Introduction
Osteosarcoma (OSA) is a common type of primary tumor
of the bone (1–3). OSA has similar worldwide incidence
rates in children and adolescents; the incidence rate is 5.5–14 per
million persons at age 15–19 years (4). The 5-year survival rate in patients
presenting with localized OSA is ~80%, whereas the prognosis is
poor in patients with metastatic disease (5). Primary tumors commonly originate from
the proximal tibia and humerus, as well as from the metaphyseal
(actively growing) regions of the distal femur; however, OSA may
develop in any bone of the body, with lungs and bone being the most
likely sites of metastasis (5).
Tetraspanins are a set of transmembrane receptor
glycoproteins with a molecular weight between 25 and 50 kDa. They
are involved in numerous important physiological processes
(6–14). Tetraspanins have been found to be
associated with the expression of various tumor prognosis factors,
such as CD9 in lung cancer (15–17),
CD82 in prostatic cancer (18–20), and
CD63 and CD9 in melanoma (21,22).
Decreased tetraspanin expression contributes to the promotion of
tumor invasion through the lymphatic system. It had been reported
that downstream regulation of tetraspanin is critical for tumor
progression in breast cancer (23).
Coding transmembrane protein 35 (TMEM35) is a
transmembrane protein that is conservatively expressed in humans,
canines, cattle, mice, rats, chicken, zebra fish and
Drosophila species (24);
however, the function of TMEM35 remains poorly understood. A
previous study in rats revealed that TMEM35 may be a candidate
regulatory factor involved in adrenal cortex-zona glomerulosa
growth following sodium consumption (24).
In the present study, the TMEM35 expression in OSA
tissues and cell lines were investigated, as well as the effect of
TMEM35 knockdown on cell cycle progression.
Materials and methods
Cell culture
Two OSA cell lines, SaOS2 and U2OS (American Type
Culture Collection, Manassas, VA, USA) were investigated in the
present study. Cells were cultured in Dulbecco's modified Eagle
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Paisley,
Scotland) containing 10% fetal calf serum, 10,000 U/ml penicillin
and 10,000 mg/ml streptomycin, at 37°C in a humidified atmosphere
containing 5% CO2 in air.
Human tissues
Tissue samples were collected from 37 patients
diagnosed with OSA at the Department of Orthopedic Surgery,
Shanghai Sixth People's Hospital (Shanghai, China). All specimens
were acquired from patients who underwent surgical resection and
provided informed consent. Among the 37 patients, 25 were male and
12 were female. The ages of the patients ranged from 14–45 years.
Specimens of tumor and adjacent normal tissue were collected from
each patient, and the diagnosis of OSA was validated by
pathological examination. Specimens were frozen at −80°C for
DNA/RNA extraction. The Ethics Committee of Shanghai Sixth People's
Hospital provided ethical approval.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells or tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Cergy
Pontoise, France). Next, 3 mg total RNA was denatured for 10 min at
70°C and then reversed transcribed into cDNA at 37°C for 90 min
using 300 U Moloney murine leukemia virus reverse transcriptase, 15
mg oligo dT primers (both Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 1 mM deoxynucleoside triphosphate
(Bioline, London, UK) in a total volume of 30 ml. qPCR was then
performed using a SYBR Green PCR Master Mix kit (ABgene; Thermo
Fisher Scientific, Inc., Courtaboeuf Cedex, France) supplemented
with 0.5 mM primers. The PCR mixture contained 7.5 µl SYBR Green,
4.5 µl water, 1 µl forward and reverse primers, respectively, and 2
µl DNA template. The primers were: Human TMEM35 forward,
5′-TGGGGACTATCAAGCTGACC-3′, and reverse,
5′-CAATGCTTTTTCGGAGGAGA-3′; β-actin forward,
5′-AATCGTGCGTGACATTAAGGAG-3′, and reverse,
5′-ACTGTGTTGGCGTACAGGTCTT-3′. The thermal cycling conditions used
were as follows: 95°C for 15 min, then 40 cycles at 95°C for 20
sec, 58°C for 15 sec, and 72°C for 15 sec. Signals with a threshold
cycle (Cq) value of >39 were considered to indicate no
transcription of the target gene. The relative expression of mRNA
was calculated using the 2−ΔΔCq method (25).
Cell transfection
siRNAs specifically targeting the TMEM35 gene
(TMEM35-si-1 and TMEM35-si-2), and negative control (si-LUC) were
transfected into SaOS2 and U2OS cells. The siRNA sequences were
TMEM35-si-1: CCAGAACCGUAACUAUUGU and TMEM35-si-2:
CAACCCUCCUUAUAUGAGA. The siRNAs and Lipofectamine 2000 (both
Invitrogen) were combined in DMEM at room temperature. The mixture
was added to the cells dropwise and incubated for 4–6 h. The medium
was then changed to fresh medium containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% antibiotic, and cells were cultured for 3 days.
Wound-healing and cell migration
assays
A wound-healing assay was performed using SaOS2
cells according to the manufacturer's protocol (ibidi GmbH,
Martinsried, Germany). Cell migration assays were also performed
using SaOS2 and U2OS cell lines, as described previously (26). Briefly, in the wound-healing assay,
cells were seeded into 3-mm cell culture dishes and when cell
confluence was ~90%, a scratch was made at the bottom of the dish.
The width of the wound was measured to assess wound healing. The
cell migration assay was a Boyden chamber assay in which cells were
seeded in an insert with a porous membrane (50,000 cells/insert).
DMEM without FBS was added to the upper chamber and DMEM with 10%
FBS was added to the bottom of the plate. The insert was put into
the well of a 12 well plate containing medium for 24 h. The number
of cells that transferred to the bottom of the membrane from the
upper side was determined. To do this, non-migrated cells were
removed from the upper surface using cotton-tipped swabs. Cells
that migrated to the underside were fixed with 3.7%
paraformaldehyde in phosphate-buffered saline at 4°C and stained
with crystal violet. Membranes were then cut from the insert and
observed under a microscope. Five fields were randomly selected and
a count for each assay was performed in duplicate.
Colony formation assays
A total of ~20,000 SaOS2 or U2OS cells were seeded
into dishes and cultured at 37°C for 2 weeks in DMEM containing 10%
fetal bovine serum and 1% antibiotic. Soft agar colony formation
assays were conducted, in which the transfected cells were seeded
in soft agar and incubated for approximately 3–4 weeks. At the end
of the incubation, the cells were fixed with 4% paraformaldehyde
and stained with 0.1% crystal violet (Hangzhou DayangChem Co.,
Ltd.; Hangzhou, China) or 1% Coomassie Brilliant Blue. The colonies
were then counted under a dissecting microscope (AmScope
SE306-AZ-E; AmScope, Irvine, CA, USA).
Cell cycle analyses
Flow cytometry with propidium iodide staining was
used to analyze cell cycle progression. The cells were fixed in 70%
ethanol and rehydrated in phosphate-buffered saline and adjusted to
a concentration of 1–5×106 cells/ml. Cells were treated
with 10 mg/ml RNase A for 30 min and 10 µg/ml PI (both
Sigma-Aldrich, St. Louis, MO, USA) for 15 min at room temperature.
The cells were kept in the dark on ice or at 4°C in a fridge until
analysis. Results were analyzed using a BD Accuri C6 Plus flow
cytometer with Flowjo software (BD Biosciences, San Jose, CA,
USA).
Bioinformatics analysis
In order to analyze the structural characteristics
and secondary structure of TMEM35, SPLIT software version 4.0
(split.pmfst.hr/split/4/) was used (27). For investigation of the poorly
understood TMEM35 function and the associated molecular mechanism,
the STRING database (http://string-db.org) (27) was used to predict the proteins
interacting with TMEM35. The database contains known and predicted
protein interactions, including directly (physical) and indirectly
(functional) relevant interactions. The data are derived from four
sources: Genomic context, high-throughput experiments, coexpression
and previous knowledge.
Statistical analysis
Two-factor analysis of variance was performed to
compare the data. Student's t-test was used to determine the
statistical significance. The results are presented as mean ±
standard deviation. SPSS statistical analysis software, version
21.0 (IBM SPSS, Armonk, NY, USA) was used to analyze the results.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Human TMEM35 gene
SPLIT software was used to analyze the structural
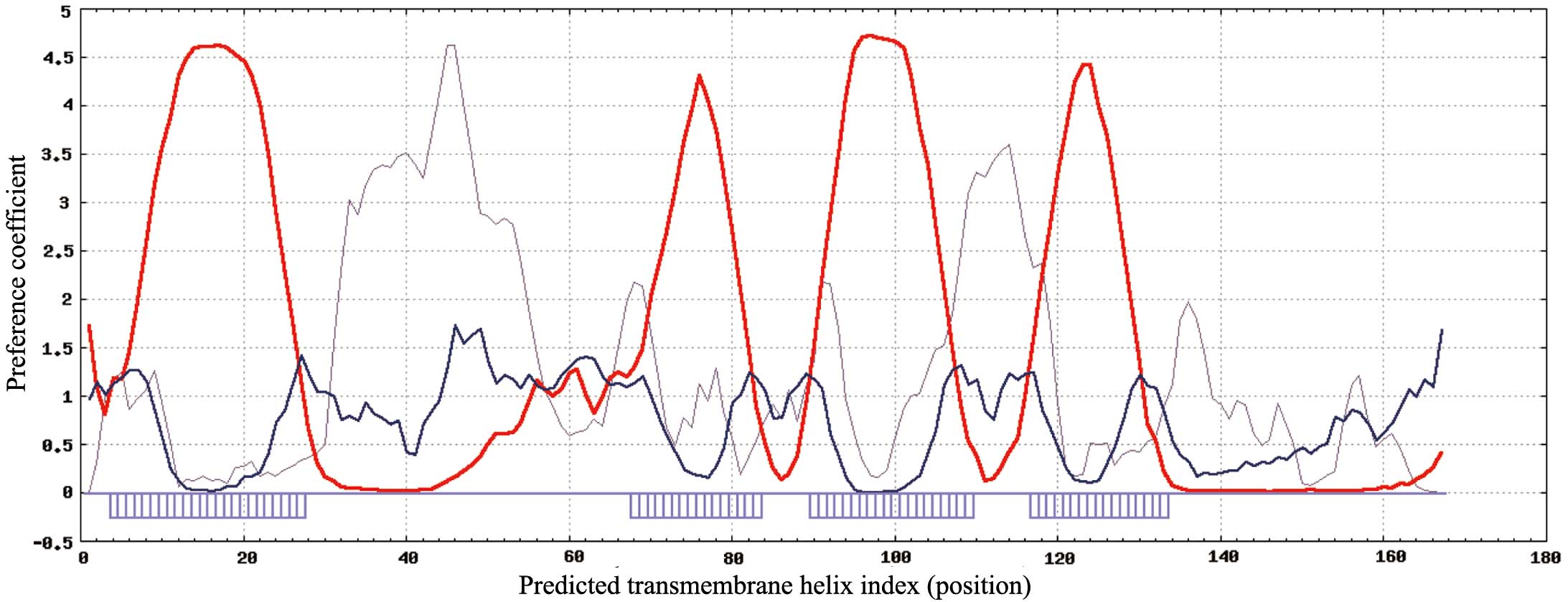
characteristics and secondary structure of TMEM35 (27). On the basis of the results presented
in Fig. 1, TMEM35 is a low molecular
weight, cell membrane surface protein with four highly hydrophobic
domains. TMEM35 has a structure typical of a tetraspanin, the
functions of which have been reviewed previously (28), and include extensive involvement in
cell proliferation, adhesion and migration. Therefore, we
hypothesize that cell signal transduction pathways are directly or
indirectly influenced by TMEM35, due to an effect on tumor cell
adhesion, differentiation, migration and invasion.
Upregulation of TEME35 in OSA
cells
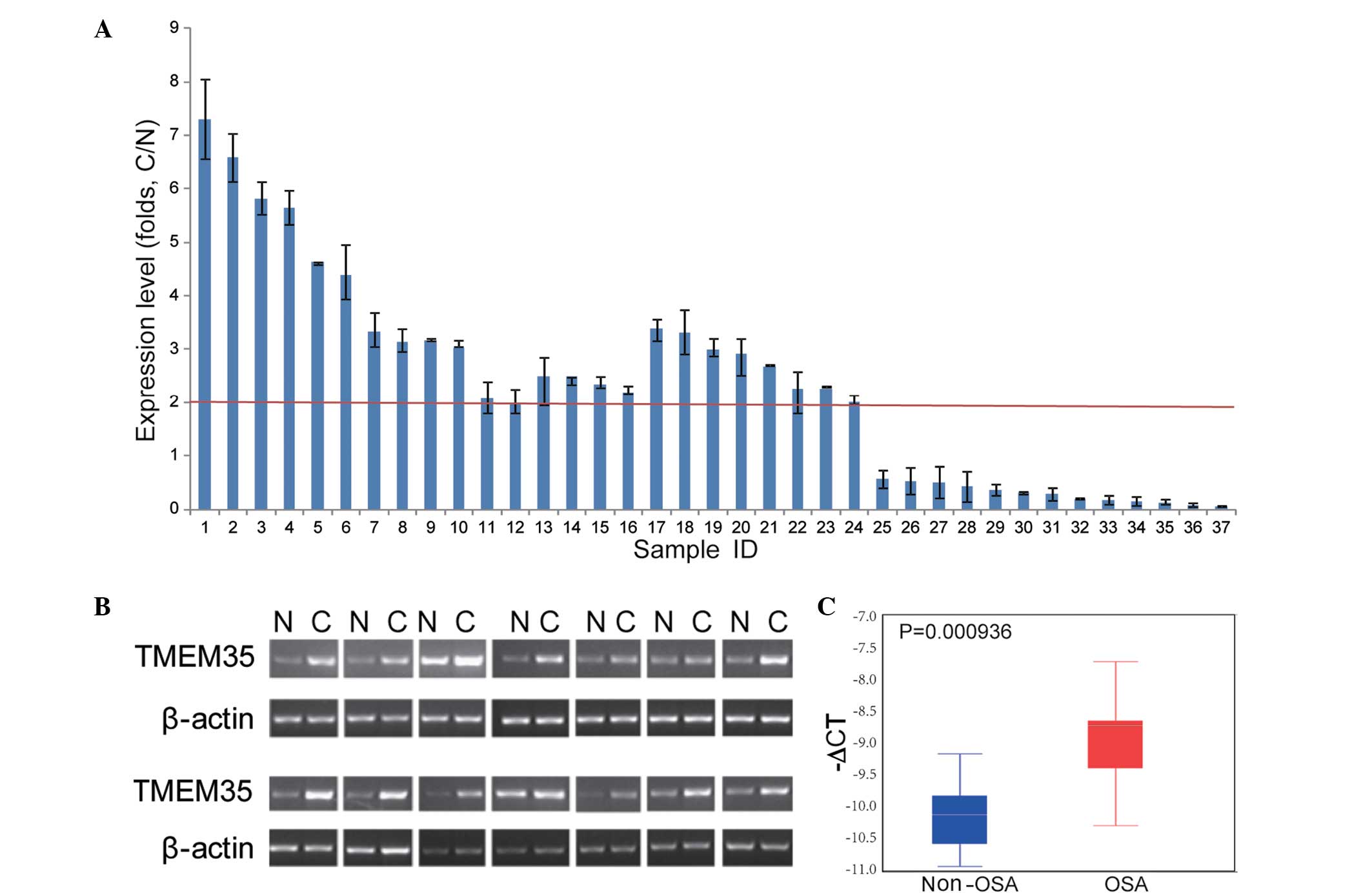
In order to investigate the TMEM35 expression in
OSA, RT-qPCR assays were performed in human OSA samples and
non-tumor tissues. As shown in Fig.
2, TMEM35 was found to be upregulated in 24/37 (64.86%) OSA
samples, among which 22 samples presented a 2-fold upregulation of
TMEM35 (two tailed t-test, P<0.05). These results indicated that
TMEM35 overexpression may play an important role in OSA.
Knockdown of TMEM35 expression
inhibits OSA cell growth
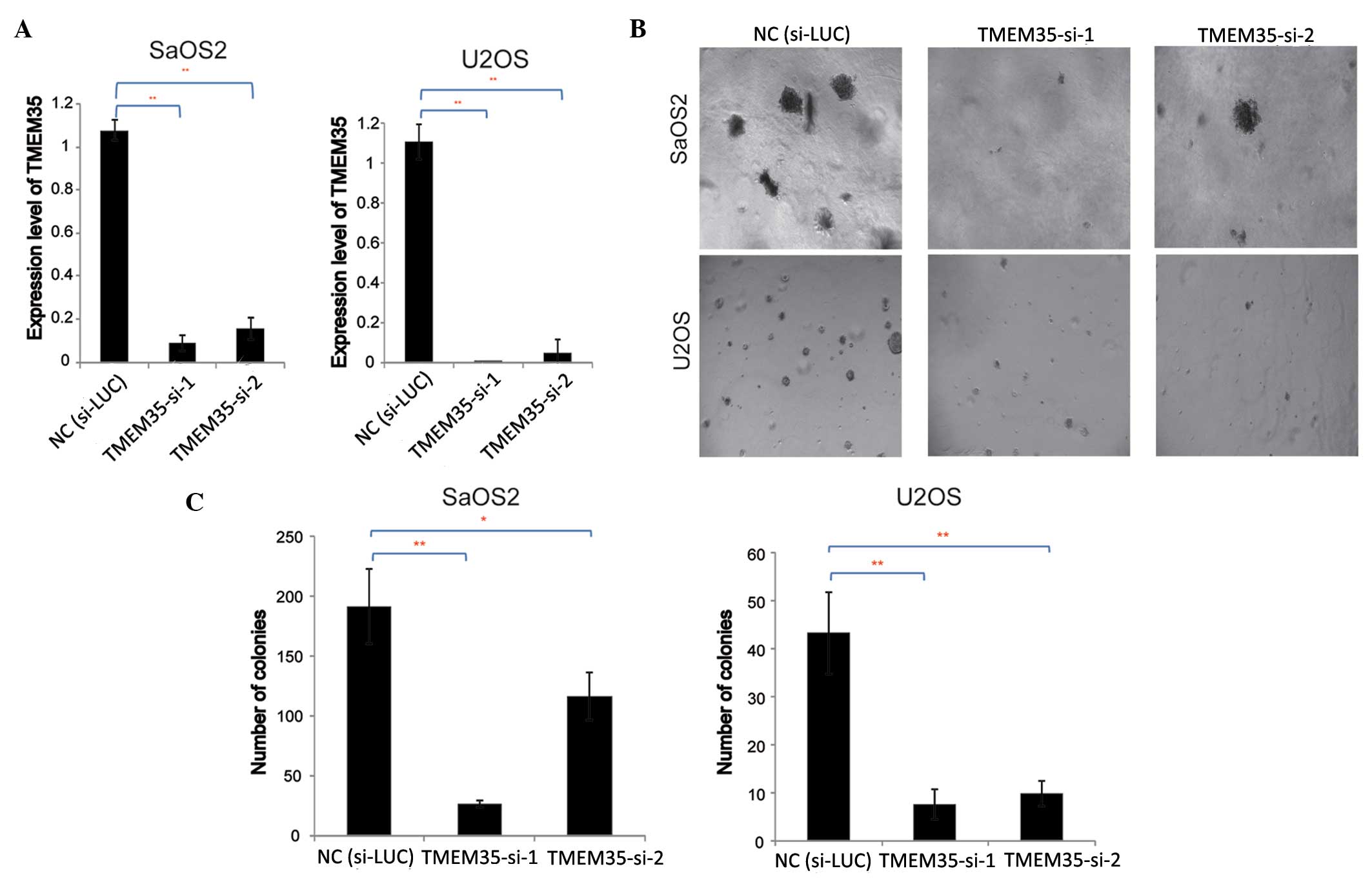
To further investigate the effects of TMEM35
expression on OSA cell growth, TMEM35 knockdown was performed by
transfection with siRNA, and the effect of TMEM35 knockdown on soft
agar colony formation was evaluated. siRNAs specifically targeting
the TMEM35 gene (TMEM35-si-1 and TMEM35-si-2) were transfected into
SaOS2 and U2OS cells, and RT-qPCR was performed to evaluate the
efficiency of gene knockdown. The results indicated that TMEM35
expression decreased significantly following transfection,
indicating that the siRNAs silenced TMEM35 expression in the SaOS2
and U2OS cells (Fig. 3A).
Subsequently, the effect of TMEM35 knockdown on OSA cell colony
formation was examined.
A soft agar colony formation assay of SaOS2 and U2OS
cells was performed to evaluate the contact inhibition effect on
these tumor cells, and the results are shown in Fig. 3B. Results indicated that the number
and size of the cell colonies decreased significantly compared with
those in the negative control (si-LUC). Subsequently, the number of
SaOS2 and U2OS cell colonies were investigated with crystal violet
staining (Fig. 3C). Results
demonstrated that TMEM35 knockdown significantly inhibited SaOS2
and U2OS cell growth in the soft agar (two tailed t-test,
P<0.05), implying that TMEM35 may play an role in the
adherence-independent growth of OSA cells.
TMEM35 knockdown arrests OSA cells in
G1 phase
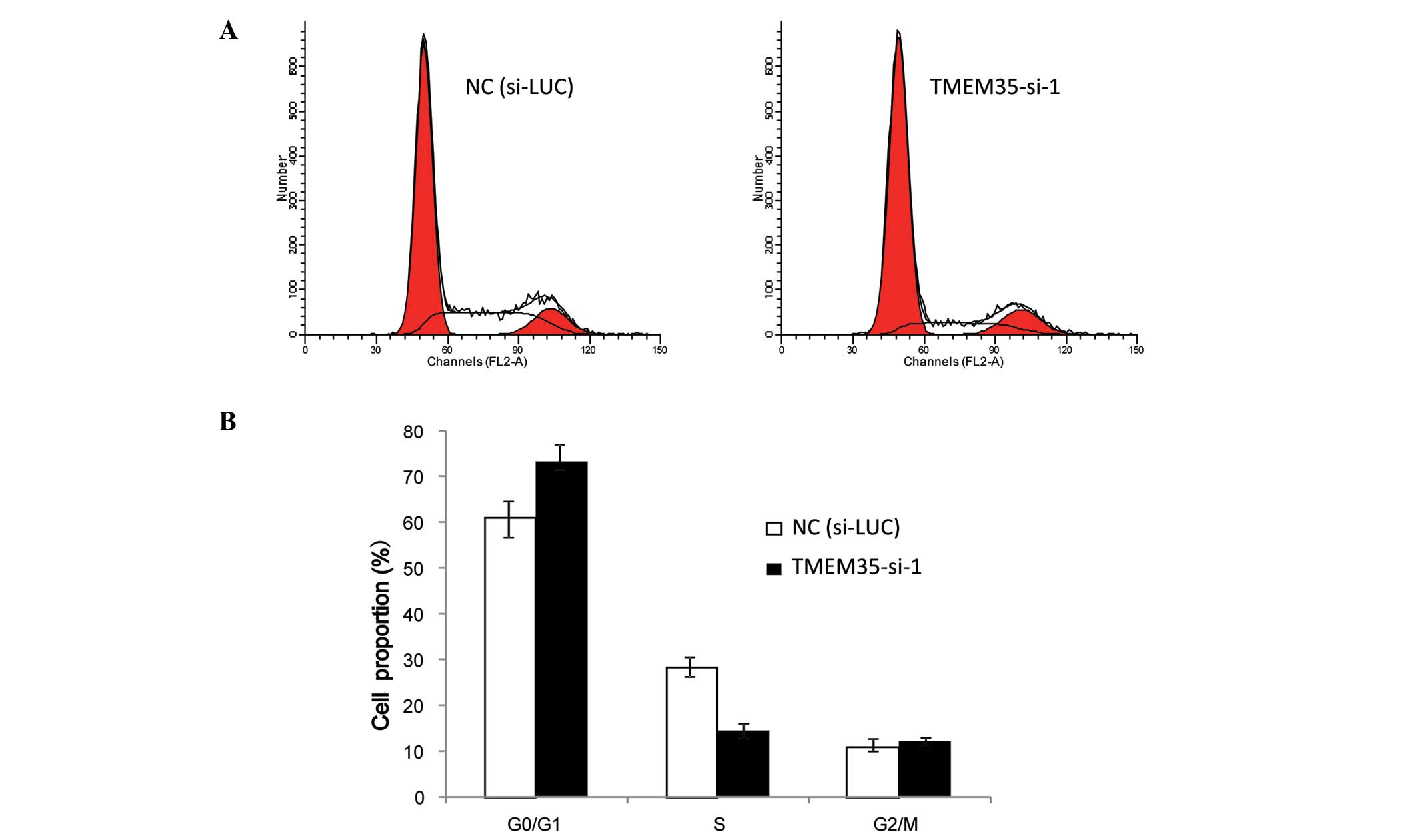
In order to explain how the cytological metabolism
of TMEM35 knockdown inhibits cell growth in OSA cells, flow
cytometric analysis was performed to analyze changes in the cell
cycle progression following TMEM35 silencing in SaOS2 cells. Cells
transfected with either si-LUC or TMEM35-si-1 were collected and
stained with PI. As shown in Fig. 4,
the proportion of cells in the G0/G1 phase increased (si-LUC,
61.07±3.21%; TMEM35-si-1, 73.35±4.52%), and the proportion of cells
in the S phase decreased (si-LUC, 28.16±1.23%; TMEM35-si-1,
14.55±2.31%) when compared with the cell population in the negative
control (si-LUC) following TMEM35 silencing in SaOS2 cells. These
collective data indicate that TMEM35 knockdown reduced the S phase
cell population and resulted in G1 arrest in SaOS2 cells, which
suggests that TMEM35 participated in cell cycle progression.
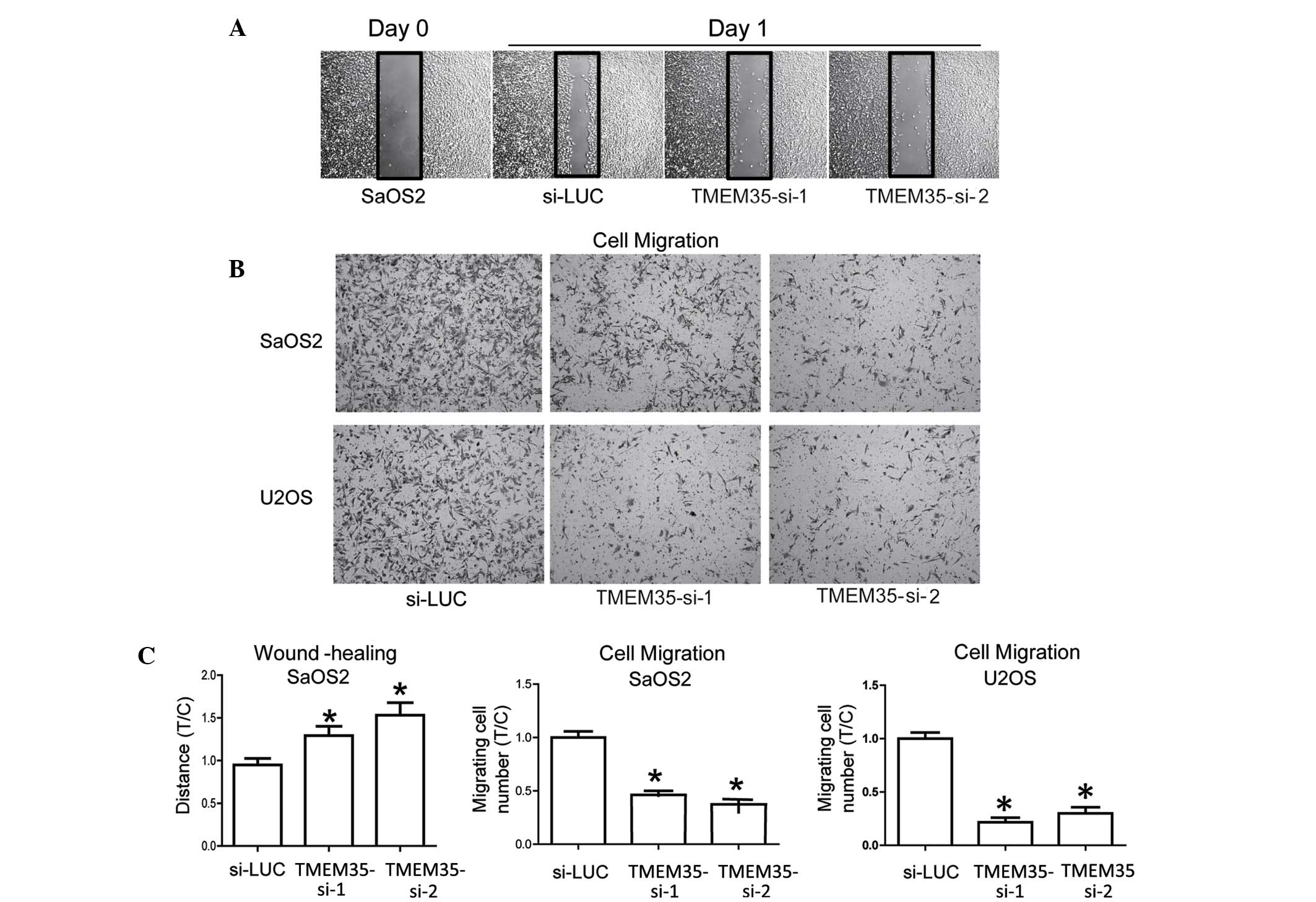
In vitro cell migration depends on
TMEM35 expression
The migratory potential of transduced OSA cell lines
was determined, since metastasis is the main deleterious
characteristic of OSA cells. The results indicated that cell
migration was reduced subsequent to TMEM35 silencing when compared
with that in the parental cells (Fig.
5A). A Boyden chamber assay confirmed the strong association
between TMEM35 expression and migratory potential (Fig. 5B and C). These data indicated that
TMEM35 expression regulates the migratory potential, in
vitro cell migration and invasive abilities of human OSA cells.
In addition, the results demonstrated that a reduced TMEM35
expression attenuates the OSA cell aggressiveness.
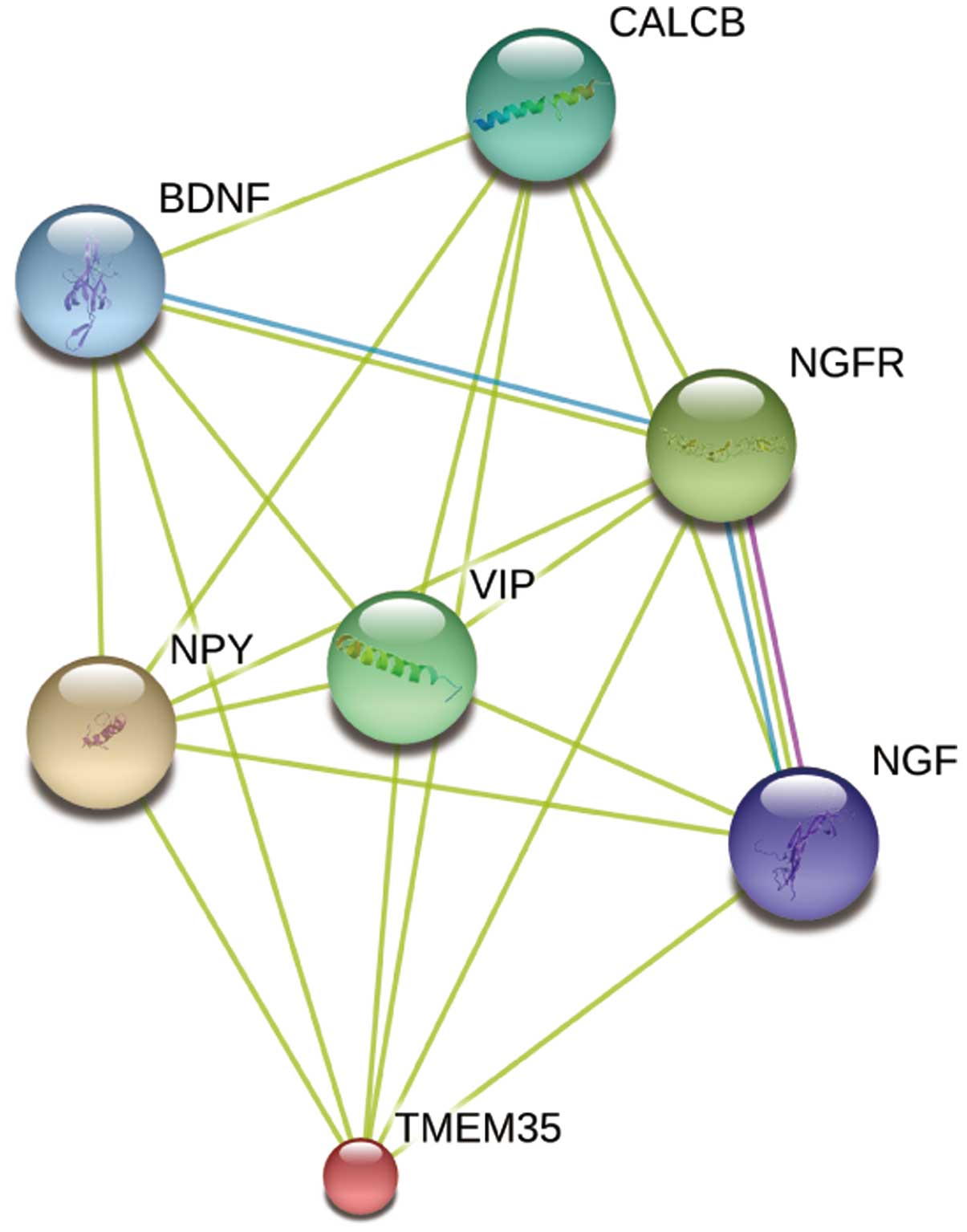
Identification of potential functional
partners of TMEM35 protein
For investigation of the poorly understood TMEM35
function and the associated molecular mechanism, the STRING
database (27) was used to predict
the proteins interacting with TMEM35. The database contains known
and predicted protein interactions, including directly (physical)
and indirectly (functional) relevant interactions. As demonstrated
in Fig. 6, TMEM35 may be associated
with neuropeptide Y (NPY), nerve growth factor (NGF), NGF receptor
(NGFR), brain-derived neurotropic factor (BDNF), vasoactive
intestinal peptide (VIP) and calcitonin-related polypeptide β
(CALCB). These predicted functional partner proteins indicate that
TMEM35 may play an important role in cell differentiation and
survival, further revealing the important function of TMEM35.
Discussion
Transmembrane proteins, coded by ~30% of genes in
humans, play critical roles in human physiological and pathological
progression (29). Transmembrane
proteins have been reported to be directly associated with numerous
serious human diseases, including Alzheimer's disease and
cardiovascular diseases (30).
Approximately 50% of drugs for such diseases target transmembrane
proteins (31), which highlights the
importance of further investigation into the structure and function
of transmembrane proteins. TMEM35 is a four-pass transmembrane
protein that exists conservatively in humans, canines, cattle,
mice, rats, chicken, zebra fish and Drosophila species
(24). A previous study in rats
revealed that TMEM35 may be a candidate regulatory factor involved
in adrenal cortex-zona glomerulosa growth following sodium
consumption (24). However, the
function of TMEM35 in OSA remains poorly understood. It has been
reported that another four-pass transmembrane protein gene, CD151
is highly associated with hepatocellular carcinoma invasion and
metastasis (32), and is
significantly upregulated in various tumors, including breast,
prostate, colorectal and pancreatic cancer, as well as in
hepatocellular carcinoma (33,34),
compared with corresponding normal tissues. Furthermore, survival
and relapse rates are significantly decreased or increased,
respectively, upon CD151 upregulation.
The human TMEM35 gene comprises 167 amino acids and
is located at the chromosome position Xq22.1. The molecular weight
and isoelectric point of TMEM35 are 18,440.23 Da and 10.09,
respectively (http://www.genecards.org/). The present study
investigated the expression of the transmembrane protein TMEM35,
and the results indicated that it was upregulated in OSA samples
compared with the normal tissues, as evaluated with RT-qPCR. TMEM35
was found to be important for OSA cell growth, and its knockdown
inhibited cell growth and adherence-independent growth in soft
agar. As shown in Fig. 4, TMEM35
knockdown inhibited cell cycle progression and resulted in cell
cycle arrest at G1 phase, which may account for the inhibition in
OSA cell growth. Migration was also inhibited by TMEM35 knockdown
in OSA cells. The aforementioned results imply that TMEM35 plays an
important role in OSA cell growth, migration and invasion through
regulation of the cell cycle progression, which indicates that it
may be a potential novel therapeutic target for drug
development.
Furthermore, the present study investigated the
potential functional partners of TMEM35. The results indicated that
TMEM35 may be associated with NPY, NGF, NGFR, BDNF, VIP and CALCB.
NPY is involved in the regulation of the gonadotropin-releasing
hormone transport and release (35).
In addition, NGF plays an important role in the development and
maintenance of the sympathetic nerve and sensorium nerve system.
Its receptor, NGFR, is a member of the tumor necrosis factor
receptor superfamily, which binds to NGF with a low affinity
(36). BDNF promotes the survival
and differentiation of neurons in the peripheral and central
nervous systems during the development process (37). Furthermore, VIP induces
vasodilatation and reduces arterial blood pressure (38), and CALCB is also known to induce
vasodilatation (39). These
observations may improve the understanding of the molecular
signaling pathway and mechanisms involved in the development of
OSA.
In conclusion, the present study found that TMEM35
kncockdown inhibited OSA cell proliferation by arresting the cell
cycle at the G1 phase. Furthermore, TMEM35 knockdown inhibited OSA
cell migration. These results provide new insight into the function
of TMEM35 in OSA initiation and progression. However, the precise
mechanism by which TMEM35 regulates cell proliferation and
migration requires further clarification. Further investigation of
the role TMEM35 in the cell cycle is worthy of investigation in
future studies.
Glossary
Abbreviations
Abbreviations:
|
OSA
|
osteosarcoma
|
|
TMEM35
|
transmembrane protein 35
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
NPY
|
neuropeptide Y
|
|
NGF
|
nerve growth factor
|
|
NGFR
|
nerve growth factor receptor
|
|
BDNF
|
brain-derived neurotropic factor
|
|
CALCB
|
calcitonin related polypeptide β
|
|
VIP
|
vasoactive intestinal peptide
|
References
|
1
|
Hashimoto K, Hatori M, Hosaka M, Watanabe
M, Hasegawa T and Kokubun S: Osteosarcoma arising from giant cell
tumor of bone ten years after primary surgery: A case report and
review of the literature. Tohoku J Exp Med. 208:157–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi K, Zhao M, Yamauchi K, Yamamoto N,
Tsuchiya H, Tomita K, Kishimoto H, Bouvet M and Hoffman RM:
Systemic targeting of primary bone tumor and lung metastasis of
high-grade osteosarcoma in nude mice with a tumor-selective strain
of Salmonella typhimurium. Cell Cycle. 8:870–875. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi A, Yamamoto N, Shirai T, Nishida
H, Hayashi K, Watanabe K, Miwa S and Tsuchiya H: Successful
correction of tibial bone deformity through multiple surgical
procedures, liquid nitrogen-pretreated bone tumor autograft,
three-dimensional external fixation, and internal fixation in a
patient with primary osteosarcoma: A case report. BMC Surg.
15:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stiller CA: International patterns of
cancer incidence in adolescents. 33:631–645. 2007.
|
|
5
|
Zhang Y, Zhang L, Zhang G, Li S, Duan J,
Cheng J, Ding G, Zhou C, Zhang J, Luo P, et al: Osteosarcoma
metastasis: Prospective role of ezrin. Tumour Biol. 35:5055–5059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
García-López MA, Barreiro O, García-Diez
A, Sánchez-Madrid F and Peñas PF: Role of tetraspanins CD9 and
CD151 in primary melanocyte motility. J Invest Dermatol.
125:1001–1009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gartlan KH, Belz GT, Tarrant JM, Minigo G,
Katsara M, Sheng KC, Sofi M, van Spriel AB, Apostolopoulos V,
Plebanski M, et al: A complementary role for the tetraspanins CD37
and Tssc6 in cellular immunity. J Immunol. 185:3158–3166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goschnick MW and Jackson DE: Tetraspanins
- structural and signalling scaffolds that regulate platelet
function. Mini Rev Med Chem. 7:1248–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gourgues M, Clergeot PH, Veneault C, Cots
J, Sibuet S, Brunet-Simon A, Levis C, Langin T and Lebrun MH: A new
class of tetraspanins in fungi. Biochem Biophys Res Commun.
297:1197–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang X, Zhang J and Huang Y: Tetraspanins
in cell migration. Cell Adh Migr. 9:406–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones EL, Demaria MC and Wright MD:
Tetraspanins in cellular immunity. Biochem Soc Trans. 39:506–511.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Köberle M, Kaesler S, Kempf W, Wölbing F
and Biedermann T: Tetraspanins in mast cells. Front Immunol.
3:1062012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krementsov DN, Weng J, Lambelé M, Roy NH
and Thali M: Tetraspanins regulate cell-to-cell transmission of
HIV-1. Retrovirology. 6:642009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perron JC and Bixby JL: Tetraspanins
expressed in the embryonic chick nervous system. FEBS Lett.
461:86–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higashiyama M, Taki T, Ieki Y, Adachi M,
Huang CL, Koh T, Kodama K, Doi O and Miyake M: Reduced motility
related protein-1 (MRP-1/CD9) gene expression as a factor of poor
prognosis in non-small cell lung cancer. Cancer Res. 55:6040–6044.
1995.PubMed/NCBI
|
|
16
|
Zheng R, Yano S, Zhang H, Nakataki E,
Tachibana I, Kawase I, Hayashi S and Sone S: CD9 overexpression
suppressed the liver metastasis and malignant ascites via
inhibition of proliferation and motility of small-cell lung cancer
cells in NK cell-depleted SCID mice. Oncol Res. 15:365–372.
2005.PubMed/NCBI
|
|
17
|
Saito Y, Tachibana I, Takeda Y, Yamane H,
He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, et al:
Absence of CD9 enhances adhesion-dependent morphologic
differentiation, survival and matrix metalloproteinase-2 production
in small cell lung cancer cells. Cancer Res. 66:9557–9565. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JJ, Jin YB, Lee YJ, Lee JS, Lee YS,
Ko YG and Lee M: KAI1 suppresses HIF-1α and VEGF expression by
blocking CDCP1-enhanced Src activation in prostate cancer. BMC
Cancer. 12:812012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jee B, Jin K, Hahn JH, Song HG and Lee H:
Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of
human prostate cancer cells through Src-dependent pathway. Exp Mol
Med. 35:30–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HA, Park I, Byun HJ, Jeoung D, Kim YM
and Lee H: Metastasis suppressor KAI1/CD82 attenuates the matrix
adhesion of human prostate cancer cells by suppressing fibronectin
expression and β1 integrin activation. Cell Physiol Biochem.
27:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radford KJ, Thorne RF and Hersey P: CD63
associates with transmembrane 4 superfamily members, CD9 and CD81
and with beta 1 integrins in human melanoma. Biochem Biophys Res
Commun. 222:13–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Si Z and Hersey P: Expression of the
neuroglandular antigen and analogues in melanoma. CD9 expression
appears inversely related to metastatic potential of melanoma. Int
J Cancer. 54:37–43. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sauer T and Suciu V: The role of
preoperative axillary lymph node fine needle aspiration in
locoregional staging of breast cancer. Ann Pathol. 32:e24–e28.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tran PV, Georgieff MK and Engeland WC:
Sodium depletion increases sympathetic neurite outgrowth and
expression of a novel TMEM35 gene-derived protein (TUF1) in the rat
adrenal zona glomerulosa. Endocrinology. 151:4852–4860. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fromigué O, Hamidouche Z and Marie PJ:
Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces
osteosarcoma cell invasion. J Biol Chem. 283:30549–30556. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuhn M, Szklarczyk D, Franceschini A,
Campillos M, von Mering C, Jensen LJ, Beyer A and Bork P: STITCH 2:
An interaction network database for small molecules and proteins.
Nucleic Acids Res. 38:D552–D556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hemler ME: Tetraspanin functions and
associated microdomains. Nat Rev Mol Cell Biol. 6:801–811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Whitelegge JP: Integral membrane proteins
and bilayer proteomics. Anal Chem. 85:2558–2568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cobbold C, Monaco AP, Sivaprasadarao A and
Ponnambalam S: Aberrant trafficking of transmembrane proteins in
human disease. Trends Cell Biol. 13:639–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pieck KI: More than 50% of drugs target
membrane proteins. http://www.irbbarcelona.org/en/news/more-than-50-of-drugs-target-membrane-proteins
(In Italian)Accessed. December 30–2014
|
|
32
|
Ke AW, Shi GM, Zhou J, Huang XY, Shi YH,
Ding ZB, Wang XY, Devbhandari RP and Fan J: CD151 amplifies
signaling by integrin α6β1 to PI3K and induces the
epithelial-mesenchymal transition in HCC cells. Gastroenterology.
140:1629–1641, e15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sadej R, Romanska H, Baldwin G,
Gkirtzimanaki K, Novitskaya V, Filer AD, Krcova Z, Kusinska R,
Ehrmann J, Buckley CD, et al: CD151 regulates tumorigenesis by
modulating the communication between tumor cells and endothelium.
Mol Cancer Res. 7:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu
MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, et al: Role of overexpression of
CD151 and/or c-Met in predicting prognosis of hepatocellular
carcinoma. Hepatology. 49:491–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roland AV and Moenter SM: Regulation of
gonadotropin-releasing hormone neurons by glucose. Trends
Endocrinol Metab. 22:443–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mantyh PW, Koltzenburg M, Mendell LM, Tive
L and Shelton DL: Antagonism of nerve growth factor-TrkA signaling
and the relief of pain. Anesthesiology. 115:189–204. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Binder DK and Scharfman HE: Brain-derived
neurotrophic factor. Growth Factors. 22:123–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nilsson SF and Bill A: Vasoactive
intestinal polypeptide (VIP): Effects in the eye and on regional
blood flows. Acta Physiol Scand. 121:385–392. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenfeld CR, White RE, Roy T and Cox BE:
Calcium-activated potassium channels and nitric oxide coregulate
estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol.
279:H319–H312. 2000.PubMed/NCBI
|