Introduction
Osteosarcoma is the most common aggressive
malignancy in children and young adolescents, which accounts for
2.4% of all pediatric malignancies worldwide. The estimated average
survival rate of patients with osteosarcoma following diagnosis at
metastatic stage is 4–5 years (1,2). Despite
recent advances and developments in osteosarcoma treatment
strategies, the overall survival rate of patients has not yet
improved. According to cancer stem cell theory, the presence of a
small population of cancer initiating cells, cancer stem cells
(CSCs), are responsible for treatment failure, tumor relapse and
metastasis (3–5). These CSCs have been demonstrated to
possess increased expression of stem cell surface proteins,
including Oct3/4A and Nanog, and ATPase binding cassette (ABC)
transporters, including MDR1, also known as ABCB1, ABCC1 and ABCG2,
also known as BCRP1, which have an essential role in the
maintenance of self-renewal and drug resistance properties of CSCs,
respectively (6,7). Therefore, understanding the underlying
molecular mechanism and signaling pathways involved in the
multi-drug and apoptosis resistance of cancer stem cells is
necessary in order to efficiently target CSCs. The Hoechst 33342
dye exclusion assay (8,9) is a well-used method for the
purification of CSCs. In this method, the Hoechst 33342 dye is
prevented from entering cancer stem cells due to ABC transporter
proteins, such as ABCG2. These cells appear as a distinct
population on the periphery of the dot plot analysis of the
fluorescence activated cell sorting (FACS) profile; hence, they are
termed side population (SP) cells (9). Previous studies have demonstrated that
SP cells possess the majority of the remarkable features of cancer
stem cells, including a high resistance to chemotherapeutic drugs,
high tumorigenic potency and the overexpression of stemness genes
(6,7,10);
therefore, they are referred to as ‘enriched cancer stem cells
(5,11,12).
Characterizing SP cells and elucidating the pathways associated
with drug resistance may help to develop anti-cancer drugs to
target CSCs.
In the present study, osteosarcoma stem-like SP
cells were purified and characterized from the aggressive human
osteosarcoma OS-65 cell line. Furthermore, the level of multi-drug
and apoptosis resistance of SP cells was evaluated, along with the
transcriptional regulation of multiple ABC transporter and stemness
genes, in order to explore the credibility of CSCs as a novel
therapeutic target for the prevention of tumor relapse.
Materials and methods
Cell lines and culture conditions
Osteosarcoma cell line OS-65 was donated by Dr
Di-Sheng Yang (Department of Orthopaedics of the Second Affiliated
Hospital of Zhejiang University (Hangzhou, China). OS-65 is an
aggressive primary human osteosarcoma (stage-III) with lung
metastasis. Cells were cultured in Dulbecco's modified Eagle medium
(DMEM) supplemented with 10% fetal bovine serum (both purchased
from Sigma-Aldrich, St. Louis, MO, USA) with antibiotics (GE
Healthcare Life Sciences, Logan, UT, USA) and maintained in T-75
flasks (Corning Inc., Corning, NY, USA) at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air. Upon 90%
confluency, cells were removed from the culture flask using
Trypsin-EDTA (0.25%-53 mM EDTA; Sigma-Aldrich), washed and
suspended in 10% DMEM. A cell count was conducted using a
hemocytometer (Sigma-Aldrich).
FACS analysis
Experimental groups
Cells were equally divided into two groups: Control
group, cells + Hoechst 33342 dye (Sigma-Aldrich) (n=9); and
drug-treated group, cells + verapamil drug + Hoechst 33342 dye
(n=9). Each sample had ~1×106 cells. A total of ~106
cells/ml in 10% DMEM were labeled with either 5 µl/ml Hoechst 33342
dye alone or in combination with 0.8 µl/ml verapamil
(Sigma-Aldrich), as outlined. Cells were resuspended in 500 µl
Hank's balanced salt solution (HBSS; Sigma-Aldrich) containing 10
mM HEPES for FACS analysis. These cells were then incubated at 37°C
for 90 min, centrifuged for 5 min at 300 × g (4°C) and resuspended
in ice-cold HBSS. Then, 2 µg/ml propidium iodide (PI;
Sigma-Aldrich) was added to identify dead cells. The cells were
then filtered through a 40 µm cell strainer (BD Falcon; BD
Pharmingen, San Diego, CA, USA) to obtain single suspension cells.
Dual-wavelength analysis and purification were performed using
dual-laser cytometry (FACSVantage; BD Biosciences, Franklin Lakes,
NJ). A 610 nm dichroic mirror short-pass was used to separate the
emission wavelengths. PI-positive dead cells were excluded from the
analysis.
Sarcosphere formation assay
Sarcosphere formation assay was performed, according
the protocol described by Gibbs et al (7). Cells were plated at a density of 60,000
cells/well in ultra-low attachment six-well plates (Corning, Inc.)
containing serum-free DMEM/F12 medium supplemented with
N2, 10 ng/ml epidermal growth factor and 10 ng/ml human
basic fibroblast growth factor (both purchased from Sigma-Aldrich).
The culture was analyzed for sphere formation daily for 7 days, and
cell proliferation was measured by checking the absorbance at 450
nm using a plate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Following 7 days of culturing, the total number of
sarcospheres generated by FACS-sorted SP and non-SP cells was
quantified by inverted phase contrast microscopy (Eclipse TS100;
Nikon Corporation, Tokyo, Japan).
Immunofluorescent staining
FACS-sorted SP and non-SP cells from the OS-55 cell
line were fixed in BD Cytofix solution (BD Biosciences) and
incubated for 20 min at 4°C. Following blocking in donkey serum
(Sigma-Aldrich) for 20 min, cells were incubated with goat
anti-Oct3/4A polyclonal primary antibody (1:100; sc-8628; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C,
and were subsequently incubated with rhodamine red-conjugated
donkey anti-goat antibody (1:200; 705-295-003; Jackson
Immunoresearch Laboratories, Inc., West Grove, PA, USA). For CD44
and Nanog immunofluorescence analysis, cells were stained with
fluorescein isothiocyanate (FITC)-conjugated anti-human Nanog (1:5;
674206; BioLegend, San Diego CA, USA) or CD44 (1:5; 8011-0441;
eBioscience, Inc., San Diego, CA, USA) antibodies. Human embryonic
stem cells were used as the positive control. Cells were observed
under a confocal microscope (LSM 700; Zeiss AG, Oberkochen,
Germany). Images were captured and processed by Adobe Photoshop CS4
(Microsoft Corporation, Redmond, WA, USA).
Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR)
Total RNA from SP and non-SP cells was extracted
using TRIzol reagent and treated with RNAase-Free DNase according
to the manufacturer's instructions (both purchased from Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Samples were then
reverse transcribed using a First-Strand cDNA Synthesis Kit
containing Oligo(dT)12–18 primers (Fermentas; Thermo
Fisher Scientific, Inc.) and 1.5 µg total RNA, according to the
manufacturer's instructions. RT-qPCR analysis was subsequently
performed using IQ Supermix with SYBR-Green (Bio-Rad Laboratories,
Inc.) and a 20 µl reaction volume containing 300 nM forward and
reverse primers and 50 ng cDNA template. The thermo cycling
conditions were as follows: Initial denaturation and enzyme
activation at 95°C for 2 min, 37 cycles of denaturation at 95°C for
15 sec, annealing at 60°C for 50 sec and extension at 72°C for 30
sec, using instrument default settings for melt curve analyses.
Sequences of the human specific primers (Sigma-Aldrich) were as
follows: ABCG2 forward, TCAATCAAAGTGCTTCTTTTTTATG and reverse,
TTGTGGAAGAATCACGTGGC; ABCB5 forward, CACAAGTTGGACTGAAAGGA and
reverse, ACCACTAGGCATGTCCTTCC; MDR1 forward, ACAGGAAGAGATTGTGAGGG
and reverse, TATCCAGAGCTGACGTGGCT; Oct3/4A forward,
TGGAGAAGGAGAAGCTGGAGCAAAA and reverse,
GGCAGATGGTCGTTTGGCTGAATAGACC; Sox2 forward, CACACTGCCCCTCTCACACAT
and reverse, CATTTCCCTCGTTTTTCTTTGAA; Nanog forward,
TCCTCCTCTTCCTCTATACTAAC and reverse, CCCACAAATCACAGGCATAG; actin,
forward GCGGGAAATCGTGCGTGACATT and reverse,
GGCAGATGGTCGTTTGGCTGAATA; Bcl-2 forward, ACACTGTTAAGCATGTGCCG and
reverse, CCAGCTCATCTCACCTCACA (13).
PCR products were electrophoresed on 1.2% agarose gel and stained
with ethidium bromide (both purchased from Sigma-Aldrich). Results
were analyzed using CFX Manager Software (version 3.0; Bio-Rad
Laboratories, Inc.).
Cell resistance assay
A total of ~1×103 cells/plate were cultured in
96-well plates and treated with the following chemotherapeutic
agents: 10 µg/ml 5-fluorouracil, 250 mM gemcitabine, 30 ng/ml
paclitaxel, 5 mg/ml cisplatin, 10 mg/ml etoposide and 2 µg/ml 100
oxaliplatin (all purchased from Sigma-Aldrich). Mean optical
density (OD) was obtained at OD450 and represented as a
graph. Cell resistance in SP and non-SP cells was calculated using
the following formula: Cell resistance rate (%) = (Experimental
group OD450/control group OD450) × 100.
Values presented in the graph are the mean of three independent
experiments.
Statistical analysis
One-way analysis of variance and Student's t-test
was performed to determine significant differences between the
treatment and control groups. Microsoft Excel (version 14.6.1;
Microsoft Corporation, Redmond, WA, USA) was used to perform
statistical analyses. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Purification and characterization of
osteosarcoma SP cells
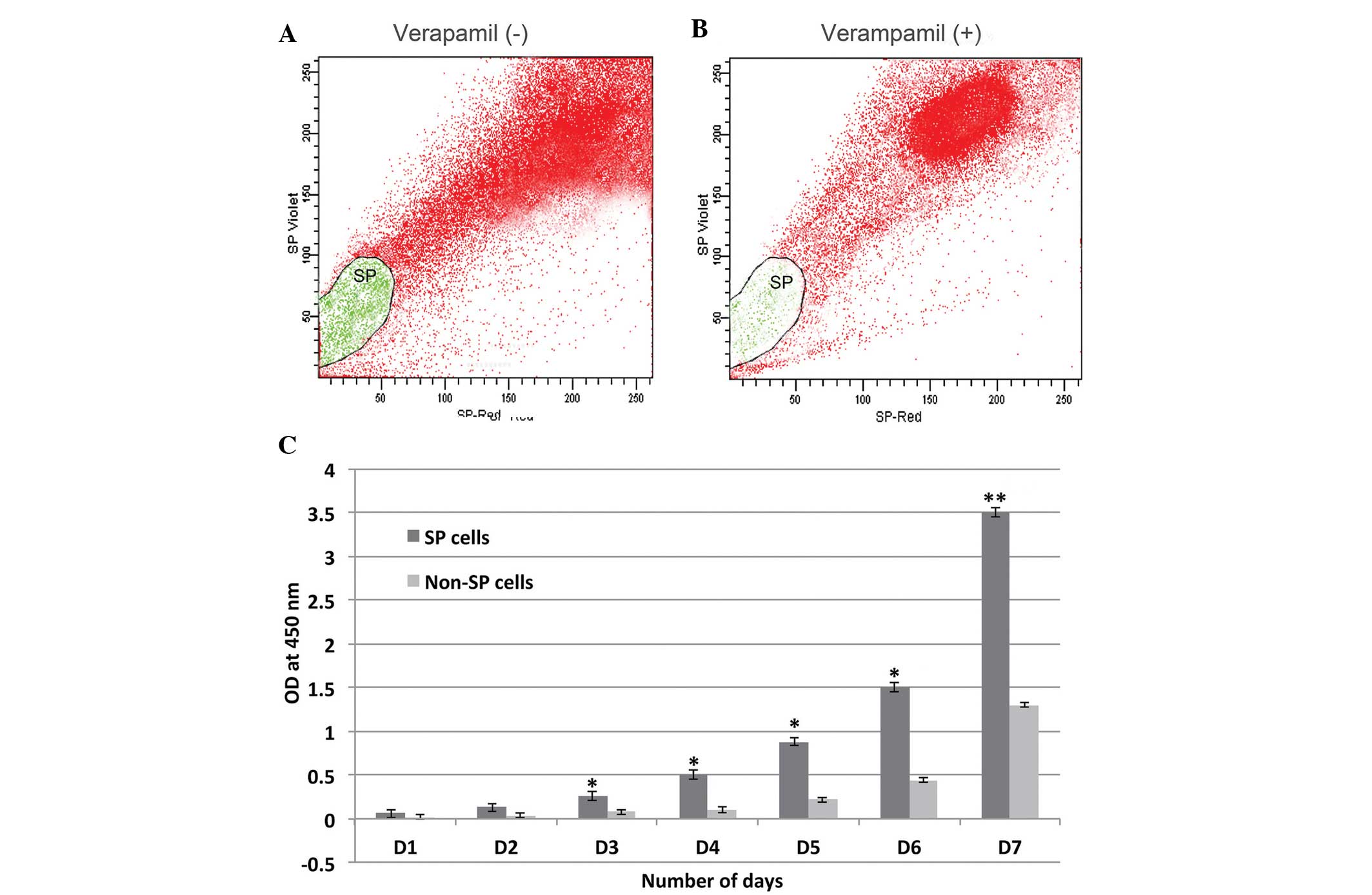
Osteosarcoma samples were examined for the presence
of cancer stem-like SP cells using a FACS-based Hoechst 33342 dye
exclusion assay. Osteosarcoma samples contained ~3.3% SP cells, and
were reduced to 0.3% upon treatment with verapamil, which is an
inhibitor of ABC transporters (Fig. 1
and B). Subsequently, the FACS-purified osteosarcoma SP and
non-SP cells were subjected to an in vitro proliferation
assay. Osteosarcoma SP cells exhibited significantly increased cell
proliferation from day 3, as compared with the non-SP cells
(P<0.05), and were most confluent on day 7, as compared with the
non-SP cells (P<0.01; Fig. 1C).
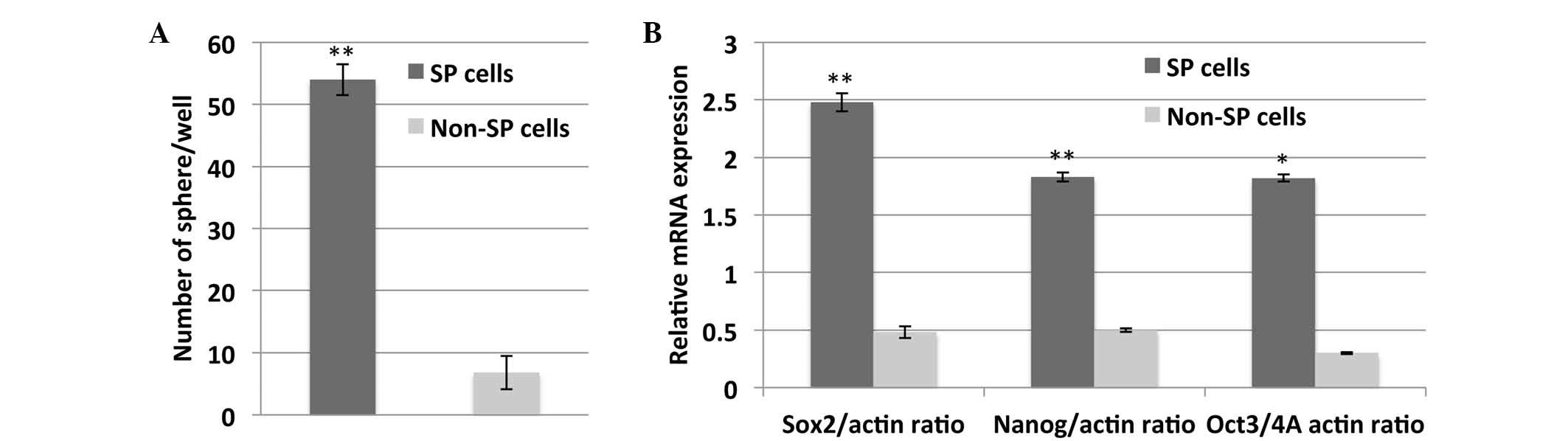
In addition, a sarcospheres formation assay was performed in order
to determine the self-renewal properties of the osteosarcoma SP
cells. SP cells generated significantly more sarcospheres, as
compared with the non-SP cells (P<0.01; Fig. 2A). Furthermore, SP cells exhibited
significantly increased mRNA expression of stem cell surface genes,
including Sox2, Nanog and Oct-3/4A, which are responsible for the
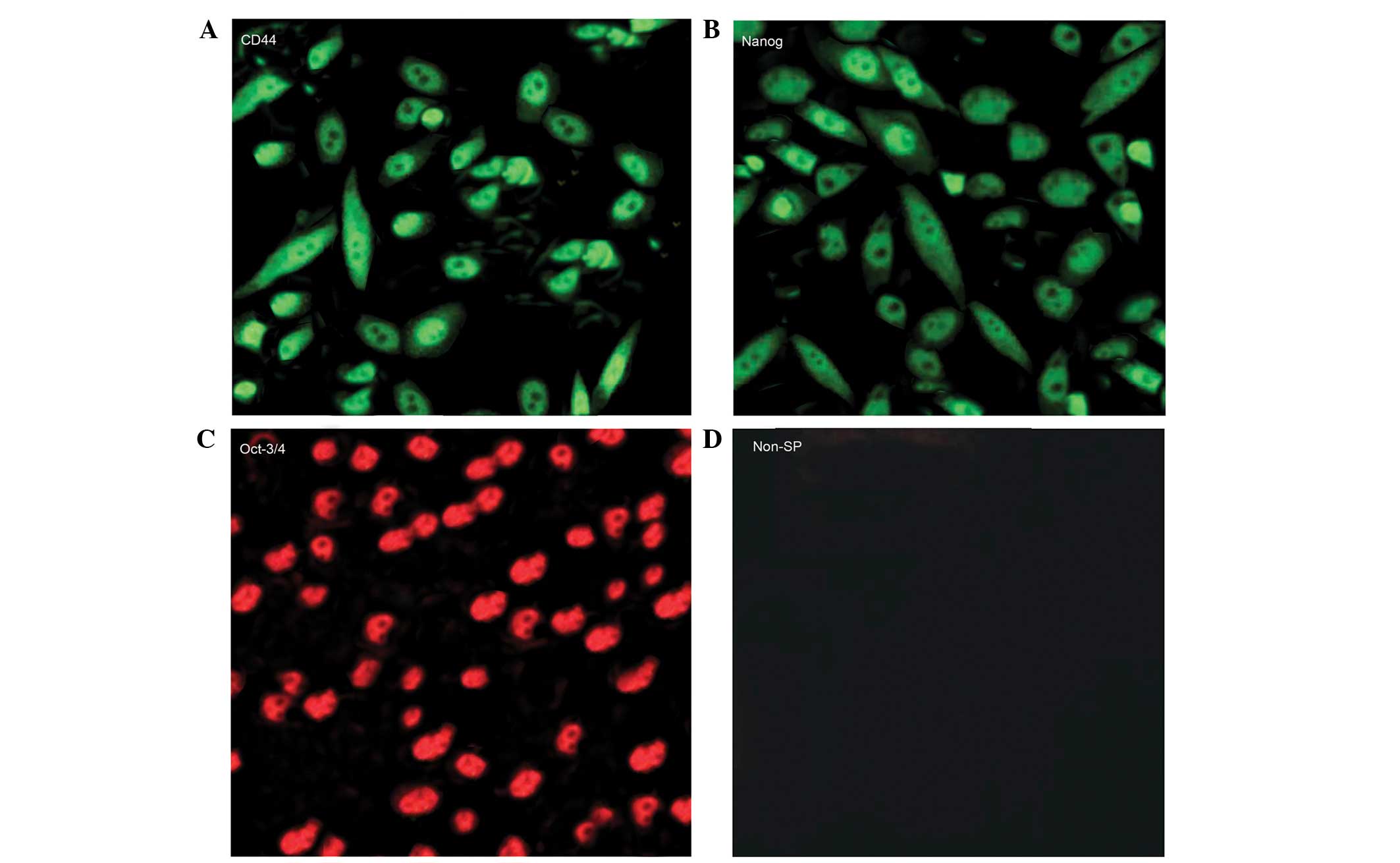
maintenance of self-renewal of SP cells (Fig. 2B). As detected by immunofluorescence,
SP cells were positive for CD44, Nanog and Oct-4 protein
expression, whereas non-SP cells were not (Fig. 3). These results confirmed the
presence of a small population of SP cells in the OS-65
osteosarcoma cell line, as demonstrated by their active role in dye
exclusion via ABC transporters and their high potency of
self-renewal.
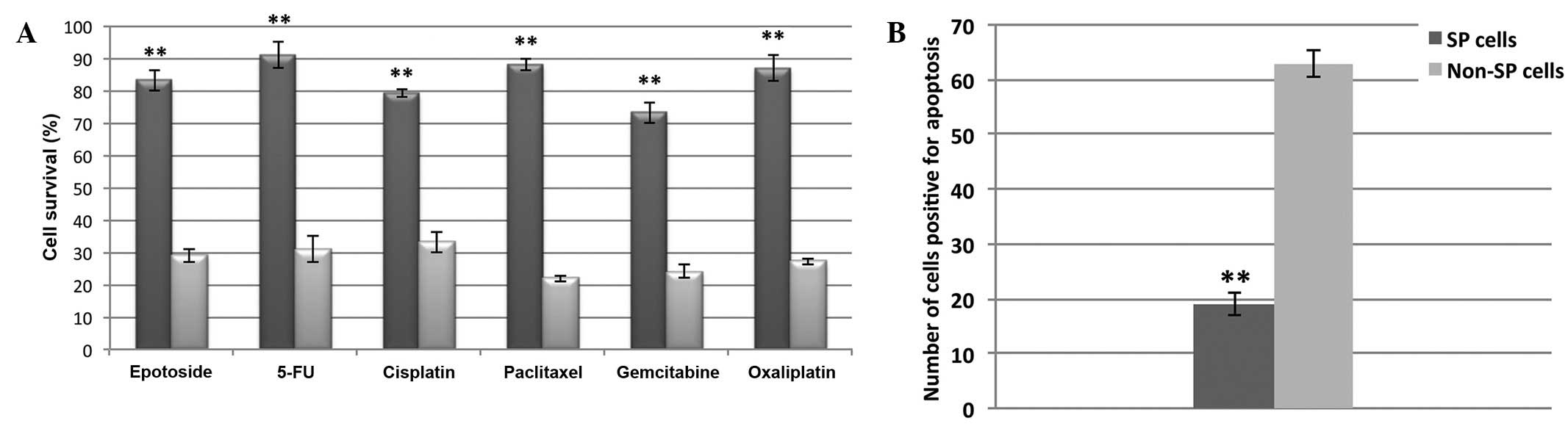
Osteosarcoma SP cells are multi-drug
and apoptosis resistant
FACS-sorted SP and non-SP cells were subjected to
drug resistance and cell death assays. As shown in Fig. 4A, the SP cells were highly resistant
to treatment with etoposide, gemcitabine, 5-fluorouracil,
cisplatin, paclitaxel and oxaliplatin and their survival rate
(>80%) was significantly increased, as compared with non-SP
cells (<30%; P<0.01). Consequently, the number of SP cells
that underwent apoptosis was significantly decreased, as compared
with the non-SP cells (P<0.01; Fig.
4B). Subsequently, in order to address the cause of the high
multi-drug and apoptosis resistance exhibited by the SP cells,
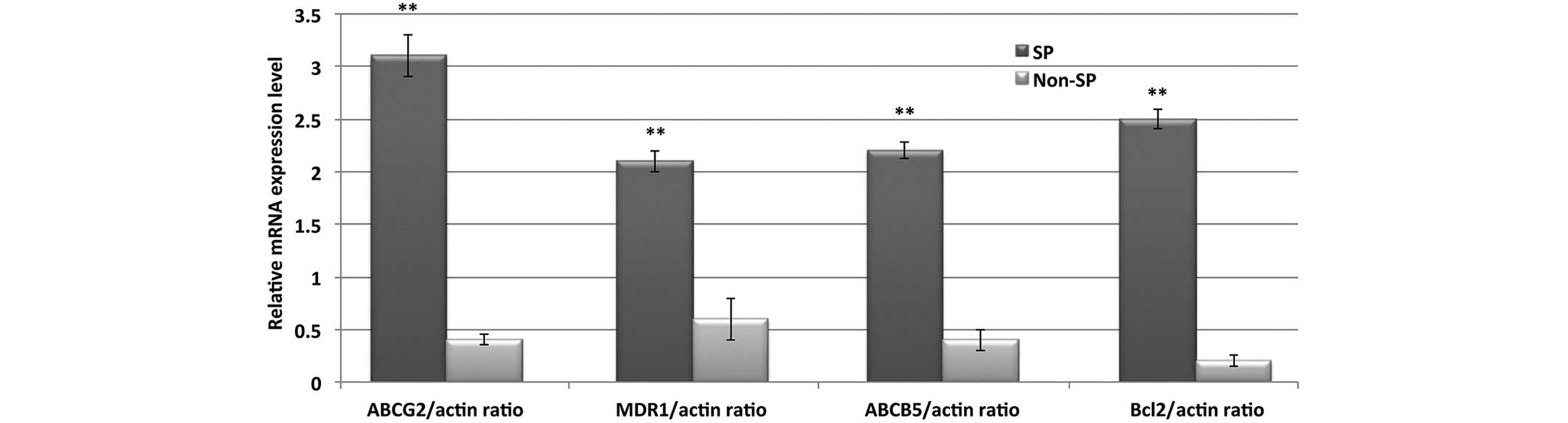
RT-qPCR was performed to evaluate the transcriptional regulation of
various ABC transporters and anti-apoptotic genes. The relative
mRNA expression levels of ABCG2, MDR1, ABCB5 and Bcl-2 were
significantly upregulated in the SP cells, as compared with the
non-SP cells (P<0.01; Fig. 5).
These results suggested that enhanced drug resistance and
downregulated apoptosis in SP cells may be responsible for
chemotherapy failure and tumor recurrence.
Discussion
Cancers are heterogeneous and the presence of small
populations of CSCs are responsible for initiating the development
of tumors. CSCs are characterized by the overexpression of stem
cell surface proteins and ABC transporter proteins, which are
associated with tumor metastasis and invasion, and multi-drug
resistance, respectively (12,14,15).
Therefore, elucidating the molecular mechanisms underlying
CSC-mediated tumorigenesis is crucial for the development of novel
anti-cancer drugs, in order to provide the effective treatment. In
order to investigate these mechanisms, it is essential to elucidate
the factors and signaling pathways involved in CSC-mediated
multi-drug resistance and cancer relapse. The present study
demonstrated that the OS-65 human osteosarcoma cell line contains
3.3% cancer stem cell-like SP cells, which were isolated using the
FACS-based Hoechst 33342 dye exclusion method. Furthermore, the
results of the present study demonstrated that these osteosarcoma
SP cells are highly efficient in generating tumor sarcospheres, as
they exhibit elevated expression of Oct3/4A, CD44 and Nanog stem
cell surface proteins, which have been reported as essential for
the maintenance of self-renewal of CSCs during tumorigenesis and
tumor invasion (3,15–17). In
line with these findings, it has previously been demonstrated that
SP cells from the OS99-1 and MG-63 osteosarcoma cell lines exhibit
relatively increased mRNA transcriptional levels and are highly
capable of generating more sarcospheres (18). Hence, the expression of these stem
cell proteins in SP cells may be used to validate the metastatic
stage of cancer.
SP cells are also resistant to chemotherapeutic
agents and apoptosis. A previous study demonstrated that these
effects are induced by the overexpression of multi-drug resistance
transporter proteins and anti-apoptotic factors (13). Furthermore, the overexpression of
stem cell surface proteoglycans and glycoproteins, including CD44,
Oct3/4A and Nanog, may also contribute to tumor metastasis and
invasion (19–22). Consistent with these previous
findings, the results of the present study demonstrated that OS-65
SP cells exhibited significantly increased expression levels of
CD44, Oct3/4A and Nanog, which may be essential for the maintenance
of self-renewal and metastasis of osteosarcoma SP cells (3,15–17). In
addition, the present study also demonstrated that SP cells possess
differential expression of ABCG2, ABCB5 and MDR1 transporter
proteins, which are predominantly upregulated, which may explain
the resistance of SP cells to chemotherapeutic agents. Similarly,
the anti-apoptotic factor, Bcl-2, was significantly downregulated,
suggesting that Bcl-2 acts as a survival factor for SP cells.
However, the functional association between stem cells surface
proteins, anti-apoptotic factors and ABC transporter proteins
requires further study.
In conclusion, the results of the present study
suggested that presence of cancer stem-like SP cells may be a
predominant cause of chemotherapy failure and tumor recurrence.
These effects are likely to be due to the overexpression of ABC
transporters and anti-apoptotic factors, as these increase the
resistance of osteosarcoma SP cells against chemotherapeutic agents
and apoptosis. Therefore, designing novel anti-cancer drugs, which
suppress the downstream signaling pathways involved in drug and
apoptosis resistance may effectively reduce tumor relapse in
patients with osteosarcoma.
Acknowledgements
The authors of the present study would like to thank
Dr Di-Sheng Yang (Department of Orthopaedics, Second Affiliated
Hospital, Zhejiang University) for providing the OS-65 osteosarcoma
cell line and for sharing the relevant protocols.
References
|
1.
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma and Ewing's sarcoma: National cancer
data base report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wu C, Wei Q, Utomo V, Nadesan P, Whetstone
H, Kandel R, Wunder JS and Alman BA: Side population cells isolated
from mesenchymal neoplasms have tumor initiating potential. Cancer
Res. 67:8216–8222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: Implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Su J, Xu XH, Huang Q, Lu MQ, Li DJ, Xue F,
Yi F, Ren JH and Wu YP: Identification of cancer stem-like CD44+
cells in human nasopharyngeal carcinoma cell line. Arch Med Res.
42:15–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properfies of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cho RW and Clarke FM: Recent advances in
cancer stem cells. Curr Opin Genet Dev. 18:48–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fan J, Li R, Zhang R, Liu HL, Zhang N,
Zhang FQ and Dou KF: Effect of Bcl-2 and Bax on survival of side
population cells from hepatocellular carcinoma cells. World J
Gastroenterol. 7(13): 6053–6059. 2007. View Article : Google Scholar
|
|
14.
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chang CC: Recent translational research:
Stem cells as the roots of breast cancer. Breast Cancer Res.
8:103–105. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lee J, Kim HK, Rho JY, Han YM and Kim J:
The human OCT-4 isoforms differ in their ability to confer
self-renewal. J Biol Chem. 281:3554–3565. 2006.
|
|
17.
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang L, Park P and Lin CY:
Characterization of stem cell attributes in human osteosarcoma cell
lines. Cancer Biol Ther. 8:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Setoguchi T, Taga T and Kondo T: Cancer
stem cells persist in many cancer cell lines. Cell Cycle.
3:414–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Pesce M and Schöler HR: Oct-4: Gatekeeper
in the beginnings of mammalian development. Stem Cells. 19:271–278.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B,
Ng HH and Robson P: Transcriptional regulation of nanog by OCT4 and
SOX2. J Biol Chem. 280:24731–24737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|