Introduction
Endoscopic procedures are of great importance for
the diagnosis and treatment of various diseases, including upper
gastrointestinal bleeding, esophageal dilatation and foreign body
removal. However, anxiety, pain, fear and gastrointestinal
reactions may cause patients to be less cooperative during
endoscopy, and may even induce harmful cardiovascular adverse
events (1); therefore, the role of
sedation in endoscopy is significant. Higher doses of sedative
drugs have been found to result in improved patient cooperation
during the procedure and satisfaction (2,3).
Although various sedative agents are commonly used, the ‘ideal’
agent for endoscopy sedation remains to be established.
Midazolam, the most common agent used for sedation,
is a benzodiazepine with rapid onset of action and short duration
of sedative effect (4). It produces
central nervous system depression effects through the stimulation
of γ-amino butyric acid receptors (5). To date, midazolam remains the
predominant intensive care unit (ICU) sedative agent (6). However, it has certain undesirable side
effects, such as delayed recovery of memory, long-term behavioral
changes such as long-term cognitive dysfunction and respiratory
depression.
Dexmedetomidine is a new-type, highly selective
α2-adrenoceptor agonist, which has sedative, amnestic,
sympatholytic and analgesic effects (7). It was first approved for use in ICU in
1999, and its use has been rapidly extended to various other
clinical situations (8). A previous
study has reported that dexmedetomidine may be a possible
alternative to midazolam in sedation (9). As the use of dexmedetomidine has
increased, associated adverse effects, such as hypotension and
bradycardia, have been reported (10). Dexmedetomidine is increasingly used
in the sedation of patients in different clinical situations.
Therefore, a meta-analysis was performed in the
present study in order to compare the effects of the two drugs,
midazolam and dexmedetomidine, in sedation during endoscopy by
analyzing the most recently-published controlled trials.
Materials and methods
Result reliability
To ensure the reliability of the present
meta-analysis, the results were reported according to the Preferred
Reporting Items for Systematic Reviews and Meta-analyses statement
(11).
Literature search strategy
The following digital databases were searched for
the identification of studies: PubMed (www.ncbi.nlm.nih.gov/pubmed), Cochrane Library
(www.cochranelibrary.com/), Ovid
(ovidsp.ovid.com/autologin.cgi) and
ClinicalTrails (https://clinicaltrials.gov/) databases. In addition,
Chinese databases were searched, including CQVIP (http://en.cqvip.com/), WanFang Data (www.wanfangdata.com/) and Chinese Biomedical
Literature databases (www.sinomed.ac.cn). All the databases were searched up
to November 2014, with no language restriction. The literature
search was performed using the relevant keywords of
‘dexmedetomidine’, ‘midazolam’, ‘Dormicum’, ‘endoscopy’. For the
Chinese databases, free-text terms were used, including ‘mi da zuo
lun’ or ‘mi zuo an ding’ (which is the translation of ‘midazolam’
in Chinese), as well as ‘nei jing’, ‘chang jing’ and ‘wei jing’
(which refer to different types of endoscopy in Chinese). The
search strategy was independently performed by two investigators.
Any disagreements were resolved by consensus and discussion.
Study selection
The inclusion criteria for the trials included in
the present meta-analysis were as follows: i) Randomized controlled
trials (RCTs); ii) the study focused on the sedation effects of
dexmedetomidine and midazolam; and iii) the study involved patients
with an American Society of Anesthesiologists (ASA) (12) grade I to III, and who presented for
outpatient endoscopy procedures under conscious sedation. Exclusion
criteria were as follows: i) Case reports, letters, reviews,
editorial articles, meta-analyses and retrospective studies; ii)
duplicates of previous published articles; and iii) studies which
included children.
Data extraction
The following variables were recorded for each of
the studies: First author, journal, publication date, country,
baseline difference, method of randomization, degree of blinding,
dropouts and withdrawals. By baseline difference we mean the basic
health condition of the patients. The degree of blinding means the
degree of blinding method, i.e. double or single-blinding used.
Finally the withdrawals indicate the patients who did not finish
the study. The primary outcome of interest were changes in vital
signs, including the continuous peripheral oxygen saturation
(SpO2), heart rate, respiration rate, mean arterial
pressure (MAP) of the patients, Ramsay sedation scale (RSS)
(13) and Alertness/Sedation scale
(OOA/S) (14). Secondary outcomes
included numeric rating scale pain scores, post-procedure
satisfaction questionnaire and adverse events.
Statistical analysis
All statistical analyses were performed using Review
Manager version 5.2 statistical software (Cochrane Collaboration,
Copenhagen, Denmark). Dichotomous data are expressed as odds ratios
(ORs) with 95% confidence intervals (CIs). Continuous variables are
presented as the standard mean difference (SMD). The statistical
significance of the pooled value was evaluated using the Z test,
while heterogeneity was analyzed via the I2 test. Where
the heterogeneity test showed no heterogeneity, the data were
processed via a fixed effects model; otherwise, a random effects
model was conducted for the analysis. Begg's funnel plots were used
to detect publication biases. P<0.05 was considered to indicate
a statistically significant difference in all the analyses. To
ensure the accuracy of the outcomes, two researchers assessed the
data independently and obtained the same results.
Assessment of study quality
The included studies were reviewed and assessed for
methodological quality using the Jadad composite scale (15). High-quality trials scored >3 out
of a maximum score of 5 (16).
Results
Study characteristics
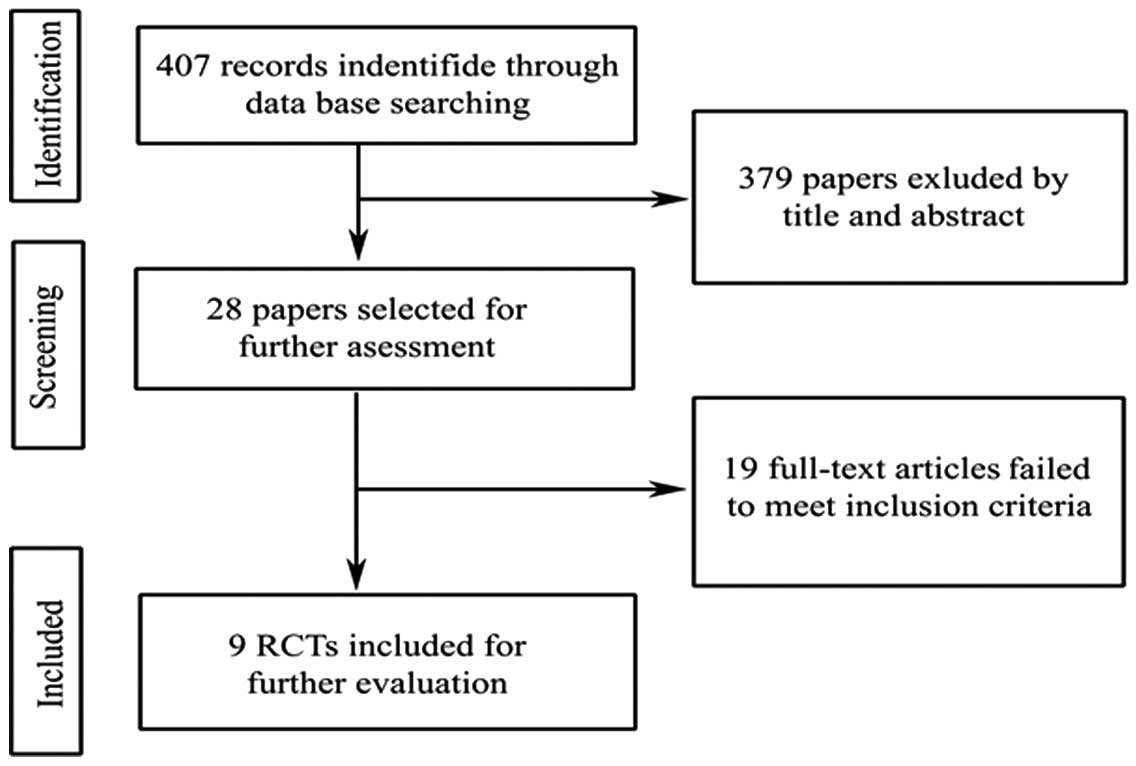
The process of study selection is shown in Fig. 1. According to the inclusion criteria,
9 studies were included in the present meta-analysis. The included
studies were published as full text between January 2007 and
December 2014. Of the 657 patients included in the 9 eligible RCTs
(17–25), 329 patients were allocated to the
dexmedetomidine group, while 328 patients comprised the midazolam
group. The sedation effects in the two groups were evaluated.
Patient characteristics, including age and gender, are shown in
Table I.
 | Table I.Patient characteristics in studies
comparing the use of dexmedetomidine with midazolam for sedation
during endoscopy. |
Table I.
Patient characteristics in studies
comparing the use of dexmedetomidine with midazolam for sedation
during endoscopy.
| Authors | Year | Patients, n | Age, years | Male, n | Female, n | Refs. |
|---|
| Wei et
al | 2014 | 30/30 | 35.3/36.6 | 16/17 | 14/13 | (17) |
| Sethi et
al | 2014 | 30/30 | 42/44 | 13/14 | 17/16 | (18) |
| Demiraran et
al | 2007 | 25/25 | 42.2/43.3 | 13/9 | 12/16 | (19) |
| Dere et
al | 2010 | 30/30 | 57.9/60.1 | 22/21 | 8/9 | (20) |
| Zhang et
al | 2013 | 30/30 | ≥65 | NR | NR | (21) |
| Li et
al | 2014 | 30/30 | 55.1 | 38 | 22 | (22) |
| Arpaci and
Bozkirli | 2013 | 20/20 | 50.4/47.1 | 6/7 | 14/13 | (23) |
| Karaaslan et
al | 2007 | 35/35 | 32.5/34.4 | 23/21 | 12/14 | (24) |
| Liao et
al | 2012 | 99/98 | 58.5/60.1 | 61/62 | 38/36 | (25) |
Quality of the included studies
The mean Jadad score of the included studies was 3
out of a maximum possible score of 5. This indicated that the
majority of the included RCTs were of moderate quality, although 2
Chinese trials (21,22) were of low quality (Jadad score of
<3), which was a result of relatively poor study design.
Trial outcomes
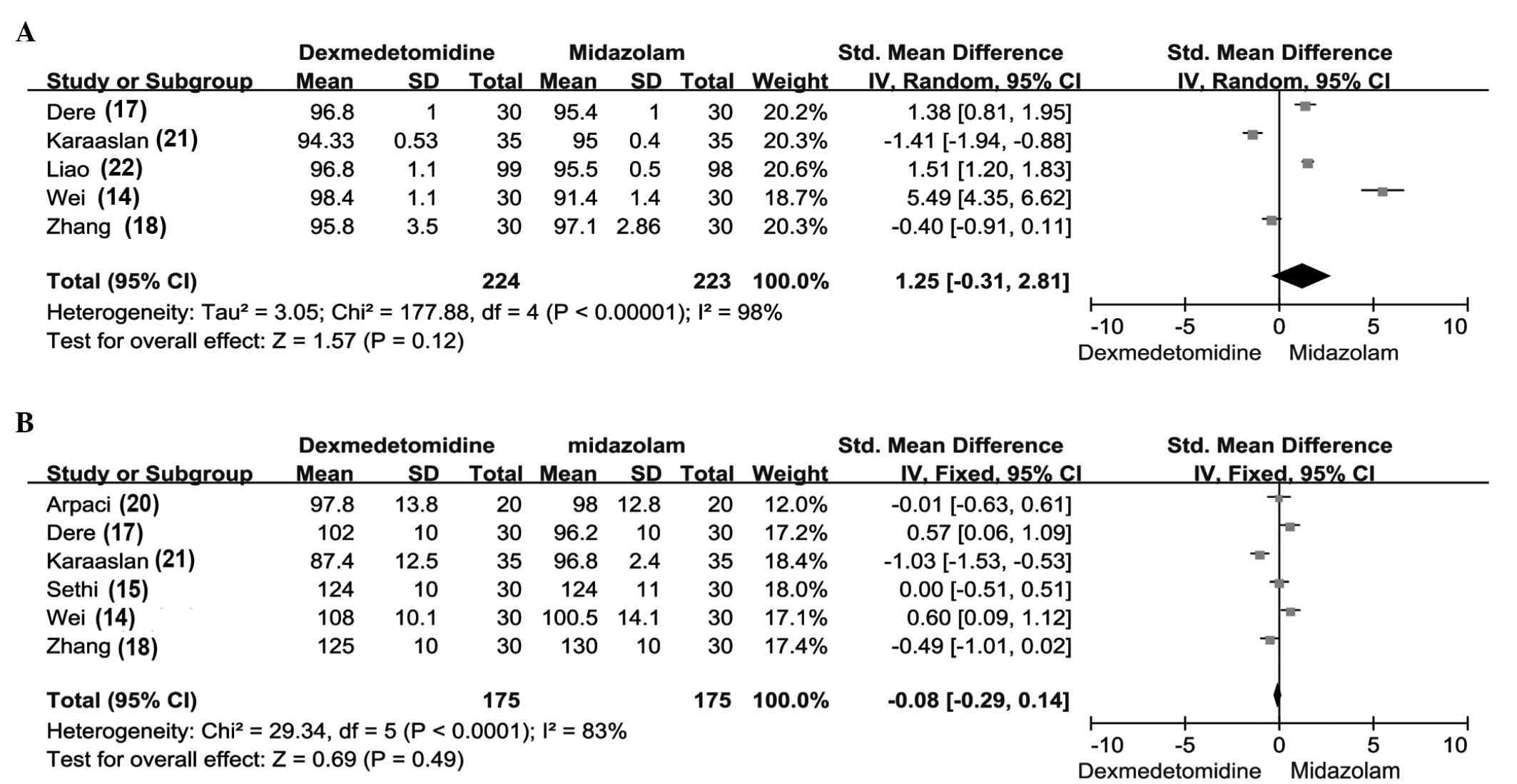
SpO2
Statistical analysis performed in 5 of the eligible
studies (17,20,21,24,25)
revealed that the SpO2 showed no statistically
significant difference between the dexmedetomidine and midazolam
groups (Fig. 2A). These 5 studies
were used for the analysis because of their similar study index
which made them comparable for a meta-analysis. In addition, there
was no evidence of significant heterogeneity (SMD, 1.25; 95% CI,
−0.31 to 2.81; P=0.12; Fig. 2A).
MAP
In total, 6 studies reported the MAP of patients
following administration of the two agents (17,18,20,21,23,24). The
results demonstrated that there was no significant difference in
the MAPs between the dexmedetomidine and midazolam groups [SMD,
−0.08; 95% CI, −0.29 to 0.14]. In addition, no significant
heterogeneity was detected (Fig.
2B).
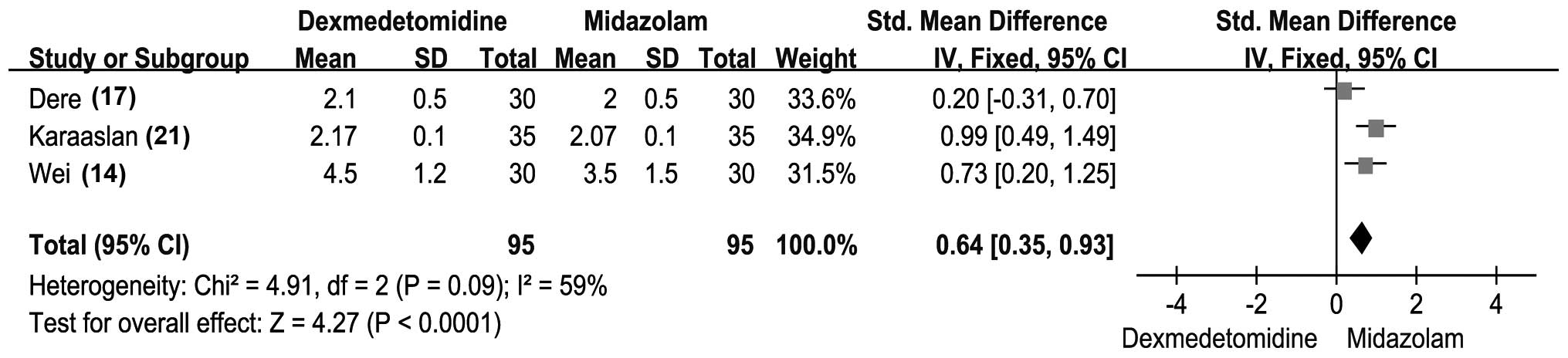
RSS
In total, 3 of the 9 included studies reported the
RSS of patients (17,20,24). The
results revealed that the RSS scores of patients administered
dexmedetomidine were significantly higher compared with those in
the midazolam group patients (SMD, 0.64; 95% CI, 0.35–0.93;
P<0.0001). However, no significant heterogeneity was detected in
the results (Fig. 3). With regard to
normal adults, 0.05–0.075 mg/kg midazolam intravenous injection
produces the effect of sedation. A higher dose of 0.1–0.15 mg/kg is
commonly used for anaesthesia induction. For dexmedetomidine, a
continuous intravenous infusion of 0.2–0.7 µg/kg/h can achieve a
good sedation outcome. General anaesthesia stages may be induced
above such a drug dose.
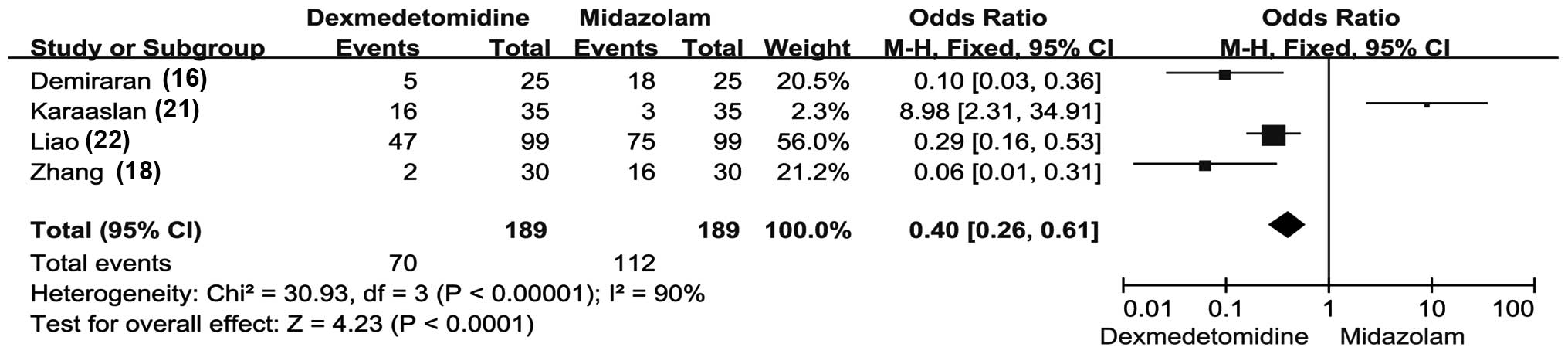
Adverse events
Out of the 9 eligible studies, 4 trials (involving
189 patients) compared the adverse events reported in patients
administered with dexmedetomidine and midazolam (19,21,24,25). The
main adverse events that were reported were respiratory depression,
nausea and vomiting, dysphoria, reflux, dizziness, abdominal
distention and pain.
The pooled results displayed a statistically
significant difference between the subgroups (Fig. 4). In the dexmedetomidine group, a
significantly lower number of adverse events were reported,
compared with the midazolam group (OR, 0.40; 95% CI, 0.26–0.61;
P<0.0001; Fig. 4). The adverse
events and main trail results were shown in Table II.
 | Table II.Main results of the
meta-analysis. |
Table II.
Main results of the
meta-analysis.
|
| Patients
included |
|
|
|---|
|
|
|
|
|
|---|
| Results | Dexmedtomidine | Midazolam | Standard deviation
Mean Difference | P-value |
|---|
|
SpO2 | 224 | 223 | 1.25
[-0.31,0.61] | 0.12 |
| MAP | 175 | 175 | −0.08
[-0.29,0.1] | 0.49 |
| RSS | 95 | 95 | 0.64
[0.35,0.93] | <0.0001 |
| Adverse events |
|
|
|
|
| Hypertension | 4 | 12 | – |
|
| Hypotension | 11 | 8 | – |
|
| Tachycardia | 13 | 12 | – |
|
| Hypoxia | 1 | 11 | – |
|
| Nausea | 10 | 2 | – |
|
| Other | 31 | 69 | – |
|
| Total | 70 | 112 | 0.40
[0.26,0.61] | <0.0001 |
Testing for publication bias
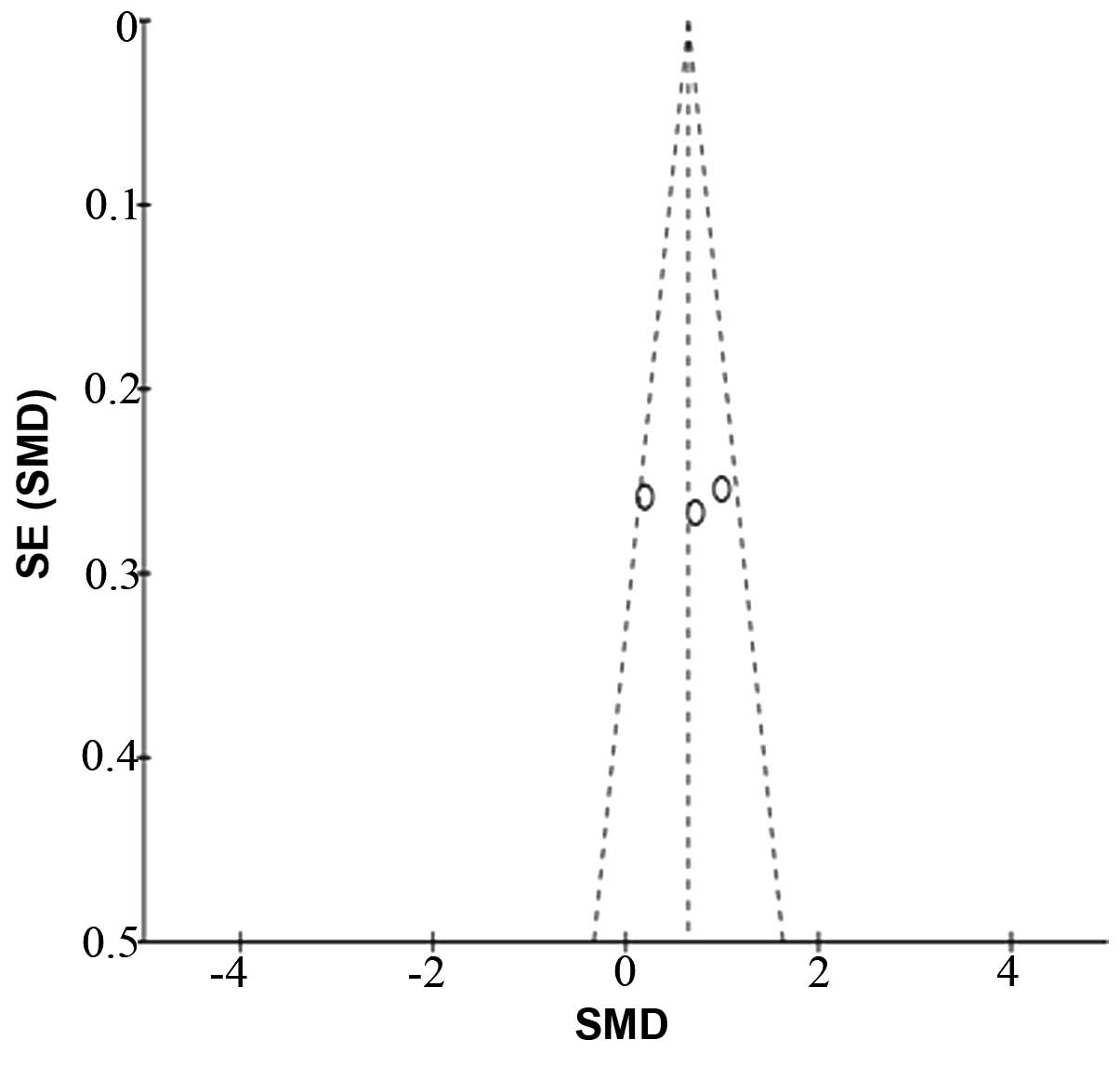
A funnel plot of the outcome of the RSS score
following sedation with dexmedetomidine and midazolam in the
included studies demonstrated there was no significant publication
bias (Fig. 5). However, the number
of trials included was <10, thus this conclusion may not be
entirely accurate.
Discussion
Endoscopy is an essential procedure for clinical
diagnosis and treatment of various diseases such as
gastrointestinal neuroendocrine tumours (26–29),
gastroesophageal reflux, lung cancer and cervical cancer. Different
endosocopes may be used to diagnose different diseases. For
example, cystoscopy is used to diagnose diseases in the urinary
system. Although conscious sedation is the most widely used method
to relieve sickness and pain during endoscopy, the sedation agents
used greatly vary among different regions (30). Midazolam has long been regarded as
the golden standard for sedation. However, dexmedetomidine has been
increasingly studied in recent years.
To the best of our knowledge, Adams et al
(31) performed the first
meta-analysis concerning the effects of dexmedetomidine and
midazolam in adult ICU patients. In addition, Sun et al
(32) performed a systemic review
regarding premedication in children using the two drugs. The
present meta-analysis evaluated the sedation effects of
dexmedetomidine and midazolam during endoscopy.
The results of the current meta-analysis suggested
that dexmedetomidine sedation may achieve a more stable respiratory
system. Patients sedated with dexmedetomidine had statistically
lower hypoxia rates in the present study. Furthermore, patients
administered dexmedetomidine have been previously shown to be
easily awaken during endoscopy and experience less respiratory
depression compared with those sedated with midazolam (33,34).
Therefore, dexmedetomidine may be advantageous compared with
midazolam for the sedation of patients with previous history of
respiratory diseases.
As reported previously (35), the most significant complications
associated with dexmedetomidine are hypotension and bradycardia.
However, the results of the present meta-analysis challenge these
conclusions, since no significant differences were observed in the
arterial pressure or heart rate of the 175 patients investigated.
This is in agreement with the results of Yu et al (36) reporting that the SBP values did not
present any differences after slow infusion of the drug over a 10
min time-frame. Instead, appropriate dose and transfusion velocity
of dexmedetomidine may achieve favorable cardiovascular stability
(37,38). However, dexmedetomidine should be
used with caution in patients diagnosed with severe sinus
bradycardia or heart block (39).
Potent sedation effects may be the main advantage of
dexmedetomidine for endoscopy. The present study results revealed
that the RSS was significantly higher in the dexmedetomidine group,
while the OOA/S of patients in the dexmedetomidine was also higher
compared with that in the midazolam group. Even a maintenance dose
of 0.5 µgkg/h dexmedetomidine was able to provide better sedation
compared with 0.05 mgkg−1 midazolam. This indicated a
superior sedation effect of dexmedetomidine compared with midazolam
(40–42). However, despite its outstanding
sedation potency, it is also able to maintain awarenesss at the
sedative dose (43,44), which may enable the patient to change
positions according to orders and make the endoscopy a more smooth
procedure (45).
There was also a significant difference between the
dexmedetomidine and midazolam groups in terms of the number of
adverse events reported. Major complications, including
hypertension, requirement for mandible support and intubation, were
mainly observed in the midazolam group (46,47).
Therefore, dexmedetomidine may be advantageous with regard to the
prognosis and outcome of endoscopy patients.
Several limitations exist in the present
meta-analysis. First, two of the included trials were published in
Chinese (21,22), and these studies were of relatively
poor quality due to unclear concealment of research details. In
addition, it was difficult to draw a definitive conclusion
regarding whether dexmedetomidine was a better sedative compared
with midazolam since no uniform criteria exist to assess the
effects of sedatives. Furthermore, a greater number of
well-designed trials are required to confirm the aforementioned
results.
In conclusion, the present meta-analysis included 9
RCTs reporting sedation during endoscopic procedures, and the
results indicated that the sedation effects of dexmedetomidine and
midazolam were comparable in patients undergoing endoscopy.
Therefore, the present study recommends that both of the
medications should be considered for patient sedation during
endoscopy.
Acknowledgements
This study was supported by a grant from the
National Special grant projects of China (grant no. 201002005).
References
|
1
|
Eqer EI, White PF and Boqetz MS: Clinical
and economic factors important to anaesthetic choice for day-case
surgery. Pharmacoeconomics. 17:245–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon SH: Sedation regimens for
gastrointestinal endoscopy. Clin Endosc. 47:135–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim KH: Safe sedation and hypnosis using
dexmedetomidine for minimally invasive spine surgery in a prone
position. Korean J Pain. 27:313–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triantafillidis JK, Merikas E, Nikolakis D
and Papalois AE: Sedation in gastrointestinal endoscopy: Current
issues. World J Gastroenterol. 19:463–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yilaz E, Hough KA, Gebhart GF, Williams BA
and Gold MS: Mechanisms underlying midazolam-induced peripheral
nerve block and neurotoxicity. Reg Anesth Pain Med. 39:525–533.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chawla R, Myatra SN, Ramakrishnan N, Todi
S, Kansal S and Dash SK: Current practices of mobilization,
analgesia, relaxants and sedation in Indian ICUs: A survey
conducted by the Indian society of critical care medicine. Indian J
Crit Care Med. 18:575–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bajwa S and Kulshrestha A:
Dexmedetomidine: An adjuvant making large lnroads into clinical
practice. Ann Med Health Sci Res. 3:475–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takrouri MS, Seraj MA, Channa AB,
el-Dawlatly AA, Thallage A, Riad W and Khalaf M: Dexmedetomidine in
intensive care unit: A study of hemodynamic changes. Middle East J
Anesthesiol. 16:587–595. 2002.PubMed/NCBI
|
|
9
|
Ihmsen H and Saari Ti: Dexmdetomidine.
pharmacokinetics and pharmacodynamics. Anaesthesist. 61:1059–1066.
2012.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bharati S, Pal A, Biswas C and Biswas R:
Incidence of cardiac arrest increases with the indiscriminate use
of dexmedetomidine: A case series and review of published case
reports. Acta Anaesthesiol Taiwan. 49:165–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moher D, Liverati A, Tetalaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sankar A, Johnson SR, Beattie WS, Tait G
and Wijeysundera DN: Reliability of the American society of
anesthesiologists physical status scale in clinical practice. Br J
Anaesth. 3:424–432. 2014. View Article : Google Scholar
|
|
13
|
Dawson R, von Fintel N and Nairn S:
Sedation assessment using the Ramsay scale. Emerg Nurse. 3:18–20.
2010. View Article : Google Scholar
|
|
14
|
Zhan-Ying G, Chang-Ming W, Shuai T,
Lin-Lin T and Yu-Feng H: Comparison of effects of different doses
of dexmedetomidine on inhibiting tracheal intubation-evoked
haemodynamic responce in the elderly patients. J Clin Diangn Res.
9:10–13. 2015.
|
|
15
|
Yang Z, Zheng Q and Wang Z: Meta-analysis
for nasogastric or nasojejunal decompression after gastrectomy for
gastric cancer. Br J Surg. 95:809–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomized trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei W, Chen Q, Zhang LC and Chen WH:
Dexmedetomidine verses midazolam for sedation in upper
gastrointestinal endoscopy. J Int Med Res. 42:516–522. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sethi P, Mohammed S, Bhatia PK and Gupta
N: Dexmedetomidine verses midazolam for conscious sedation in
endoscopic retrograde cholangiopancreatography: An open-lable
randomized controlled trial. Indian J Anaesth. 58:18–24. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demiraran Y, Korkut E, Tamer A, Yorulmaz
I, Kocaman B, Sezen G and Akcan Y: The comparison of
dexmedetomidine and miazolam used for sedation of patients during
upper endoscopy: A prospective, randomized study. Can J
Gastroenterol. 21:25–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dere K, Sucullu I, Budak ET, Yeyen S,
Filiz AI, Ozkan S and Dagli G: A comparison of dexmedetomidine
versus midazolam for sedation, pain and hemodynamic control, during
colonoscopy under conscious sedation. Eur J Anaesthesiol.
27:648–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang G, Zheng FL, Ouyang W and Xiao DH:
Small dose of dexmedetomidine in elderly patients for conscious
sedation undergoing colonoscopy. Zhong Guo Nei Jing Za Zhi.
19:685–688. 2013.(In Chinese).
|
|
22
|
Li YX, Qu XH, Li HY, Luo ZH, Cong S, Liu B
and Cui XG: The comparison of sedation with dexmedetomidine and
midazolam used for enteroscopy. Xian Dai Sheng Wu Yi Xue Jin Zhan.
14:2293–2225. 2014.(In Chinese).
|
|
23
|
Arpaci AH and Bozkirli F: comparison of
sedation effectiveness of remifentanil-dexmedetomidine and
remifentanil-midazolam combinations and their effects on
postoperative cognitive functions in cstoscopies: A randomized
clinical trial. J Res Med Sci. 18:107–114. 2013.PubMed/NCBI
|
|
24
|
Karaaslan K, Yilmaz F, Gulcu N, Colak C,
Sereflican M and Kocoglu H: Comparison of dexmedetomidine and
midazolam for monitored anesthesia care combined with tramadol via
patient-controlled analgesia in endoscopic nasal surgery: A
prospective, randomized, double-blind, clinical study. Curr Ther
Res Clin Exp. 68:69–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao W, Ma G, Su QG, Fang Y, Gu BC and Zou
XM: Dexmedetomidine versus midazolam for conscious sedaton in
postoperative patients undergoing flexible bronchoscopy: A
randomized study. J Int Med Res. 40:1371–1380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koca T, Dereci S, Karaham N and Akcam M:
Gastrointestinal neuroendocrine tumors in two children. Indian
Pediatr. 1:70–72. 2016.
|
|
27
|
Kenshi Yao: The endoscopic diagnosis of
early gastric cancer. Ann Gastroenterol. 1:11–22. 2013.
|
|
28
|
Yu H, Yang AM, Lu WX, Zhou WX, Yao F, Fei
GJ, Guo T, Yao LQ, He LP and Wang BM: Magnifying narrow-band
imaging endoscopy is superior in diagnosis of early gastric cancer.
World J Gastroenterol. 30:9156–9162. 2015. View Article : Google Scholar
|
|
29
|
Wang D, Wei XE, Yan L, Zhang YZ and Li WB:
Enhanced CT and CT virtual endoscopy in diagnosis of heterotopic
pancreas. World J Gastroenterol. 33:3850–3855. 2011. View Article : Google Scholar
|
|
30
|
Bell GD: Premedication, preparation and
surveillance. Endoscopy. 34:2–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adams R, Brown GT, Davidson M, Fisher E,
Mathisen J, Thomson G and Webster NR: Efficacy of dexmedetomidine
compared with midazolam for sedation in adult intensive care
patients: A systematic review. Br J Anaesth. 111:703–710. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Lu Y, Huang Y and Jiang H: Is
dexmedetomidine superior to midazolam as a premedication in
children? A meta-analysis of randomized controlled trials. Paediatr
Anaesth. 24:863–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tellor BR, Arnold HM, Micek ST and Kollef
MH: Occurrence and predictors of dexmedetomidine infusion
intolerance and failure. Hosp Pract (1985). 40:186–192. 2012.
View Article : Google Scholar
|
|
34
|
Huang Z, Chen YS, Yang ZL and Liu JY:
Dexmedetomidine versus midazolam for the sedation of patients with
non-invasive ventilation failure. Intern Med. 51:2299–2305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jalowiecki P, Rudner R, Gonciarz M,
Kawecki P, Petelenz M and Dziurdzik P: Sole use of dexmedetomidine
has limited utility for conscious sedation during outpatient
colonoscopy. Anesthesiology. 103:269–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu C, Li S, Deng F, Yao Y and Qian L:
Comparison of dexmedetomidine/fentanyl with midazolam/fentanyl
combination for sedation and analgesia during tooth extraction. Int
J Oral Maxillofac Surg. 43:1148–1113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schafrath E, Kuhlen R and Tonner PH:
Analgesia and sedation in intensisve care medicine. Anaesthesist.
53:1111–1130. 2004.(In German). PubMed/NCBI
|
|
38
|
Cheung CW, Ying CL, Chiu WK, Wong GT, Ng
KF and Irwin MG: A comparison of dexmedetomidine and midazolam for
sedation in third molar surgery. Anaesthesia. 62:1132–1138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arain SR and Ebert TJ: The efficacy, side
effects and recovery characteristics of dexmedetomidine versus
propofol when used for intraoperative sedation. Anesth Analg.
95:461–466. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Snapir A, Posti J, Kentala E, Koskenvuo J,
Sundell J, Tuunanen H, Hakala K, Scheinin H, Knuuti J and Scheinin
M: Effects of low and high plasma concentrations of dexmedetomidine
on myocardial perfusion and cardiac function in healthy male
subjects. Anesthesiology. 105:902–910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nelson LE, Lu J, Guo T, Saper CB, Franks
NP and Maze M: The a2-adrenoceptor agonist dexmedetomidine
converges on an endogenous sleep promoting pathway to exert its
sedative effects. Anesthesiology. 98:428–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prielipo RC, Wall MH, Tobin JR, Groban L,
Cannon MA, Fahey FH, Gage HD, Stump DA, James RL, Bennett J and
Butterworth J: Dexmedetomidine induced sedation in volunteers
decreases regional and global cerebral blood flow. Anesth Analg.
95:1052–1059. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gerlach AT, Dasta J, Armen S, Smith J,
Steinberg S, Martin L and Cook C: Titration protocol reduces
hypotension during dexmedetomidine infusion in critically ill
surgical patients (abstract). Crit Care Med. 34:A1482006.
View Article : Google Scholar
|
|
44
|
Mack PF, Perrine K, Kobylarz E, Schwartz
TH and Lien CA: Dexmedetomidine and neurocognitive testing in awake
craniotomy. J Neurosurg Anesthesiol. 16:20–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wijeysundera DN, Bender JS and Beattie WS:
Alpha-2 adrenergic agonists for the prevention of cardiac
complications among patients undergoing surgery. Cochrane Database
Syst Rev. 7:CD0041262009.
|
|
46
|
Coull JT, Jones ME, Ecan TD, Frith CD and
Maze M: Attentional effects of noradrenaline vary with arousal
level: Slective activation of thalamic pulvinar in humans.
Neuroimage. 22:315–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Menda FK, Köner O, Sayin M, Türe H, Imer P
and Aykaç B: Dexmedetomidine as an adjunct to anesthetic induction
to attenuate hemodynamic response to endotracheal intubation in
patients undergoing fast-track GABG. Ann Card Anaesth. 3:16–21.
2010.
|