Introduction
Sjogren's syndrome type B antigen (SSB)/La antibody
is one of the autoantibodies generally observed in the connective
tissue diseases such as Sjögren's syndrome, neonatal lupus
erythematosus and sub-acute cutaneous lupus erythematosus. Patients
with anti-SSB antibodies are more likely to experience less severe
conditions and improved prognosis (1,2). On the
other hand, double-stranded deoxyribonucleic acid (dsDNA)
antibodies were identified as the most characteristic
autoantibodies in patients with systemic lupus erythematosus (SLE)
and their vital roles in the pathogenesis of lupus and other
autoimmune diseases have been described (3–5). In
general, multiple autoantibodies may be detected simultaneously in
patients with lupus erythematosus. Smith (Sm) antibodies are often
associated with u1-ribonucleoprotein (RNP) antibodies whereas SSA
antibodies are usually found with SSB antibodies. This type of
antibody co-prevalence was related to the phenomenon of epitope
spreading, during which, epitopes that are non-crossreactive and
distinct from an inducing epitope become the main target of the
ongoing immune response e.g., as a result of acute/persistent
infection or secondary to chronic tissue destruction during
progressive autoimmune disease (5).
The relationship among these autoantibodies remains unclear. In the
present study, we determined the antibody profile in rabbits that
were immunized with synthetic SSB peptides alone or in combination
with dsDNA. The results showed that beside the production of target
antibodies, coimmunization was associated with epitope
spreading.
Materials and methods
Reagents
The main reagents used in this study included:
fluorenylmethyloxycarbonyl (Fmoc) amino acids (Amersham Pharmacia
Biotech, Piscataway, NJ, USA), hemocyanin (Sigma-Aldrich, Hong
Kong, China), calf thymus DNA (DingGuo ChangSheng Biotechnology
Co., Ltd., Beijing, China), and dsDNA immunofluorescence assay kit
and extractable nuclear antigens (ENA) polypeptide spectrum kit
(Euroimmun AG, Lübeck, Germany).
Prediction and design of amino acid
sequences containing polypeptide antigens
The primary sequence of SSB/La protein was obtained
based on web-based resources, and prediction and analysis were
conducted by using specific structural analysis software. An SSB/La
polypeptide was designed and synthesized after comprehensive
consideration of its secondary structure, hydrophilicity, and
surface accessibility in combination with immunogenic epitopes. The
sequence of this polypeptide was: SSB/La 214–225aa KQKLEEDAEMKS-Y.
The C-terminal tyrosine (Y) was coupled with keyhole limpet
hemocyanin (KLH) vector to yield the KQKLEEDAEMKS-Y-KLH
conjugate.
Synthesis and purification of
epitope-containing polypeptide
The target polypeptide with antigenic epitopes was
synthesized from C- to N-terminus by using the solid-phase
synthesis approach (with Fmoc-protected amino acids as the starting
materials on solid supporter-resin) using an automated peptide
synthesizer (ABI Mode 433A; Applied Biosystems, Foster City, CA
USA). The yielded peptide-resin was hydrolyzed to obtain the
peptide crude product. The crude product was then purified by
C18-reverse phase high-performance liquid chromatography (600E;
Waters Corp., Milford, MS, USA) and the purified product (>95%)
was freeze-dried (Virtis Company, Inc., Cardiner, NY, USA).
Rabbit immunizations
For this purpose, 10 healthy New Zealand white
rabbits (average body weight: ~2 kg) were procured from the animal
hold unit, at the People's Hospital affiliated to Peking University
(Beijing, China) and randomized into 5 groups, containing 2 rabbits
each: i) SSB immunization group, animals were labeled as SSB1 and
SSB2; ii) dsDNA immunization group, animals were labeled as DNA1
and DNA2; iii) SSB+dsDNA immunization group, animals were labeled
as co-immunization 1 and co-immunization 2; iv) KLH immunization
group, animals were labeled as KLH1 and KLH2; and v)
phosphate-buffered saline (PBS) group, animals were labeled as
control no. 1 and control no. 2.
Rabbits were immunized with SSB polypeptide at a
concentration of 1 mg/ml. During the primary immunization, 1 ml of
antigenic polypeptide was emulsified with 1 ml of complete Freund's
adjuvant (CFA). For emulsification, one part of adjuvant and one
part of antigen were aspirated into a syringe and emulsified by
repeated plunger movements. A successful emulsification was yielded
when no spreading of emulsified droplets was observed in water. The
mixture was administered subcutaneously through multiple injections
(40–50 injection sites) in the back. Booster immunization comprised
the antigenic polypeptide and incomplete Freund's adjuvant (IFA).
Rabbits were immunized for 4 times with a 20-day interval. After 2
weeks of the 4th immunization, rabbits were sacrificed by
exsanguination.
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples were collected at 10 days following
the 2nd, 3rd and 4th immunizations and serum samples were
separated. Serum antibody titers were determined by indirect ELISA.
Antigenic SSB peptide fragment (free of hemocyanin; 0.2 µg/0.1
ml/well), DNA (2 µg/0.1 ml/well) and hemocyanin in carbonate buffer
(pH 9.6) were added to 96-well microtiter plates and incubated at
4°C overnight, followed by washing with PBS-T (0.01 M PBS pH 7.4
with 0.05% Tween-20) 4 times and dried. Plates were blocked by
incubation at 37°C for 1 h with 1% bovine serum albumin (BSA) in
PBS-T buffer (pH 9.6; 0.1 ml/well), followed by washing 4–5 times
and dried. Rabbit serum was diluted in 1% BSA PBS-T buffer to give
the following dilutions: 1:100; 1:200; 1:400; 1:800; 1:1,600;
1:3,200; and 1:6,400. Using normal rabbit serum (1:1,000 dilution;
0.1 ml/well) as negative control, the plates were incubated at 37°C
for 1 h, followed by repeated washes as before. Then, secondary
antibody i.e., horseradish peroxidase-labeled goat anti-rabbit
immunoglobulin G (IgG) was added (0.1 ml/well) and incubated at
37°C for 40 min, followed by repeated washes. Chromogenic reaction
was developed by incubating for 10 min in the dark at 37°C with
solution A (10 mg temporomandibular disorder + 1 ml
dimethylformamide), diluted 1:100 with citrate buffer (pH 5.0) and
solution B (12 ml distilled water+5 ml H2O2).
The reaction was terminated with 2N H2SO4 by
adding 2 drops/well and optical density (OD) was read at 450 nm
ultraviolet wavelength. OD values >1.5-fold the negative control
OD were considered as positive. For positive results, the highest
dilution was considered to be the antibody titer.
ENA and dsDNA antibody assays
ENA antibody assay was performed using ENA
polypeptide spectrum kit, based on western blotting, according to
the manufacturer's instructions. Similarly, dsDNA antibody assay
was carried out using dsDNA assay kit, based on fundamental
procedure of indirect immunofluorescence assay, according to the
manufacturer's instructions.
Results
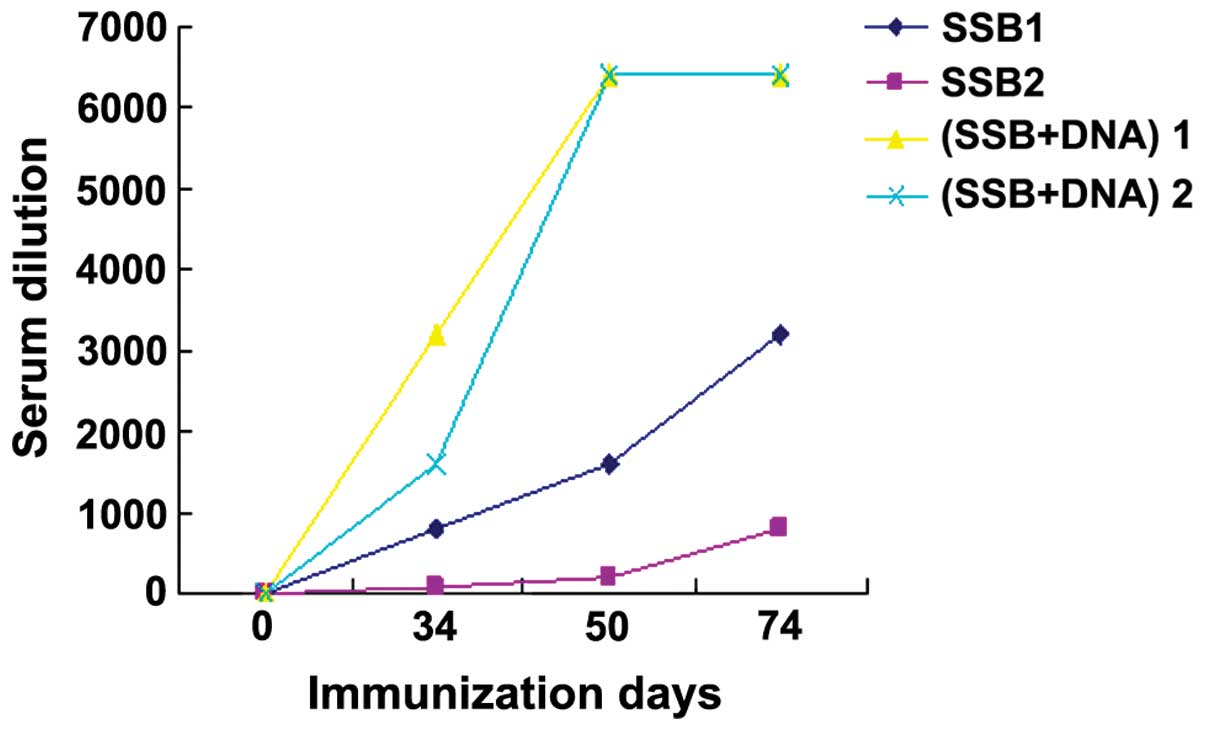
Anti-SSB polypeptide antibody
titers
Serum samples were collected at the 2nd, 3rd and 4th
immunization and anti-SSB polypeptide antibodies were measured by
ELISA. Although no anti-SSB polypeptide antibodies were detected in
PBS, hemocyanin, and the dsDNA immunization groups, anti-SSB
polypeptide antibody titers at 2nd, 3rd and 4th immunizations in
rabbit SSB1 were 1:800, 1:1,600 and 1:3,200, respectively. The
corresponding antibody titers in rabbit SSB2 were 1:100, 1:200 and
1:800, respectively. In the SSB+dsDNA co-immunization group,
respective anti-SSB titers in the co-immunization group rabbit 1
were 1:3,200, 1:6,400 and 1:6,400, and in co-immunization group
rabbit 2 were 1:1,600, 1:6,400 and 1:6,400. Thus, anti-SSB
polypeptide antibody titers in the co-immunization groups were
significantly higher than those of the SSB alone group at each time
point (Fig. 1).
ENA and dsDNA antibody profiles
The induction of anti-SSB polypeptide antibody was
concomitant with the positive detection of anti-Sm antibody in the
co-immunization group rabbit 1 and with anti-RNP and anti-Sm
antibodies in co-immunization group rabbit 2. A positive detection
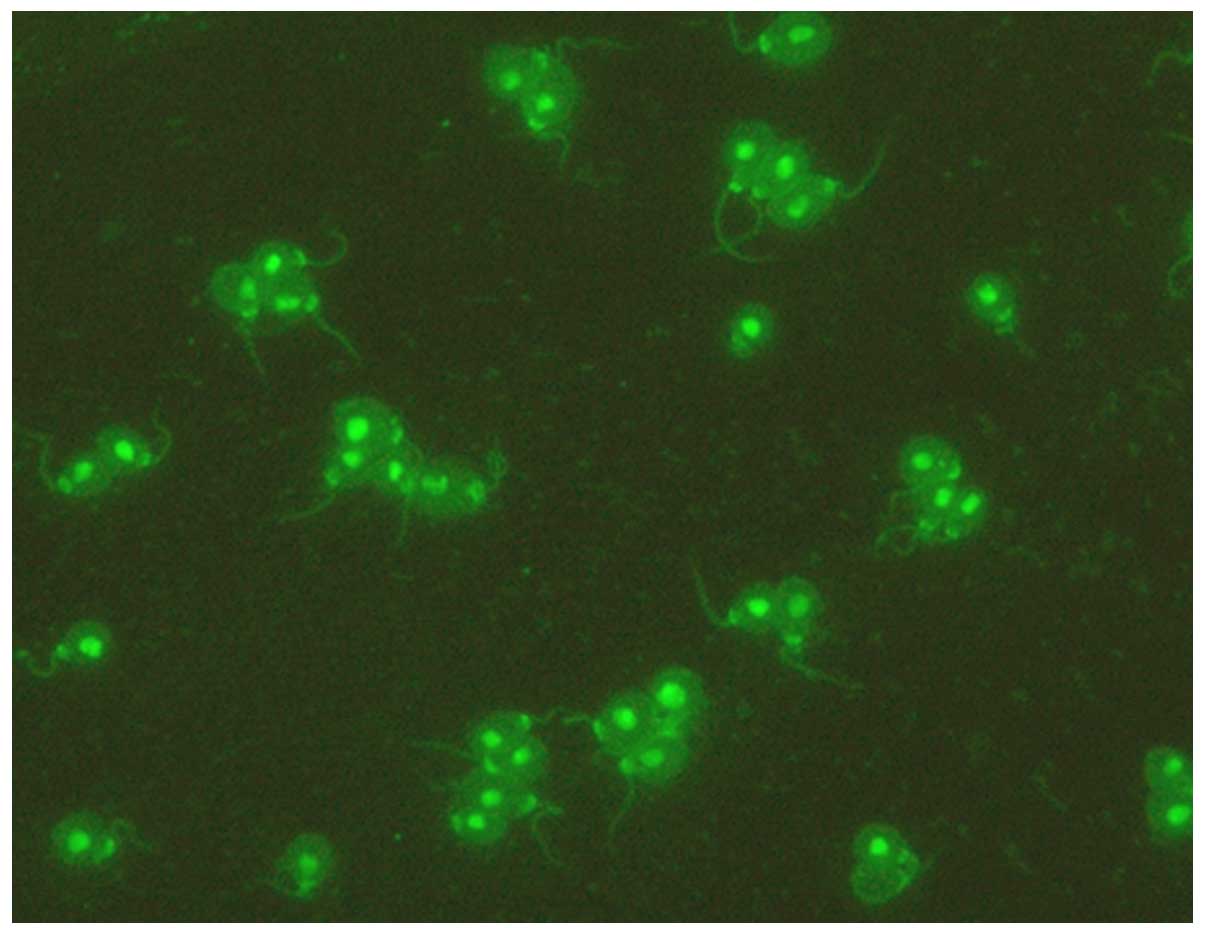
of anti-dsDNA antibody in rabbit SSB1 (1:160 titer; Fig. 2) was consistent with its detection in
the co-immunization group rabbit 1. No anti-dsDNA antibody was
detected in rabbits from the other groups.
Discussion
Autoantibodies of different types and clinical
relevance are commonly found in the sera of patients with SLE and
Sjögren's syndrome (5). Certain
autoantibodies even play a role in the pathogenesis of diseases
(5). It is, however, unclear whether
any antibody interactions occur during the course of their
production in the host. In the present study, SSB and dsDNA
antigens were used, alone or in combination, to immunize rabbits
and determine their antibody profiles. First, the human SSB/La
antigen (214–225aa) corresponding to the immunodominant epitope
region (179–242aa) was selectively designed and synthesized by
following standard peptide synthesis method (6–10). The
result showed that anti-SSB polypeptide antibodies were produced in
the SSB and SSB+dsDNA coimmunization groups, indicating the
presence of epitopes in the synthesized polypeptide fragment.
Anti-SSB antibody peak titers were observed at the 3rd immunization
in the SSB+dsDNA group. In the SSB group, relatively lower antibody
titers were observed. Mammalian chromatin, nucleosomes, DNA, and
histones rarely induce autoimmune responses (11–13), and
CD4+ and CD8+ T-cell populations with
suppressive activities against anti-DNA antibody production were
observed in normal mice, but not in mice with lupus (14). Of note, we also did not detect
anti-dsDNA antibodies following immunization with dsDNA alone,
suggesting that the dsDNA was incapable of inducing antibody
production. Notably, although the DNA had no immunogenicity,
anti-SSB antibody titers were higher in the SSB+dsDNA
coimmunization group as compared with the SSB group. It may be
possible that the coimmunization with DNA antigen had enhanced the
immunogenicity of SSB antigen or induced the antigenic stimulation
for higher antibody production.
In the current study, the rabbits also produced RNP
and Sm antibodies in addition to anti-SSB polypeptide antibodies
following co-immunization. Furthermore, anti-dsDNA antibodies were
detected in SSB1 and coimmunization 1 rabbits, suggesting the
induction of intra- and inter-molecular epitope spreading following
SSB plus dsDNA coimmunization. Since the mammalian DNA is devoid of
auto-immunogenicity, the induction of anti-dsDNA antibody may,
therefore, be a result of SSB polypeptide-induced epitope
spreading. Similar findings of SSB antigen-induced epitope
spreading have been reported previously (15). Epitope spreading is defined as the
development of immune response to a specific epitope as well as
other epitopes in the early stages of T- or B-cell-mediated
responses, spanning from monoclonal or oligoclonal to polyclonal T
or B cell activation. From the perspective of anti-infection,
epitope spreading is considered an efficient mechanism to generate
effective immune response against alien epitopes of invading
pathogens or tumor cells.
Notably, an autoimmune response may also be
triggered by exogenous antigen(s) that progresses to a truly
autoimmune reaction, resulting in the induction of self-epitope
specific antibodies and lymphocytes. Epitope spreading may occur
from dominant epitopes to subdominant or recessive epitopes, from
intra- to intermolecular epitopes, or from exoepitopes (e.g.,
viruses) to endoepitopes. Epitope spreading may occur at the
following positions: i) inside of an antigenic molecule i.e.,
intramolecular; ii) different antigen or antigenic determinants
inside of a macromolecule, i.e., related intermolecular; and iii)
epitopes with no direct interconnections and special anatomical
distribution under physical or physiological conditions i.e.,
intermolecular. These types of epitope spreading have been
previously reported (16–19). For instance, the presence of
cross-reactivity between Epstein-Barr virus (EBV) nuclear antigen-1
during EBV infection and Ro, Sm B/B' and Sm D1 antigens was
reported in lupus patients (16). A
cross-reactivity between Ro60 and SmD autoantigens (17) as well as antigenic cross-reactivity
among full-length SSB, other epitopes, and myelin (18) were also reported. SSB
antigen-immunized animals developed intra- or intermolecular
epitope spreading (17–19). High titers of anti-dsDNA IgG
antibodies were observed, in addition to anti-histone and
anti-cardiolipin antibodies, in BALB/c mice immunized with
DWEYSVWLSN and adjuvant (20).
Scofield et al (21) reported
the development of Ro intramolecular immune spreading and
intermolecular epitope spreading for SSB, ds DNA, nRNP and Sm in
animals immunized with HNE-Ro antigen. Epitope spreading can be
related to multiple factors, such as the physical characteristics
of antigens, the presence of modified antibody, effect of genetic
background, and the established level of immune tolerance. It was
suggested that the intermolecular immune spreading was
antigen-dependent while the induction of an autoimmune response was
antigen-driven (22). Epitope
spreading is widely presented in autoimmune diseases, which are
closely associated with their pathogenesis.
In conclusion, the findings show that after rabbit
coimmunization with SSB polypeptide and dsDNA, besides the target
antibody production, unexpected antibodies (anti-ENA and
anti-dsDNA) were also induced, suggesting the intra- and
intermolecular epitope spreading. However, in-depth studies are
required to elucidate the mechanisms and pathogenesis of autoimmune
diseases.
Acknowledgements
The present study was supported by grant from the
National Natural Science Foundation (no. 30271194). We would like
to thank Professor Qianjin Lu from Central South University for
critical comments and technical review of the manuscript.
Glossary
Abbreviations
Abbreviations:
|
CFA
|
Freund's complete adjuvant
|
|
DMF
|
dimethylformamide
|
|
dsDNA
|
double-stranded deoxyribonucleic
acid
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ENA
|
extractable nuclear antigen
|
|
FITC
|
fluorescein isothiocyanate
|
|
IFA
|
Freund's incomplete adjuvant
|
|
KLH
|
keyhole limpet hemocyanin
|
|
PBS
|
phosphate-buffered saline
|
|
RNP
|
ribonucleoprotein
|
|
SLE
|
systemic lupus erythematosus
|
|
SSA
|
Sjogren's syndrome type A antigen
|
|
SSB
|
Sjogren's syndrome type B antigen
|
References
|
1
|
Wasicek CA and Reichlin M: Clinical and
serological differences between systemic lupus erythematosus
patients with antibodies to Ro versus patients with antibodies to
Ro and La. J Clin Invest. 69:835–843. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang JZ, Jin J, Guo LS and Ma SQ:
Different prognoses of two types of sub-acute cutaneous lupus
erythematosus: A follow-up of 40 cases. Chin J Dermatol. 34:9–11.
2001.
|
|
3
|
Balow JE and Austin HA III: Renal disease
in systemic lupus erythematosus. Rheum Dis Clin North Am.
14:117–133. 1988.PubMed/NCBI
|
|
4
|
Arbuckle MR, James JA, Kohlhase KF,
Rubertone MV, Dennis GJ and Harley JB: Development of anti-dsDNA
autoantibodies prior to clinical diagnosis of systemic lupus
erythematosus. Scand J Immunol. 54:211–219. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiffer LE, Hussain N, Wang X, Huang W,
Sinha J, Ramanujam M and Davidson A: Lowering anti-dsDNA antibodies
- what's new? Lupus. 11:885–894. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chambers JC, Kenan D, Martin BJ and Keene
JD: Genomic structure and amino acid sequence domains of the human
La autoantigen. J Biol Chem. 263:18043–18051. 1988.PubMed/NCBI
|
|
7
|
Kenan DJ, Query CC and Keene JD: RNA
recognition: Towards identifying determinants of specificity.
Trends Biochem Sci. 16:214–220. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gordon TP, Greer M, Reynolds P, Guidolin A
and McNeilage LJ: Estimation of amounts of anti-La(SS-B) antibody
directed against immunodominant epitopes of the La(SS-B)
autoantigen. Clin Exp Immunol. 85:402–406. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McNeilage LJ, Macmillan EM and Whittingham
SF: Mapping of epitopes on the La(SS-B) autoantigen of primary
Sjögren's syndrome: Identification of a cross-reactive epitope. J
Immunol. 145:3829–3835. 1990.PubMed/NCBI
|
|
10
|
Kohsaka H, Yamamoto K, Fujii H, Miyasaka
N, Miura H, Tanaka Y, Nishioka K and Miyamoto T: Molecular cloning
of cDNAs expressing SS-B/La protein. J Autoimmun. 2:353–357. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madaio MP, Hodder S, Schwartz RS and
Stollar BD: Responsiveness of autoimmune and normal mice to nucleic
acid antigens. J Immunol. 132:872–876. 1984.PubMed/NCBI
|
|
12
|
Stollar BD: Immunochemistry of DNA. Int
Rev Immunol. 5:1–22. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohan C, Adams S, Stanik V and Datta SK:
Nucleosome: A major immunogen for pathogenic autoantibody-inducing
T cells of lupus. J Exp Med. 177:1367–1381. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mamula MJ and Janeway CA Jr: Do B cells
drive the diversification of immune responses? Immunol Today.
14:151–152; discussion 153–154. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Routsias JG, Dotsika E, Touloupi E,
Papamattheou M, Sakarellos C, Sakarellos-Daitsiotis M, Moutsopoulos
HM and Tzioufas AG: Idiotype-anti-idiotype circuit in
non-autoimmune mice after immunization with the epitope and
complementary epitope 289–308aa of La/SSB: implications for the
maintenance and perpetuation of the anti-La/SSB response. J
Autoimmun. 21:17–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poole BD, Scofield RH, Harley JB and James
JA: Epstein-Barr virus and molecular mimicry in systemic lupus
erythematosus. Autoimmunity. 39:63–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pal R, Deshmukh US, Ohyama Y, Fang Q,
Kannapell CC, Gaskin F and Fu SM: Evidence for multiple shared
antigenic determinants within Ro60 and other lupus-related
ribonucleoprotein autoantigens in human autoimmune responses. J
Immunol. 175:7669–7677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terzoglou AG, Routsias JG, Sakarellos C,
Sakarellos-Daitsiotis M, Moutsopoulos HM and Tzioufas AG: Linear
epitopes of two different autoantigens-La/SSB and myelin basic
protein-with a high degree of molecular similarity, cause different
humoral immune responses. J Autoimmun. 21:47–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang SH, Huh MS, Kim HR, Kim IS, Kim S,
Lee JS, Semsei I, Grölz D and Bachmann M: Cross-reactivity of
antibodies immunoadsorbed to laminin with recombinant human La
(SS-B) protein. J Autoimmun. 11:163–167. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Putterman C and Diamond B: Immunization
with a peptide surrogate for double-stranded DNA (dsDNA) induces
autoantibody production and renal immunoglobulin deposition. J Exp
Med. 188:29–38. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scofield RH, Kurien BT, Ganick S, McClain
MT, Pye Q, James JA, Schneider RI, Broyles RH, Bachmann M and
Hensley K: Modification of lupus-associated 60-kDa Ro protein with
the lipid oxidation product 4-hydroxy-2-nonenal increases
antigenicity and facilitates epitope spreading. Free Radic Biol
Med. 38:719–728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deshmukh US, Gaskin F, Lewis JE, Kannapell
CC and Fu SM: Mechanisms of autoantibody diversification to
SLE-related autoantigens. Ann N Y Acad Sci. 987:91–98. 2003.
View Article : Google Scholar : PubMed/NCBI
|