Introduction
The innate immune system and inflammation are
crucial for protecting host organisms from invasive pathogens and
injurious stimuli (1). Gao and Hong
(1) reported that inflammation is
part of the non-specific immune response that occurs in reaction to
harmful stimuli, such as pathogenic microbes, damaged cells,
irritants or bodily injury, with a primary aim of neutralizing
infectious agents and initiating repair to damaged tissue.
Conversely, uncontrolled inflammatory responses lead to the
development of acute or chronic inflammatory diseases (1).
Macrophages are inflammatory cells implicated in the
initiation of inflammatory responses, and have critical roles in
the pathogenesis of numerous inflammatory disease processes by
secreting various proinflammatory mediators, including nitric oxide
(NO) and proinflammatory cytokines (2). Therefore, the modulation of
macrophage-mediated inflammatory responses may be useful in the
development of novel therapeutic approaches against these
inflammatory diseases (3). Medina
et al (4) have reported that
macrophages have an important role in inflammatory disease via the
release of factors such as NO, reactive oxygen species,
inflammatory cytokines, chemokines, growth factors and
prostaglandin mediators involved in the immune response.
However, Ulevitch and Tobias (5) have reported that excessive and
uncontrolled production of inflammatory mediators such as NO and
cytokines may lead to serious systemic complications such as
microcirculatory dysfunction, tissue damage and septic shock, which
may result in mortality.
Carrithers (6)
reported that innate immune responses mediated by mononuclear
phagocytes represent the initial host response to acute viral
infection and pattern recognition receptors recognize viral nucleic
acid and localized injury signals to initiate proinflammatory
responses and activation of adaptive immunity.
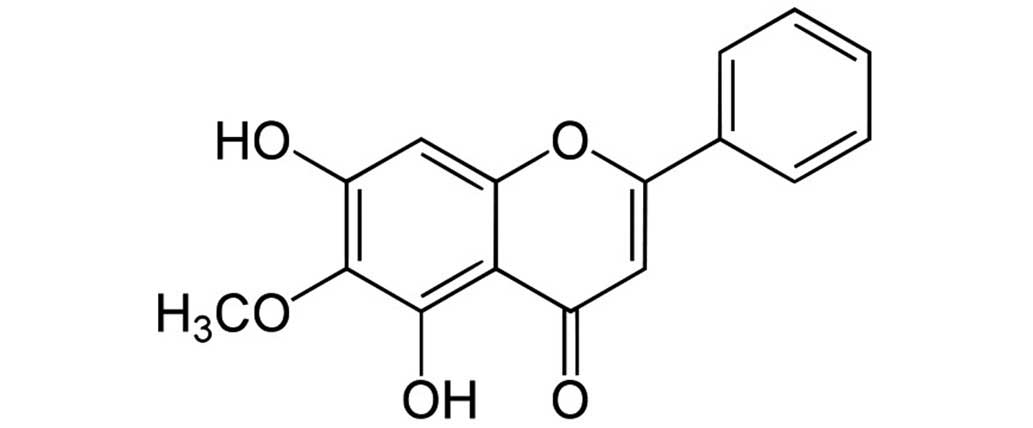
Oroxylin A
(5,7-dihydroxy-6-methoxy-2-phenylchromen-4-one; Baicalein 6-methyl
ether; Fig. 1) is an active
flavonoid compound isolated from Scutellaria radix, which has been
used to treat pulmonary infection traditionally in Korea, China and
Japan (7,8).
Tran et al (9)
have reported that oroxylin A is known to have dopamine reuptake
inhibitor activity and an inhibitory effect on nuclear factor-κB
activation. Singh and Kakkar (10)
have reported that oroxylin A enhanced lipolysis and decreased Akt
phosphorylation in mature adipocytes, suggesting that oroxylin A
may exert its anti-obesity effect by affecting the adipocyte life
cycle at critical points of differentiation and maturity. Akinyi
et al (11) have reported
that oroxylin A could significantly reduce coronary perfusion
pressure in a Langendorff preparation assay of isolated rat heart
tissue.
However, the effects of oroxylin A on virus-induced
macrophages have not been fully reported.
Double stranded (ds)RNA, which accumulates at
various stages of viral replication, stimulates macrophages to
produce inflammatory mediators (12). Polyinosinic-polycytidylic acid (PIC)
is a synthetic analog of dsRNA. Like other pathogenic endotoxins,
dsRNA activates macrophages to provoke the production of numerous
inflammatory mediators, including NO, cytokines, chemokines and
growth factors, resulting in acute or chronic inflammation
(12).
The aim of the present study was to investigate the
inhibitory effects of oroxylin A on PIC-induced inflammation using
RAW 264.7 mouse macrophages. The impact of oroxylin A was evaluated
on a range of variables, including the production of NO,
interleukin (IL)-1α, IL-1β, IL-6, IL-10, interferon gamma-inducible
protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1),
granulocyte colony-stimulating factor (G-CSF), granulocyte
macrophage-CSF (GM-CSF), leukemia inhibitory factor (LIF; IL-6
class cytokine), lipopolysaccharide-induced CXC chemokine (LIX),
macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-2, Regulated
on Activation, Normal T Expressed and Secreted (RANTES), tumor
necrosis factor (TNF)-α and vascular endothelial growth factor
(VEGF), as well as calcium release and mRNA expression of signal
transducer and activated transcription 1 (STAT1) in PIC-induced RAW
264.7 mouse macrophages.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin, streptomycin, phosphate-buffered
saline (PBS) and trypsin were purchased from Gibco (Thermo Fisher
Scientific, Inc., Grand Island, NY, USA). Oroxylin A, indomethacin,
Griess Reaction and MTT Assay kits were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell viability assay
RAW 264.7 mouse macrophages were obtained from the
Korea Cell Line Bank (Seoul, Korea). RAW 264.7 cells
(2×104/well) were seeded into 96-well plates and
cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in a 5% CO2 humidified
incubator. Cell viability was evaluated using an MTT Assay kit. The
macrophages were divided into four groups, as follows: i) The
normal group (Nor), which was treated with media only; ii) the
control group (Con), which was treated with PIC; iii) the oroxylin
A group, which was treated with PIC and various concentrations of
oroxylin A; and iv) the indomethacin group (IN), which was treated
with PIC and indomethacin. Indomethacin was used as a positive
control.
Quantification of NO production
NO concentration in culture medium was determined
using the Griess Reaction kit. Briefly, after incubation of the
cells with PIC and/or oroxylin A for 24 h, 100 µl supernatant from
each well was mixed with 100 µl Griess reagent in 96-well plates.
After an incubation of 15 min at room temperature, the optical
density was determined at 540 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Multiplex bead-based cytokine
assay
After 24 h treatment with PIC and/or oroxylin A, the
levels of various cytokines released from treated cells were
measured in cell culture supernatants using a Luminex assay based
on xMAP technology. This assay was performed using Milliplex kits
(EMD Millipore, Billerica, MA, USA) and a Bio-Plex 200 suspension
array system (Bio-Rad Laboratories, Inc.) as described previously
(13,14). Standard curves for each cytokine were
generated using the kit-supplied reference cytokine samples.
Intracellular calcium assay
After RAW 264.7 cells (2×104/well) were
seeded in wells of 96-well plates, PIC and/or oroxylin A were added
to the culture medium and incubation was conducted for 24 h at
37°C. Thereafter, the medium was removed and cells were incubated
with 100 µl Fluo-4 dye loading solution (Molecular Probes; Thermo
Fisher Scientific, Inc., Eugene, OR, USA) for 30 min at 37°C.
Following incubation, the fluorescence intensity of each well was
determined spectrofluorometrically (Dynex Technologies, West
Sussex, UK) with excitation and emission filters of 485 and 535 nm,
respectively.
STAT1 mRNA expression
At the end of 24 h incubation with PIC and/or
oroxylin A, RAW 264.7 mouse macrophages were lysed using lysis
buffer (Bio-Rad Laboratories, Inc.). To simultaneously quantify
multiple RNA targets directly from cell lysate, a QuantiGene Plex
2.0 Reagent System (Panomics, Inc., Redwood City, CA, USA) based on
branched DNA signal amplification technology with xMAP beads was
used according to manufacturer's instructions (15) and mRNA expression of STAT1 (GenBank
no. NM_009283) was determined. Data were normalized against the
control, GAPDH (GenBank no. NM_001001303).
Statistical analysis
The results shown are summarized from three
independent experiments and represent the mean ± standard
deviation. Differences were evaluated using Student's t-test with
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
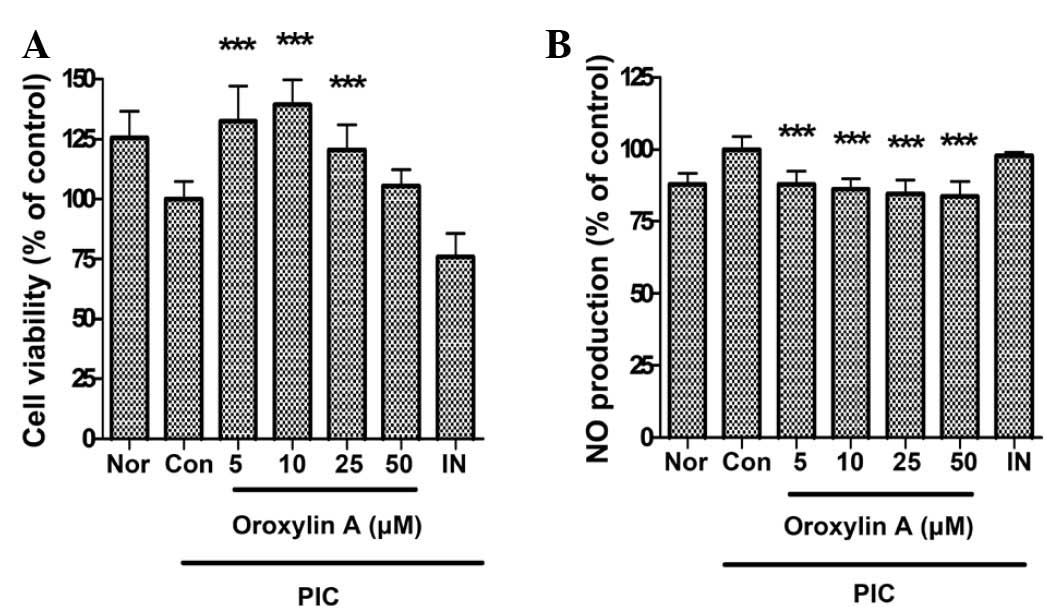
Effect of oroxylin A on cell
viability
In this study, oroxylin A at a concentration of 50
µM restored the cell viability in PIC-induced RAW 264.7 mouse
macrophages. Cell viabilities in PIC-induced RAW 264.7 mouse
macrophages incubated with oroxylin A at concentrations of 5, 10,
25 and 50 µM for 24 h were 132.46±14.56, 139.4±10.28, 120.58±10.32
and 105.38±6.76% of the control (50 µg/ml PIC alone) value,
respectively. Based on this result, oroxylin A concentrations of up
to 50 µM were selected for subsequent experiments, as the
cytotoxicity of oroxylin A was not obvious (Fig. 2A).
Effect of oroxylin A on NO
production
The results showed that oroxylin A significantly
inhibits overproduction of NO in PIC-induced RAW 264.7 mouse
macrophages (P<0.001) (Fig. 2B).
The production of NO in PIC-induced RAW 264.7 mouse macrophages
incubated with oroxylin A at concentrations of 5, 10, 25 and 50 µM
for 24 h were 87.84±4.47, 86.18±3.66, 84.41±4.89 and 83.68±5.28% of
the control value (50 µg/ml PIC alone), respectively.
Effect of oroxylin A on cytokine
production
Oroxylin A significantly reduces the overproduction
of IL-1α, G-CSF, IL-1β, GM-CSF, IL-6, VEGF, LIF, IL-10 (Fig. 3), TNF-α, MIP-1α, MIP-1β, MIP-2,
MCP-1, RANTES, IP-10 and LIX (Fig.
4) in PIC-induced RAW 264.7 mouse macrophages.
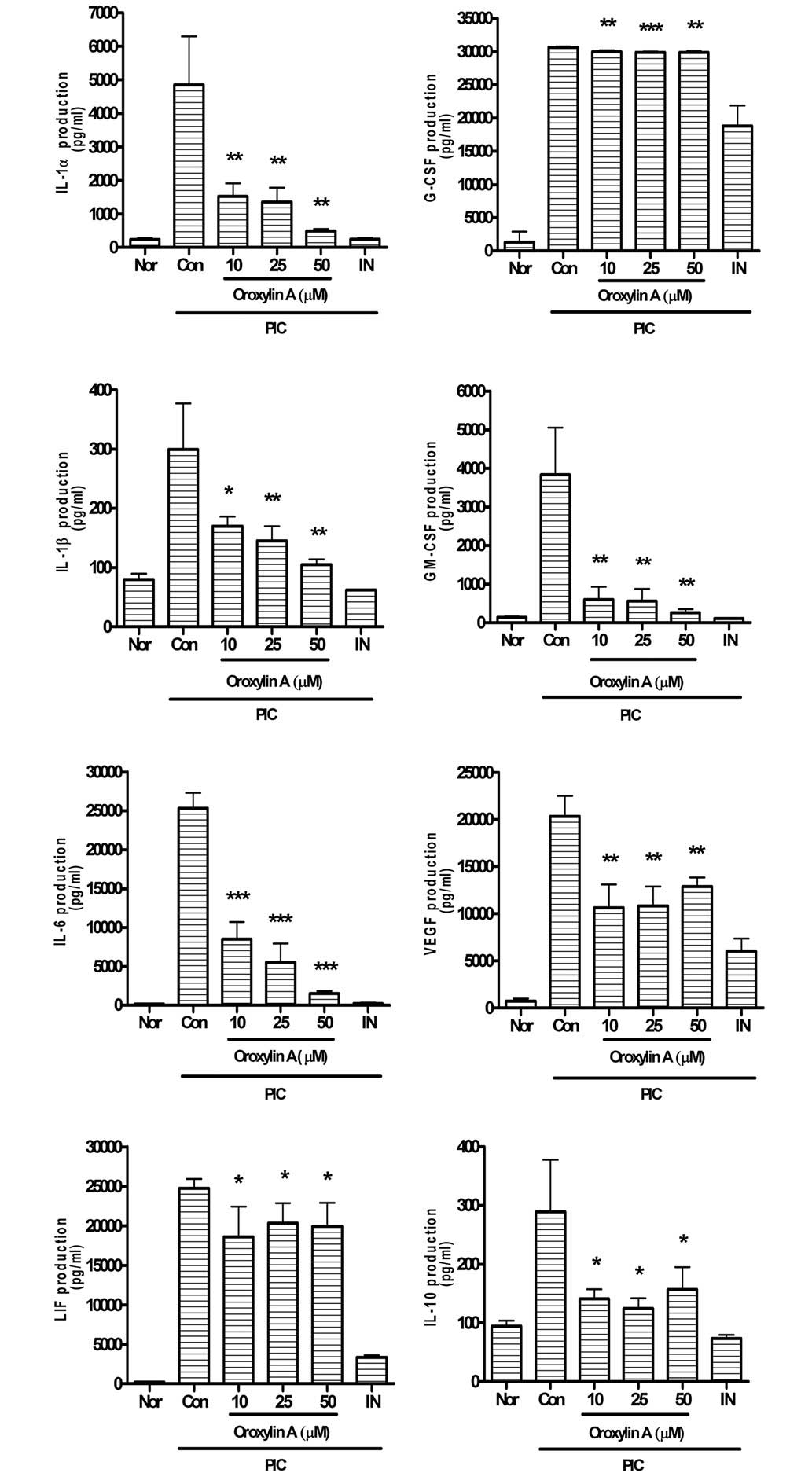 | Figure 3.Effects of oroxylin A on production of
cytokines such as IL-1α, IL-1β, IL-6, LIF (IL-6 class cytokine),
IL-10, G-CSF, GM-CSF and VEGF in PIC-induced RAW 264.7 mouse
macrophages. Fluorescence intensity of each cytokine in the culture
medium was measured using a Multiplex bead-based cytokine assay
after 24 h treatment. Values are the mean ± standard deviation of
three independent experiments. *P<0.05, **P<0.01;
***P<0.001 vs. Con. IL, interleukin; Nor, normal group; Con,
control group; IN, indomethacin (0.5 µM); PIC,
polyinosinic-polycytidylic acid; G-CSF, granulocyte-colony
stimulating factor; GM-CSF, granulocyte macrophage-colony
stimulating factor; VEGF, vascular endothelial growth factor; LIF,
leukemia inhibitory factor. |
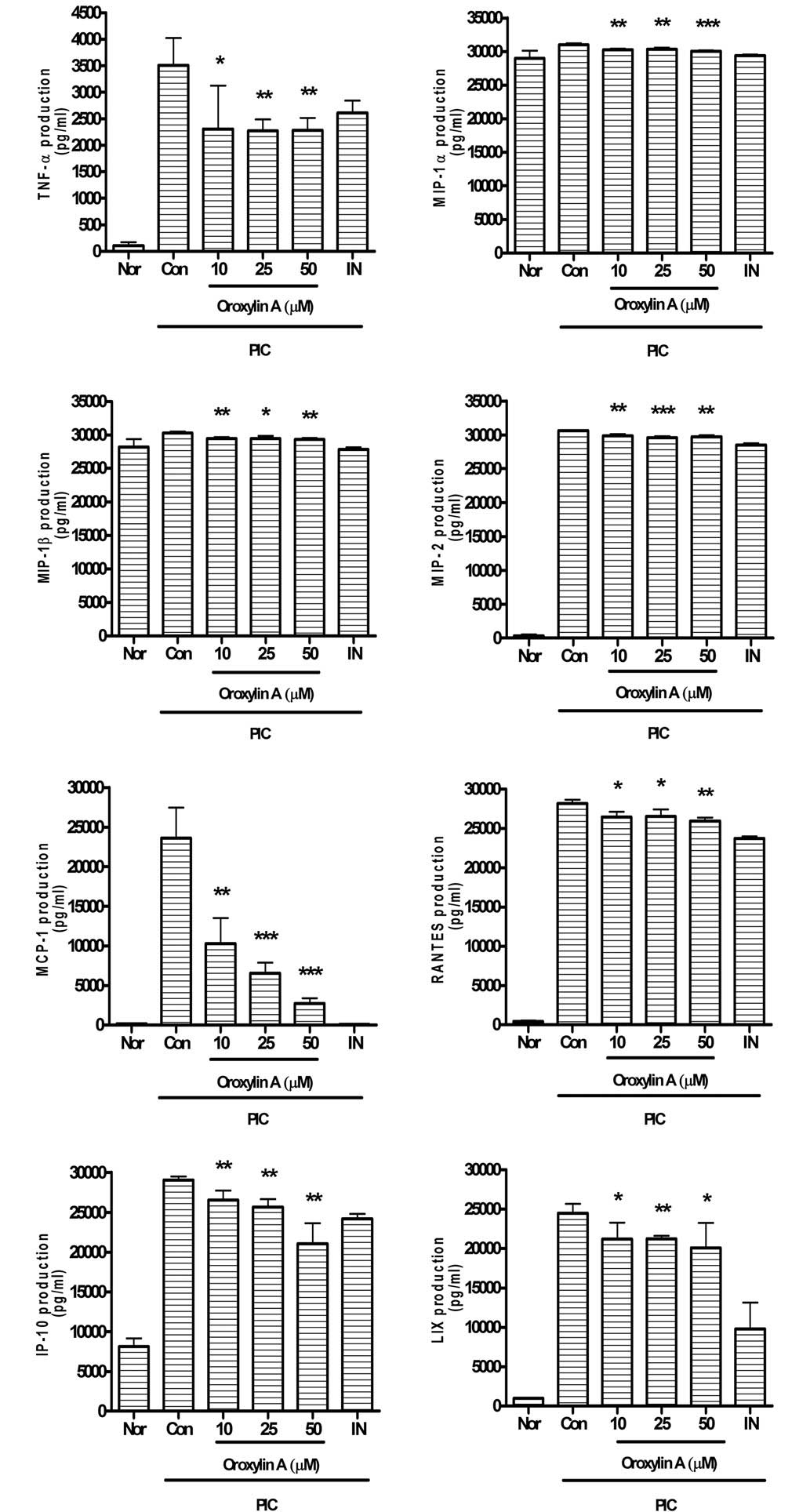 | Figure 4.Effects of oroxylin A on production of
cytokines such as TNF-α, IP-10, MCP-1, LIX, MIP-1α, MIP-1β, MIP-2
and RANTES in PIC-induced RAW 264.7 mouse macrophages. Fluorescence
intensity of each cytokine in the culture medium was measured by a
Multiplex bead-based cytokine assay after 24 h treatment. Values
are the mean ± standard deviation of three independent
experiments.*P<0.05; **P<0.01; ***P<0.001 vs. Con. Nor,
normal group; Con, control group; IN, indomethacin (0.5 µM); PIC,
polyinosinic-polycytidylic acid; TNF-α, tumor necrosis factor-α;
IP-10, interferon gamma-induced protein 10; MCP-1, monoctye
chemoattractant protein 1; LIX, lipopolysaccharide-induced CXC
chemokine; MIP, macrophage inflammatory protein; RANTES, Regulated
on Activation, Normal T Expressed and Secreted. |
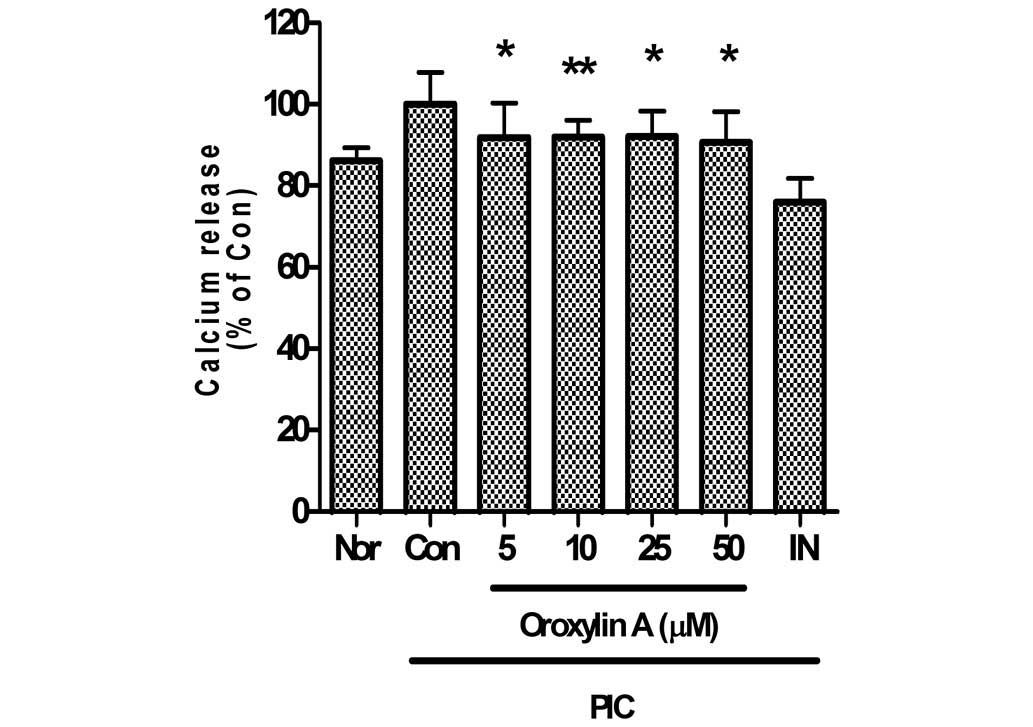
Effect of oroxylin A on intracellular
calcium release
In the present study, oroxylin A significantly
inhibited calcium release in PIC-induced RAW 264.7 mouse
macrophages (Fig. 5). Calcium
release in PIC-induced RAW 264.7 mouse macrophages incubated with
oroxylin A at concentrations of 5, 10, 25 and 50 µM for 24 h were
91.87±8.47, 91.99±4.1, 92.07±6.25 and 90.61±7.52% of the control
(50 µg/ml PIC alone), respectively.
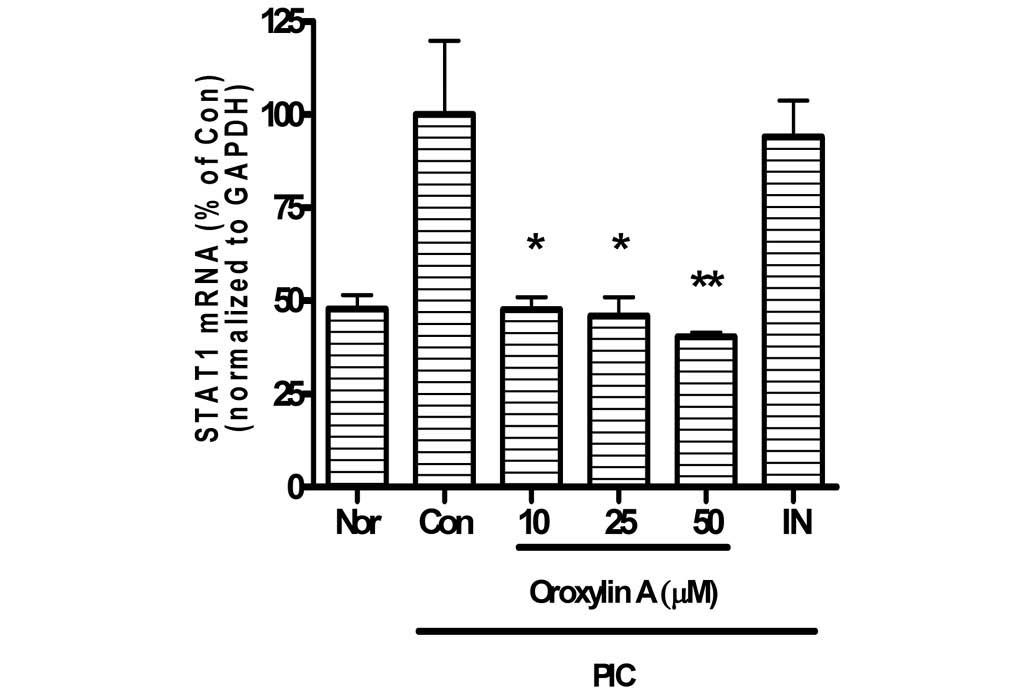
Effects of oroxylin A on expression of
STAT1 mRNA
In the present study, oroxylin A significantly
reduced STAT1 mRNA expression of PIC-activated mouse macrophages in
a dose-dependent manner (Fig. 6).
Expression levels of STAT1 mRNA in PIC-induced RAW 264.7 mouse
macrophages incubated with oroxylin A at concentrations of 10, 25
and 50 µM for 24 h were 47.7±3.33, 46.02±4.98 and 40.34±1.12% of
the control value (50 µg/ml PIC alone), respectively.
Discussion
According to a previous report by Tran et al,
oroxylin A has a dopamine reuptake inhibitor activity, and the
inhibitory effect of nuclear factor-κB activation (9). Singh and Kakkar (10) reported that oroxylin A enhanced
lipolysis and decreased Akt phosphorylation in mature adipocytes,
suggesting that oroxylin A may exert its anti-obesity effect by
influencing adipocyte life cycle at critical points of
differentiation and maturity. Akinyi et al (11) have reported that oroxylin A may
significantly lower the coronary perfusion pressure in a
Langendorff preparation assay of isolated rat heart tissue.
Previously, Qiao et al (16)
reported that oroxylin A induces apoptosis by regulating uncoupling
protein 2 in human colon cancer cells. Ye et al (17) reported that regulating inflammation
could be an important measure for the effective treatment of
cancer; oroxylin A inhibits lipopolysaccharide-induced mRNA and
protein expression of cyclooxygenase-2 and nitric oxide synthase in
RAW 264.7 cells. Zhou et al (18) reported that oroxylin A attenuated
ovalbumin-induced lung histopathologic changes and airway
hyperresponsiveness, and reduced the number of inflammatory cells.
Hui et al (19) reported that
production of IL-6 constitutes the primary cause of mortality in
patients with retinoic acid syndrome; oroxylin A possesses
abilities of inhibiting the all-trans retinoic acid-induced IL-6
production in leukemia cell lines, which may provide a therapeutic
strategy for retinoic acid syndrome. Wang et al (20) reported that oroxylin A improves the
sensitivity of multidrug-resistant leukemic cells by increasing
apoptosis in leukemic cells and decreasing the expression of
chemokine receptor 4, and may serve as a potential agent for
chronic myeloid leukemia. However, the effects of oroxylin A on
virus-induced macrophages have not been fully elucidated.
Innate immunity and inflammation are crucial for
protecting the host against various pathogens, including bacteria,
viruses and fungi (2). Mosser and
Edwards (21) have reported that
macrophages, which are a type of white blood cell that engulf and
digest cellular debris, foreign substances, microbes and cancer
cells in a process called phagocytosis, play a critical role in
innate immunity, and also help initiate adaptive immunity by
recruiting other immune cells such as lymphocytes. Canna et
al (22) have reported that
inflammasomes are innate immune sensors that respond to pathogen-
and damage-associated signals with caspase-1 activation, IL-1β and
IL-18 secretion, and macrophage pyroptosis, a proinflammatory and
lytic mode of cell death.
In the case of viral infection, dsRNA from
pathogenic viruses is a strong initiator of inflammation and
stimulates macrophages to produce inflammatory mediators including
NO, cytokines, chemokines and growth factors in immune responses
and organ injuries (12).
Unregulated inflammation is an underlying component
of various diseases, such as sepsis, cardiovascular disease,
diabetes and other chronic inflammatory diseases (23). Furthermore, Ulloa and Tracey
(24) have reported that viral and
bacterial infections contribute to the pathogenesis of severe
sepsis, which is characterized by an overwhelming production of NO
and proinflammatory cytokines, such as IL-1, IL-6 and TNF-α.
Riedemann et al (25) have
reported that the excessive production of IL-1 and IL-6 may be more
dangerous than the original stimulus, causing capillary leakage,
tissue injury and lethal organ failure, although these cytokines
trigger a beneficial inflammatory response that promotes local
coagulation to confine tissue damage.
Srivastava et al (26) have reported that various chemokines,
such as MCP-1, IP-10, M-CSF, G-CSF and GM-CSF, are increased in
bronchoalveolar fluid during lung inflammation. Wareing et
al (27) have reported that the
expression level of MIP-1α, MIP-1β, MIP-2 and RANTES is increased
in lung tissue of influenza infection.
By contrast, Sakaguchi and Wing (28) reported that superfluous or misguided
human immune responses may lead to harmful outcomes, as associated
with autoimmune disease, chronic inflammation and allergies.
Ballara et al (29) have
reported that the proangiogenic cytokine VEGF in the persistence of
inflammatory arthritis supports the hypothesis that expansion of
the synovial vasculature is important for the development of joint
destruction in rheumatoid arthritis. Recently, Reddy et al
(30) reported that LIX expression
was induced in arthritis and periodontal disease. Schon and
Boehncke (31) have reported that
the central role of cytokines and their functional interaction with
adhesion molecules in the recruitment of tissue-specific
lymphocytes has been clearly shown, primarily involving LIF (IL-6
class cytokine) and IL-10 in psoriasis (a chronic immune-mediated
skin disease). Previously, Dace et al (32) reported that IL-10, although
traditionally considered to be an anti-inflammatory cytokine that
modulates the function of adaptive immune-related cells, has also
been implicated in promoting abnormal angiogenesis in the eye and
in the pathobiology of autoimmune diseases such as lupus and
encephalomyelitis.
In the present study, the data indicate that
oroxylin A has inhibitory effects against the viral inflammation
associated with macrophage inflammasomes.
Cuschieri and Maier (33) have reported that pathogenic oxidative
stress with infection results in macrophage reprogramming with a
transient increase in intracellular calcium via the lipid membrane
dissociation of the calcium-bound protein annexin VI; this
increased cytosolic calcium, in turn, results in the activation of
calcium-dependent kinases, leading to enhanced proinflammatory
activation. Furthermore, Timmins et al (34) have reported that the endoplasmic
reticulum (ER) calcium stores are reduced in oxidative stress and
intracellular calcium concentration is increased, resulting in ER
stress-mediated STAT1 activation. In the present study, oroxylin A
inhibited the calcium release and STAT1 mRNA expression in
PIC-induced RAW 264.7 mouse macrophages. Thus, it is possible that
oroxylin A downregulates the excessive production of inflammatory
mediators in pathogenic toxicant-induced macrophages through the
calcium-STAT pathway.
However, whether intracellular calcium concentration
is increased via the calcium-bound membrane protein mobilization or
release of calcium from ER could not be confirmed in this
study.
Although the precise mechanism underlying the
regulation of the anti-inflammatory activity of oroxylin A are not
yet known, the current study demonstrates that oroxylin A has
anti-inflammatory effects associated with its inhibition of NO,
IL-1α, IL-1β, IL-6, IL-10, IP-10, G-CSF, GM-CSF, LIF (IL-6 class
cytokine), LIX, MCP-1, MIP-1α, MIP-1β, MIP-2, RANTES, TNF-α and
VEGF in PIC-induced macrophages via calcium-STAT pathway. Actual
effect of oroxylin A on acute and chronic inflammatory diseases
requires further study.
Acknowledgements
The authors thank Dr Young-Jin Kim and Ms. Hyun Joo
Kim (College of Korean Medicine, Gachon University) for their
technical assistance.
References
|
1
|
Gao HM and Hong JS: Why neurodegenerative
diseases are progressive: Uncontrolled inflammation drives disease
progression. Trends Immunol. 29:357–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JY and Park W: Anti-inflammatory
effect of wogonin on RAW 264.7 mouse macrophages induced with
polyinosinic-polycytidylic acid. Molecules. 20:6888–6900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zong Y, Sun L, Liu B, Deng YS, Zhan D,
Chen YL, He Y, Liu J, Zhang ZJ, Sun J and Lu D: Resveratrol
inhibits LPS-induced MAPKs activation via activation of the
phosphatidylinositol 3-kinase pathway in murine RAW 264.7
macrophage cells. PLoS One. 7:e441072012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medina EA, Morris IR and Berton MT:
Phosphatidylinositol 3-kinase activation attenuates the
TLR2-mediated macrophage proinflammatory cytokine response to
Francisella tularensis live vaccine strain. J Immunol.
185:7562–7572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ulevitch RJ and Tobias PS:
Receptor-dependent mechanisms of cell stimulation by bacterial
endotoxin. Annu Rev Immunol. 13:437–457. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carrithers MD: Innate immune viral
recognition: Relevance to CNS infections. Handb Clin Neurol.
123:215–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DH, Kim S, Jeon SJ, Son KH, Lee S,
Yoon BH, Cheong JH, Ko KH and Ryu JH: The effects of acute and
repeated oroxylin A treatments on abeta (25-35)-induced memory
impairment in mice. Neuropharmacology. 55:639–647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HN, Nie FF, Liu W, Dai QS, Lu N, Qi Q,
Li ZY, You QD and Guo QL: Apoptosis induction of oroxylin A in
human cervical cancer HeLa cell line in vitro and in vivo.
Toxicology. 257:80–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tran TV, Malainer C, Schwaiger S, Hung T,
Atanasov AG, Heiss EH, Dirsch VM and Stuppner H: Screening of
vietnamese medicinal plants for NF-kB signaling inhibitors:
Assessing the activity of flavonoids from the stem bark of
Oroxylum indicum. J Ethnopharmacol. 159:36–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh J and Kakkar P: Oroxylin A, a
constituent of Oroxylum indicum inhibits adipogenesis and
induces apoptosis in 3T3-L1 cells. Phytomedicine. 21:1733–1741.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akinyi M, Gao XM, Li YH, Wang BY, Liu EW,
Chai LJ, JawoBah A and Fan GW: Vascular relaxation induced by
Eucommiae ulmoides oliv. and its compounds oroxylin A and
wogonin: Implications on their cytoprotection action. Int J Clin
Exp Med. 7:3164–3180. 2014.PubMed/NCBI
|
|
12
|
Lee JY and Park W: Anti-inflammatory
effect of myristicin on RAW 264.7 macrophages stimulated with
polyinosinic-polycytidylic acid. Molecules. 16:7132–7142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon SB, Lee YJ, Park SK, Kim HC, Bae H,
Kim HM, Ko SG, Choi HY, Oh MS and Park W: Anti-inflammatory effects
of Scutellaria baicalensis water extract on LPS-activated
RAW 264.7 macrophages. J Ethnopharmacol. 125:286–290. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuk SS, Lim EM, Lee JY, Lee YJ, Kim YS,
Lee TH, Park SK, Bae H, Kim HM, Ko SG, et al: Antiinflammatory
effects of Epimedium brevicornum water extract on
lipopolysaccharide-activated RAW264.7 macrophages. Phytother Res.
24:1781–1787. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flagella M, Bui S, Zheng Z, Nguyen CT,
Zhang A, Pastor L, Ma Y, Yang W, Crawford KL, McMaster GK, et al: A
multiplex branched DNA assay for parallel quantitative gene
expression profiling. Anal Biochem. 352:50–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao C, Wei L, Dai Q, Zhou Y, Yin Q, Li Z,
Xiao Y, Guo Q and Lu N: UCP2-related mitochondrial pathway
participates in oroxylin A-induced apoptosis in human colon cancer
cells. J Cell Physiol. 230:1054–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye M, Wang Q, Zhang W, Li Z, Wang Y and Hu
R: Oroxylin A exerts anti-inflammatory activity on
lipopolysaccharide-induced mouse macrophage via Nrf2/ARE
activation. Biochem Cell Biol. 92:337–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou DG, Diao BZ, Zhou W and Feng JL:
Oroxylin A Inhibits Allergic Airway Inflammation in Ovalbumin
(OVA)-Induced Asthma Murine Model. Inflammation. 39:867–872. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui H, Yang H, Dai Q, Wang Q, Yao J, Zhao
K, Guo Q and Lu N: Oroxylin A inhibits ATRA-induced IL-6 expression
involved in retinoic acid syndrome by down-regulating CHOP. Gene.
551:230–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Miao H, Li W, Yao J, Sun Y, Li Z,
Zhao L and Guo Q: CXCL12/CXCR4 axis confers adriamycin resistance
to human chronic myelogenous leukemia and oroxylin A improves the
sensitivity of K562/ADM cells. Biochem Pharmacol. 90:212–225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canna SW, de Jesus AA, Gouni S, Brooks SR,
Marrero B, Liu Y, DiMattia MA, Zaal KJ, Sanchez GA, Kim H, et al:
An activating NLRC4 inflammasome mutation causes autoinflammation
with recurrent macrophage activation syndrome. Nat Genet.
46:1140–1146. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spite M, Norling LV, Summers L, Yang R,
Cooper D, Petasis NA, Flower RJ, Perretti M and Serhan CN: Resolvin
D2 is a potent regulator of leukocytes and controls microbial
sepsis. Nature. 461:1287–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ulloa L and Tracey KJ: The ‘cytokine
profile’: A code for sepsis. Trends Mol Med. 11:56–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riedemann NC, Neff TA, Guo RF, Bernacki
KD, Laudes IJ, Sarma JV, Lambris JD and Ward PA: Protective effects
of IL-6 blockade in sepsis are linked to reduced C5a receptor
expression. J Immunol. 170:503–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Srivastava M, Jung S, Wilhelm J, Fink L,
Bühling F, Welte T, Bohle RM, Seeger W, Lohmeyer J and Maus UA: The
inflammatory versus constitutive trafficking of mononuclear
phagocytes into the alveolar space of mice is associated with
drastic changes in their gene expression profiles. J Immunol.
175:1884–1893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wareing MD, Lyon AB, Lu B, Gerard C and
Sarawar SR: Chemokine expression during the development and
resolution of a pulmonary leukocyte response to influenza A virus
infection in mice. J Leukoc Biol. 76:886–895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakaguchi S and Wing K: Immunology.
damping by depletion. Science. 332:542–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ballara S, Taylor PC, Reusch P, Marmé D,
Feldmann M, Maini RN and Paleolog EM: Raised serum vascular
endothelial growth factor levels are associated with destructive
change in inflammatory arthritis. Arthritis Rheum. 44:2055–2064.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruddy MJ, Shen F, Smith JB, Sharma A and
Gaffen SL: Interleukin-17 regulates expression of the CXC chemokine
LIX/CXCL5 in osteoblasts: Implications for inflammation and
neutrophil recruitment. J Leukoc Biol. 76:135–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schön MP and Boehncke WH: Psoriasis. N
Engl J Med. 352:1899–1912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dace DS, Khan AA, Stark JL, Kelly J, Cross
AH and Apte RS: Interleukin-10 overexpression promotes
Fas-ligand-dependent chronic macrophage-mediated demyelinating
polyneuropathy. PLoS One. 4:e71212009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cuschieri J and Maier RV: Oxidative
stress, lipid rafts, and macrophage reprogramming. Antioxid Redox
Signal. 9:1485–1497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Timmins JM, Ozcan L, Seimon TA, Li G,
Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson
ME and Tabas I: Calcium/calmodulin-dependent protein kinase II
links ER stress with Fas and mitochondrial apoptosis pathways. J
Clin Invest. 119:2925–2941. 2009. View
Article : Google Scholar : PubMed/NCBI
|