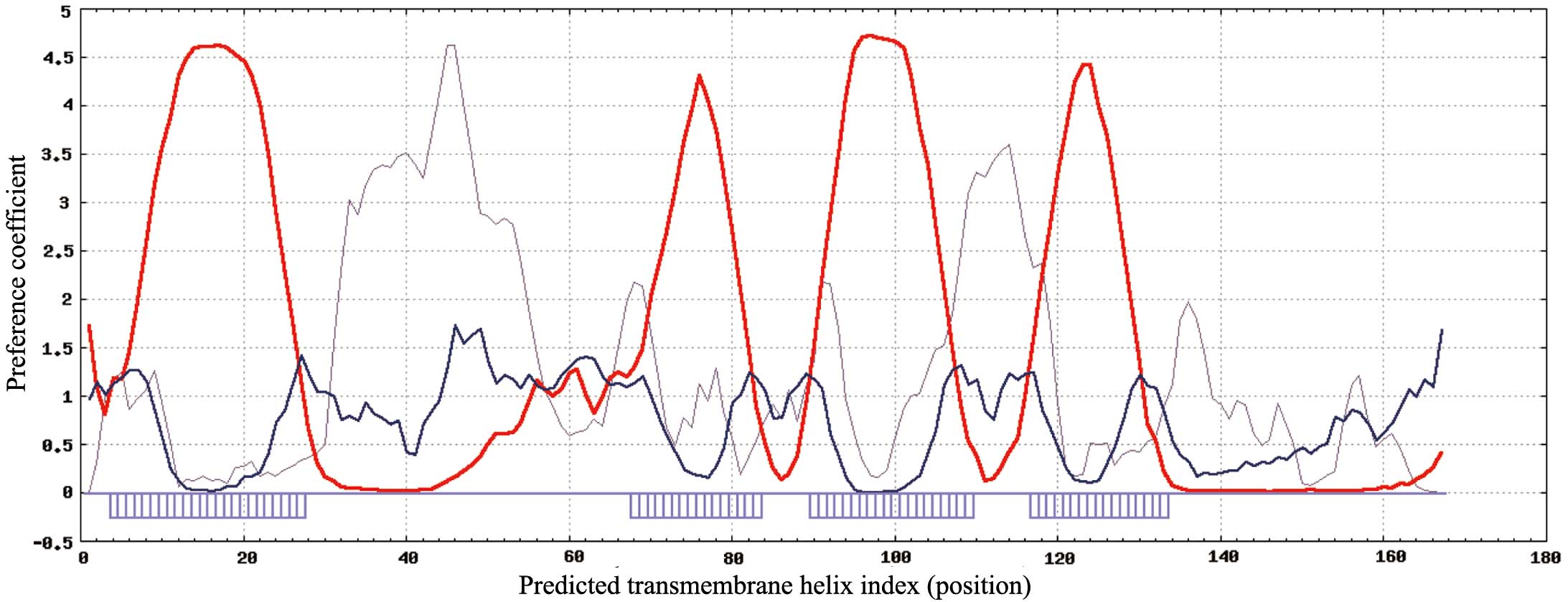
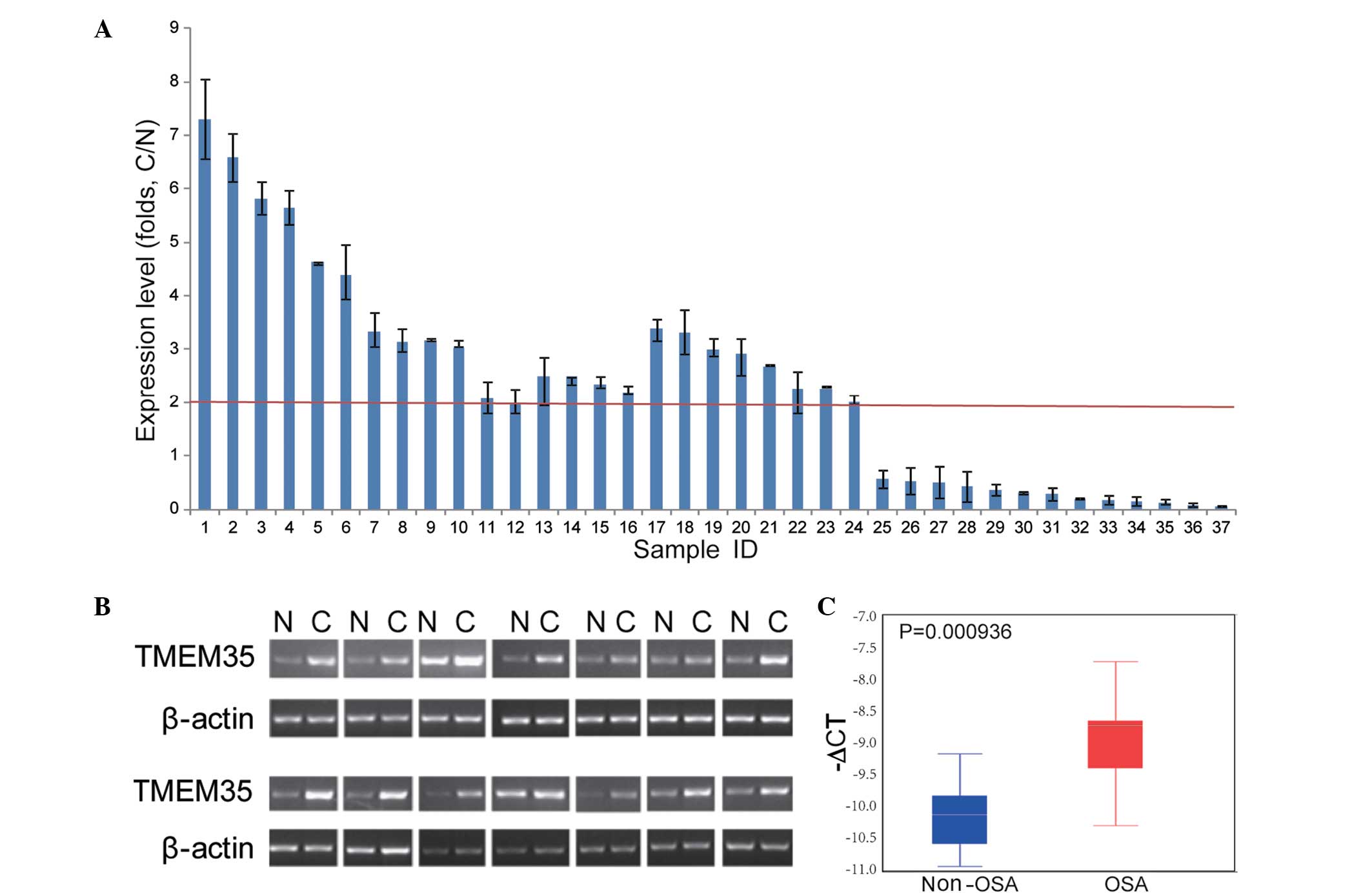
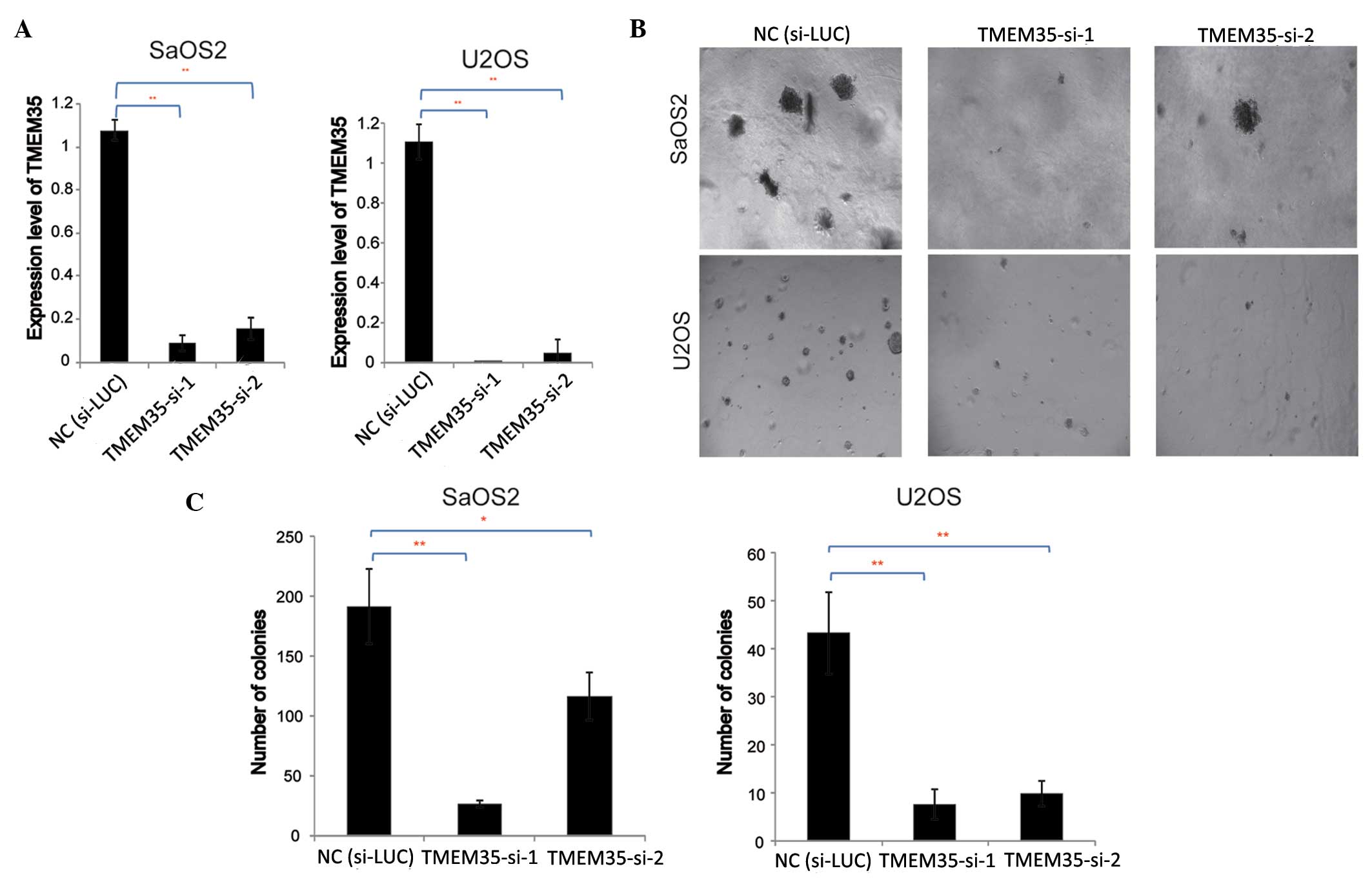
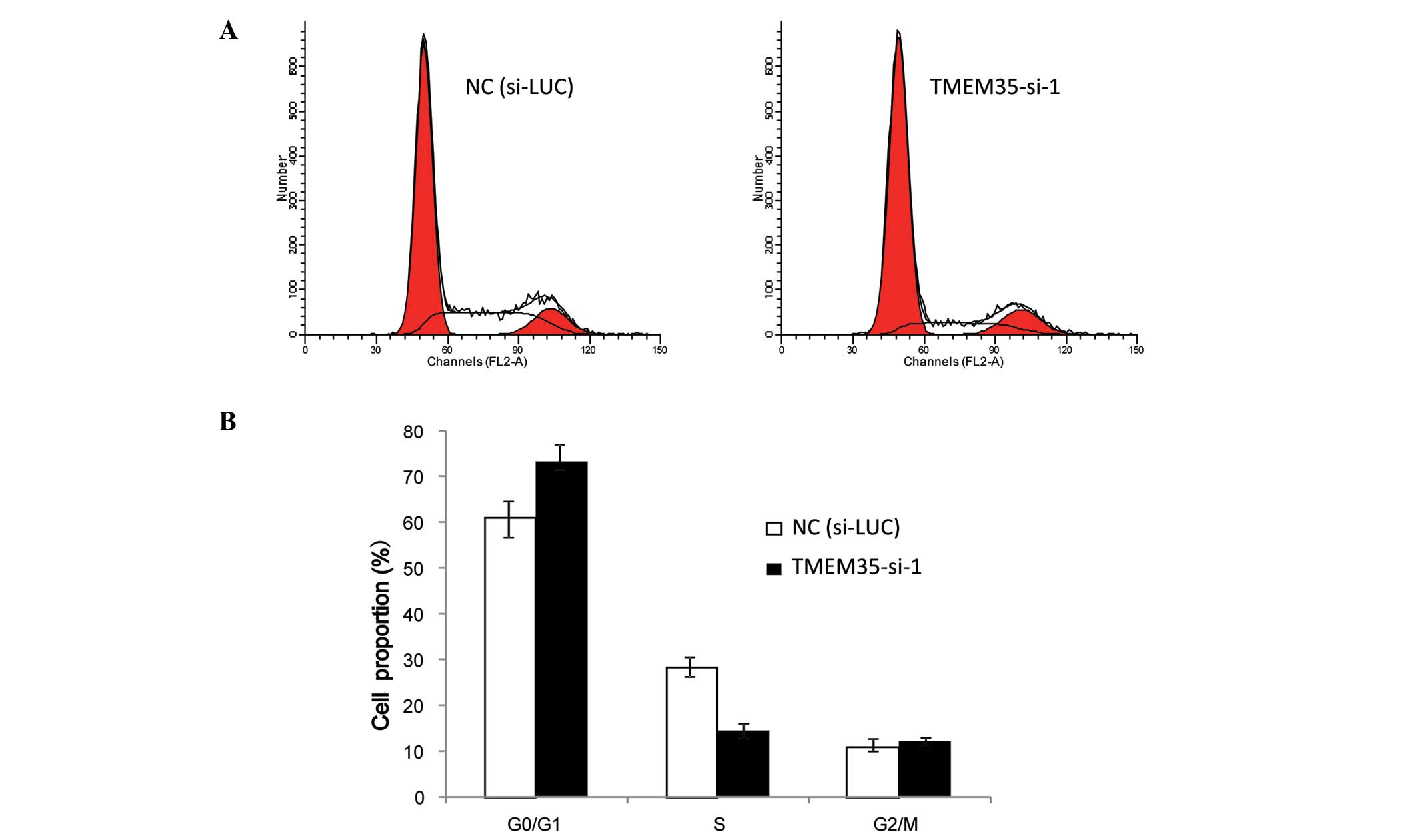
|
1
|
Hashimoto K, Hatori M, Hosaka M, Watanabe
M, Hasegawa T and Kokubun S: Osteosarcoma arising from giant cell
tumor of bone ten years after primary surgery: A case report and
review of the literature. Tohoku J Exp Med. 208:157–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi K, Zhao M, Yamauchi K, Yamamoto N,
Tsuchiya H, Tomita K, Kishimoto H, Bouvet M and Hoffman RM:
Systemic targeting of primary bone tumor and lung metastasis of
high-grade osteosarcoma in nude mice with a tumor-selective strain
of Salmonella typhimurium. Cell Cycle. 8:870–875. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi A, Yamamoto N, Shirai T, Nishida
H, Hayashi K, Watanabe K, Miwa S and Tsuchiya H: Successful
correction of tibial bone deformity through multiple surgical
procedures, liquid nitrogen-pretreated bone tumor autograft,
three-dimensional external fixation, and internal fixation in a
patient with primary osteosarcoma: A case report. BMC Surg.
15:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stiller CA: International patterns of
cancer incidence in adolescents. 33:631–645. 2007.
|
|
5
|
Zhang Y, Zhang L, Zhang G, Li S, Duan J,
Cheng J, Ding G, Zhou C, Zhang J, Luo P, et al: Osteosarcoma
metastasis: Prospective role of ezrin. Tumour Biol. 35:5055–5059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
García-López MA, Barreiro O, García-Diez
A, Sánchez-Madrid F and Peñas PF: Role of tetraspanins CD9 and
CD151 in primary melanocyte motility. J Invest Dermatol.
125:1001–1009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gartlan KH, Belz GT, Tarrant JM, Minigo G,
Katsara M, Sheng KC, Sofi M, van Spriel AB, Apostolopoulos V,
Plebanski M, et al: A complementary role for the tetraspanins CD37
and Tssc6 in cellular immunity. J Immunol. 185:3158–3166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goschnick MW and Jackson DE: Tetraspanins
- structural and signalling scaffolds that regulate platelet
function. Mini Rev Med Chem. 7:1248–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gourgues M, Clergeot PH, Veneault C, Cots
J, Sibuet S, Brunet-Simon A, Levis C, Langin T and Lebrun MH: A new
class of tetraspanins in fungi. Biochem Biophys Res Commun.
297:1197–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang X, Zhang J and Huang Y: Tetraspanins
in cell migration. Cell Adh Migr. 9:406–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones EL, Demaria MC and Wright MD:
Tetraspanins in cellular immunity. Biochem Soc Trans. 39:506–511.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Köberle M, Kaesler S, Kempf W, Wölbing F
and Biedermann T: Tetraspanins in mast cells. Front Immunol.
3:1062012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krementsov DN, Weng J, Lambelé M, Roy NH
and Thali M: Tetraspanins regulate cell-to-cell transmission of
HIV-1. Retrovirology. 6:642009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perron JC and Bixby JL: Tetraspanins
expressed in the embryonic chick nervous system. FEBS Lett.
461:86–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higashiyama M, Taki T, Ieki Y, Adachi M,
Huang CL, Koh T, Kodama K, Doi O and Miyake M: Reduced motility
related protein-1 (MRP-1/CD9) gene expression as a factor of poor
prognosis in non-small cell lung cancer. Cancer Res. 55:6040–6044.
1995.PubMed/NCBI
|
|
16
|
Zheng R, Yano S, Zhang H, Nakataki E,
Tachibana I, Kawase I, Hayashi S and Sone S: CD9 overexpression
suppressed the liver metastasis and malignant ascites via
inhibition of proliferation and motility of small-cell lung cancer
cells in NK cell-depleted SCID mice. Oncol Res. 15:365–372.
2005.PubMed/NCBI
|
|
17
|
Saito Y, Tachibana I, Takeda Y, Yamane H,
He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, et al:
Absence of CD9 enhances adhesion-dependent morphologic
differentiation, survival and matrix metalloproteinase-2 production
in small cell lung cancer cells. Cancer Res. 66:9557–9565. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JJ, Jin YB, Lee YJ, Lee JS, Lee YS,
Ko YG and Lee M: KAI1 suppresses HIF-1α and VEGF expression by
blocking CDCP1-enhanced Src activation in prostate cancer. BMC
Cancer. 12:812012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jee B, Jin K, Hahn JH, Song HG and Lee H:
Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of
human prostate cancer cells through Src-dependent pathway. Exp Mol
Med. 35:30–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HA, Park I, Byun HJ, Jeoung D, Kim YM
and Lee H: Metastasis suppressor KAI1/CD82 attenuates the matrix
adhesion of human prostate cancer cells by suppressing fibronectin
expression and β1 integrin activation. Cell Physiol Biochem.
27:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radford KJ, Thorne RF and Hersey P: CD63
associates with transmembrane 4 superfamily members, CD9 and CD81
and with beta 1 integrins in human melanoma. Biochem Biophys Res
Commun. 222:13–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Si Z and Hersey P: Expression of the
neuroglandular antigen and analogues in melanoma. CD9 expression
appears inversely related to metastatic potential of melanoma. Int
J Cancer. 54:37–43. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sauer T and Suciu V: The role of
preoperative axillary lymph node fine needle aspiration in
locoregional staging of breast cancer. Ann Pathol. 32:e24–e28.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tran PV, Georgieff MK and Engeland WC:
Sodium depletion increases sympathetic neurite outgrowth and
expression of a novel TMEM35 gene-derived protein (TUF1) in the rat
adrenal zona glomerulosa. Endocrinology. 151:4852–4860. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fromigué O, Hamidouche Z and Marie PJ:
Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces
osteosarcoma cell invasion. J Biol Chem. 283:30549–30556. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuhn M, Szklarczyk D, Franceschini A,
Campillos M, von Mering C, Jensen LJ, Beyer A and Bork P: STITCH 2:
An interaction network database for small molecules and proteins.
Nucleic Acids Res. 38:D552–D556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hemler ME: Tetraspanin functions and
associated microdomains. Nat Rev Mol Cell Biol. 6:801–811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Whitelegge JP: Integral membrane proteins
and bilayer proteomics. Anal Chem. 85:2558–2568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cobbold C, Monaco AP, Sivaprasadarao A and
Ponnambalam S: Aberrant trafficking of transmembrane proteins in
human disease. Trends Cell Biol. 13:639–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pieck KI: More than 50% of drugs target
membrane proteins. http://www.irbbarcelona.org/en/news/more-than-50-of-drugs-target-membrane-proteins
(In Italian)Accessed. December 30–2014
|
|
32
|
Ke AW, Shi GM, Zhou J, Huang XY, Shi YH,
Ding ZB, Wang XY, Devbhandari RP and Fan J: CD151 amplifies
signaling by integrin α6β1 to PI3K and induces the
epithelial-mesenchymal transition in HCC cells. Gastroenterology.
140:1629–1641, e15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sadej R, Romanska H, Baldwin G,
Gkirtzimanaki K, Novitskaya V, Filer AD, Krcova Z, Kusinska R,
Ehrmann J, Buckley CD, et al: CD151 regulates tumorigenesis by
modulating the communication between tumor cells and endothelium.
Mol Cancer Res. 7:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu
MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, et al: Role of overexpression of
CD151 and/or c-Met in predicting prognosis of hepatocellular
carcinoma. Hepatology. 49:491–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roland AV and Moenter SM: Regulation of
gonadotropin-releasing hormone neurons by glucose. Trends
Endocrinol Metab. 22:443–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mantyh PW, Koltzenburg M, Mendell LM, Tive
L and Shelton DL: Antagonism of nerve growth factor-TrkA signaling
and the relief of pain. Anesthesiology. 115:189–204. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Binder DK and Scharfman HE: Brain-derived
neurotrophic factor. Growth Factors. 22:123–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nilsson SF and Bill A: Vasoactive
intestinal polypeptide (VIP): Effects in the eye and on regional
blood flows. Acta Physiol Scand. 121:385–392. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenfeld CR, White RE, Roy T and Cox BE:
Calcium-activated potassium channels and nitric oxide coregulate
estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol.
279:H319–H312. 2000.PubMed/NCBI
|