.20, respectively;
P<0.01). No statistically significant difference was identified
in the P-STAT5 MFI values between the latter two groups (P>0.05;
Fig. 3).
Increases in P-STAT5 expression in
GPI+ and GPI− HSCs following stimulation with
G-CSF or SCF
The increased MFI values for P-STAT5 in G-CSF- or
SCF-stimulated GPI− HSCs of PNH/AA patients were 3 and
5, respectively, which were significantly lower than those in the
G-CSF- or SCF-stimulated GPI+ HSCs of PNH/AA patients
(47 and 31, respectively) and normal controls (44 and 46,
respectively) (P<0.01; Fig.
3).
Discussion
PNH is an acquired clonal disease characterized by
complement-mediated hemolysis, bone marrow failure and thrombosis.
Some patients present with markedly increased levels of
GPI− HSCs and signs of bone marrow failure, which lead
to a diagnosis of PNH/AA syndrome (7). The conventional methods for the
treatment of PNH/AA syndrome include glucocorticoids, androgens,
cyclosporine, anti-T-lymphocyte globulin (ATG) and hemopoietic stem
cell transplantation (8–10). A new targeted and disease-modifying
treatment strategy is the inhibition of the terminal complement
cascade with the humanized monoclonal anti-C5 antibody, eculizumab,
which can effectively control hemolysis and thrombosis (11–13).
Economic reasons cause many patients with PNH/AA syndrome to
undergo immunosuppressive treatment and treatment with hemopoietic
stimulating factors, such as G-CSF and EPO. However, at present,
little is known about the effects of these hemopoietic stimulating
factors on GPI− HSCs. It has been demonstrated that
adding EPO and TPO to chemotherapy may promote the proliferation
and differentiation of normal clone cells, and eliminate the
abnormal PNH clone fundamentally (4,5). In the
present study, the aim was to explore the effects of G-CSF and SCF
in PNH/AA syndrome.
The cytokine G-CSF promotes the proliferation,
differentiation, survival and functional maturation of cells within
the neutrophilic granulocyte lineage (14). Its cell-surface receptor (G-CSFR;
CD114) plays an important role in the production, survival and
activation of neutrophilic granulocytes during normal and emergency
hematopoiesis (14). This has led to
several important clinical applications for its ligand, G-CSF,
which binds to the G-CSFR causing activation via homodimerization
and subsequent phosphorylation on four tyrosine residues of the
receptor intracellular domain (15).
This initiates a range of intracellular signaling events including
the activation of Janus kinase (JAK)/STAT pathways (15,16).
Ward et al (17) further
highlighted the importance of STAT5 in the mediation of
proliferative responses to G-CSF after studying the association
between STAT5 and severe congenital neutropenia.
SCF is a critical cytokine during hematopoiesis,
which regulates stem and progenitor cell survival and
proliferation. The receptor for SCF, c-Kit (CD117), is a member of
the tyrosine kinase family of receptors and undergoes
autophosphorylation upon binding with SCF, resulting in the
activation of multiple signaling proteins such as JAK/STAT,
phosphoinositide 3-kinase, Src kinases, Shc and Ras (18,19).
Brizzi et al (20) found that
STAT1α, STAT5A and STAT5B participate in the signaling transduction
of SCF.
In the present study, the expression levels of CD114
and CD117 on HSCs from the BM of PNH/AA patients were detected, and
it was found that the expression levels on GPI− HSCs
were significantly lower than those on the GPI+ HSCs of
PNH/AA patients or normal controls. Therefore, this characteristic
can be utilized in the treatment of PNH/AA to promote the
proliferation of the normal clone instead of the PNH clone.
In order to further investigate the functions of
CD114 and CD117, the signaling pathway protein STAT5 was measured
in the HSCs by FCM. No significant difference in the STAT5 MFI was
observed among the three groups, that is, among the GPI−
and GPI+ HSCs of patients with PNH/AA and the
GPI+ HSCs of normal controls. This indicates that there
were no abnormal quantities of intracellular pathway proteins among
the three groups. Furthermore, the expression levels of P-STAT5
were measured in the BMMNCs of PNH/AA patients and normal controls
prior to and following stimulation with G-CSF or SCF in
vitro. It was found that the MFI of P-STAT5 in the unstimulated
or G-CSF- or SCF-stimulated PNH (GPI+) clone cells was
lower than that in normal clone cells, and no significant
difference was observed between the GPI+ HSCs of the PNA
patients and normal controls. This indicates that G-CSF or SCF can
significantly increase the proliferation and differentiation of
normal clones, while having little effect on abnormal PNH clones.
In a previous study (21), it was
found that in vitro, the BMMNCs of normal controls had
better proliferative capacity and gave a stronger response to G-CSF
than those of patients with PNH, which is consistent with the
results of the present study. The present study also found that the
expression level of P-STAT5 in PNH clone cells was lower than that
in normal clone cells prior to stimulation with G-CSF or SCF.
However, normal clone cells did not acquire a proliferative
advantage. By contrast, PNH clone cells were amplified, which would
eventually lead to a series of clinical manifestations. This may be
due to a complex mechanism, leading to PNH clones evading immune
attacks, undergoing a reduction in apoptosis decrease and gaining a
proliferative advantage (22,23).
Through the application of hematopoiesis-stimulating factors in
PNH/AA patients, the degree of phosphorylation of normal clone
cells can be significantly increased, which may overcome the
various factors that lead to proliferation of the abnormal clone,
leading to gradual proliferation of the normal clone and the
restoration of normal hematopoiesis.
In conclusion, PNH clone cells responded poorly to
stimulation by the hematopoiesis-stimulating factors G-CSF and SCF.
The findings of the present study may facilitate the deeper
development of hematopoiesis-stimulating factors in PNH/AA
patients. However, further studies are required in order to
investigate the mechanism in more detail.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grants nos. 30971285, 30971286 and
81170472), Tianjin Municipal Natural Science Foundation (grant nos.
14JCYBJC25400 and 12JCZDJC21500), Tianjin Medical University
Foundation (grant nos. 2011ky07 and 2010ky20), Science and
Technology Foundation of Tianjin Municipal Health Bureau (grant
nos. 2011kz115 and 2010kz105), Tianjin Municipal Health Industry
Key Project (grant no. 11KG135) and Tianjin Municipal Anticancer
Major Project (grant nos. 122ZCDSY18000 and 12ZCDZSY17900).
References
|
1
|
de Latour RP, Mary JY, Salanoubat C,
Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn
JY and Socié G: French Society of Hematology; French Association of
Young Hematologists: Paroxysmal nocturnal hemoglobinuria: Natural
history of disease subcategories. Blood. 112:3099–3106. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Latour Peffault R, Amoura Z and Socié
G: Paroxysmal nocturnal hemoglobinuria. Rev Med Interne.
31:200–207. 2010.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kinoshita T and Inoue N: Relationship
between aplastic anemia and paroxysmal nocturnal hemoglobinuria.
Int J Hematol. 75:117–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D, Fu R, Ruan EB, Qu W, Liang Y, Wang
HQ, Wang J, Li LJ, Liu H, Wang HL, et al: EPOR and TPOR expressions
on CD34+ CD59− and CD34+
CD59+ bone marrow cells from patients with paroxysmal
nocturnal hemoglobinuria. Zhonghua Xue Ye Xue Za Zhi. 32:543–547.
2011.(In Chinese). PubMed/NCBI
|
|
5
|
Wang D, Fu R, Ruan EB, Qu W, Liang Y, Wang
HQ, Wang J, Li LJ, Liu H, Wang HL, et al: STAT5 phosphorylation
levels of erythropoietin and thrombopoietin receptors in
CD34(+)CD59(−) and CD34(+) CD59(+) bone marrow cells of patients
with paroxysmal nocturnal hemoglobinuria. Zhonghua Yi Xue Za Zhi.
91:2129–2131. 2011.(In Chinese). PubMed/NCBI
|
|
6
|
Borowitz MJ, Craig FE, Digiuseppe JA,
Illingworth AJ, Rosse W, Sutherland DR, Wittwer CT and Richards SJ:
Guidelines for the diagnosis and monitoring of paroxysmal nocturnal
hemoglobinuria and related disorders by flow cytometry. Cytometry B
Clin Cytom. 78:211–230. 2010.PubMed/NCBI
|
|
7
|
Raza A, Ravandi F, Rastogi A, Bubis J, Lim
SH, Weitz I, Castro-Malaspina H, Galili N, Jawde RA and Illingworth
A: A prospective multicenter study of paroxysmal nocturnal
hemoglobinuria cells in patients with bone marrow failure.
Cytometry B Clin Cytom. 86:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arruda MM, Rodrigues CA, Yamamoto M and
Figueiredo MS: Paroxysmal nocturnal hemoglobinuria: From
physiopathology to treatment. Rev Assoc Med Bras. 56:214–221.
2010.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brodsky RA: How I treat paroxysmal
nocturnal hemoglobinuria. Blood. 113:6522–6527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raiola AM, Van Lint MT, Lamparelli T,
Gualandi F, Benvenuto F, Figari O, Mordini N, Berisso G, Bregante
S, Frassoni F and Bacigalupo A: Bone marrow transplantation for
paroxysmal nocturnal hemoglobinuria. Haematologica. 85:59–62.
2000.PubMed/NCBI
|
|
11
|
Röth A and Dührsen U: Treatment of
paroxysmal nocturnal hemoglobinuria in the era of eculizumab. Eur J
Haematol. 87:473–479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charneski L and Patel PN: Eculizumab in
paroxysmal nocturnal haemoglobinuria. Drugs. 68:1341–1346. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindorfer MA, Pawluczkowycz AW, Peek EM,
Hickman K, Taylor RP and Parker CJ: A novel approach to preventing
the hemolysis of paroxysmal nocturnal hemoglobinuria: Both
complement-mediated cytolysis and C3 deposition are blocked by a
monoclonal antibody specific for the alternative pathway of
complement. Blood. 115:2283–2291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scholz M, Schirm S, Wetzler M, Engel C and
Loeffler M: Pharmacokinetic and -dynamic modelling of G-CSF
derivatives in humans. Theor Biol Med Model. 9:322012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liongue C, Wright C, Russell AP and Ward
AC: Granulocyte colony-stimulating factor receptor: Stimulating
granulopoiesis and much more. Int J Biochem Cell Biol.
41:2372–2375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marino VJ and Roguin LP: The granulocyte
colony stimulating factor (G-CSF) activates Jak/STAT and MAPK
pathways in a trophoblastic cell line. J Cell Biochem.
103:1512–1523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ward AC, Gits J, Majeed F, Aprikyan AA,
Lewis RS, O'Sullivan LA, Freedman M, Shigdar S, Touw IP, Dale DC
and Dror Y: Functional interaction between mutations in the
granulocyte colony-stimulating factor receptor in severe congenital
neutropenia. Br J Haematol. 142:653–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roskoski R Jr: Structure and regulation of
Kit protein-tyrosine kinase - The stem cell factor receptor.
Biochem Biophys Res Commun. 338:1307–1315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Z, Zhang R, Joachimiak A,
Schlessinger J and Kong XP: Crystal structure of human stem cell
factor: Implication for stem cell factor receptor dimerization and
activation. Proc Natl Acad Sci USA. 97:7732–7737. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brizzi MF, Dentelli P, Rosso A, Yarden Y
and Pegoraro L: STAT protein recruitment and activation in c-Kit
deletion mutants. J Biol Chem. 274:16965–16972. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao YR, Shao ZH, Liu H, Shi J, Bai J, Tu
MF, Wang HQ, Xing LM, Cui ZZ, Sun J, et al: The response of bone
marrow hematopoietic cells to G-CSF in paroxysmal nocturnal
hemoglobinuria patients. Zhonghua Xue Ye Xue Za Zhi. 26:235–238.
2005.(In Chinese). PubMed/NCBI
|
|
22
|
Savage WJ, Barber JP, Mukhina GL, Hu R,
Chen G, Matsui W, Thoburn C, Hess AD, Cheng L, Jones RJ and Brodsky
RA: Glycosylphosphatidylinositol-anchored protein deficiency
confers resistance to apoptosis in PNH. Exp Hematol. 37:42–51.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao YR, Shao ZH, Liu H, Zhao MF, He GS,
Shi J, Bai J, Fu R, Tu MF, Wang HQ, et al: Expression of
apoptosis-related proteins in bone marrow neutrophils of patients
with paroxysmal nocturnal hemoglobinuria. Zhongguo Shi Yan Xue Ye
Xue Za Zhi. 13:871–874. 2005.(In Chinese). PubMed/NCBI
|