Introduction
Thyroid-associated ophthalmopathy (TAO), which is
also called Graves' ophthalmopathy or thyroid eye disease, is a
complex inflammatory disorder of the eye that is usually associated
with thyroid disorder (1). The
annual incidence of TAO is ~16/100,000 in women and 3/100,000 in
men in Olmsted county (2).
Glucocorticoids are a commonly used agent in the treatment of TAO
since the 1950s (3–5). Recent evidence has suggested that
high-dose IVMP therapy is superior to oral glucocorticoid for the
treatment of active TAO, with an excellent curative effect, quick
response and fewer adverse effects. In previous studies, IVMP has
predominantly been used in combination with other treatments, and
only the short term effects (before and immediately after
treatment) have been observed. No definite IVMP therapy was
recommended in the consensus statement of the European Group on
Graves' Orbitopathy (EUGOGO), as evidence for the superiority of
any of the different IVMP schedules is lacking (6).
The aim of the present study was to prospectively
evaluate the long-term effects of high-dose IVMP therapy in a
cohort of patients with moderately to severely active TAO.
Additionally, as serum TRAb has a vital role in the occurrence and
progression of TAO (7,8) and sICAM-1 is as an indicator of the
progress of TAO and a predictor of its prognosis (9,10), serum
TRAb and sICAM-1 were evaluated in 23 patients with TAO.
Materials and methods
Experimental design
The present prospective clinical study was performed
to evaluate the long-term effects and safety of high-dose IVMP
therapy on TAO. A total of 73 patients with TAO were recruited from
the Department of Endocrinology at the First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China) between October 2006 and
November 2010. A total of 15 participants were excluded or lost to
follow-up. Informed consent was obtained from the remaining 58
participants, or from the parents or legal guardians of minors or
incapacitated adults. The present protocol was approved by the
local ethical committee.
Inclusion criteria
Diagnosis of TAO was defined by the presence of
typical clinical features, including protruding and watery eyes,
retrobulbar pain, photophobia, and double and blurred vision, and
detailed ophthalmological examination, including exophthalmometry,
measurement of eye movements, eyelid retraction and tonometry
(11). In addition, a routine
orbital computed tomography scan (Philips Brilliance 64 CT scanner,
Philips Medical Systems, Inc., Bothell, WA, USA) was performed for
all patients before commencement of any systemic medical treatment
to evaluate the extraocular muscles and exclude the orbital
space-occupying lesions.
Moderate-to-severe TAO with a clinical activity
score (CAS) ≥4 is defined as moderately to severely active TAO. The
CAS is based on four classical signs (pain, redness, swelling and
impaired function) which were proposed by Mourits et al
(12) and consists of 10 items.
These are the following: a painful, oppressive feeling on or behind
the globe; pain on attempting an up, side or down gaze; redness of
the eyelids; diffuse redness of the conjuctiva; chemosis; swollen
caruncle; oedema of the eyelid(s); increase of proptosis by ≥2 mm
during a period between 1 and 3 months; decrease in visual acuity
of one or more times on the Snellen chart during 1 and 3 months and
a decrease in eye movements in any direction ≥5° during a period
between 1 and 3 months. TAO is defined as active eye disease when
CAS is ≥4, otherwise it is classified as inactive eye disease.
Moderate-to-severe TAO has a sufficient impact on the daily life of
patients, including any one or more of the following symptoms: Lid
retraction ≥2 mm, moderate or severe soft tissue involvement,
exophthalmos ≥3 mm above normal for race and gender and inconstant
or constant diplopia (6).
Patients
A total of 73 patients who suffered from
moderately-to-severely active TAO were recruited for the present
study. A total of 15 patients were not included. Three patients
with serious phthisis or hepatitis were excluded prior to
enrollment and five patients were lost during follow-up. Two
patients received oral glucocorticoids during the course of
high-dose IVMP therapy. One patient only underwent one cycle of
IVMP therapy due to a relapse of hepatitis, and four patients
received other treatment after IVMP therapy, including retrobulbar
injection of triamcinolone acetonide (n=2), surgery (n=2) and
99Tc-MDP therapy (n=2). The remaining 58 patients received
high-dose IVMP therapy, and six participants underwent subsequent
orbital irradiation after high-dose IVMP therapy due to
unsatisfying effects. The levels of serum TRAb and sICAM-1 were
evaluated in 23 patients who received three cycles of pulse
therapy. Follow-up was conducted for 12–57 months after the
therapy, with a mean of 28.4 months. A summary of the clinical
characteristics of the patients is shown in Table I.
 | Table I.Baseline characteristics of the 58
patients enrolled in the present study. |
Table I.
Baseline characteristics of the 58
patients enrolled in the present study.
| Characteristic | N (%) | Mean ± SD | Median | Range |
|---|
| Female | 32 (55.2) |
|
|
|
| Male | 26 (44.8) |
|
|
|
| Cigarette
smokers | 19 (32.8) |
|
|
|
| Age (years) |
| 46.2±13.7 | 46.5 | 11–74 |
| Duration of thyroid
disease (months) |
| 27.9±41.5 | 16.0 | 0–240 |
| Duration of eye
disease (months) |
| 15.8±20.0 | 9.0 | 1–108 |
| Thyroid diseases |
|
|
|
|
| Graves
disease | 50 (86.2) |
|
|
|
|
Hashimoto's thyroiditis | 6 (10.4) |
|
|
|
|
Hypothyroidism | 1 (1.7) |
|
|
|
|
Euthyroidism | 1 (1.7) |
|
|
|
| Thyroid function |
|
|
|
|
| TSH
<0.25 | 25 (43.1) |
|
|
|
| 0.25≤
TSH ≤5.0 | 21 (36.2) |
|
|
|
| TSH
>5.0 | 12 (20.7) |
|
|
|
| Serum
T4 (ug/dl) |
| 9.1±3.8 | 8.6 | 1.5–19.8 |
| Serum
T3 (ng/ml) |
| 1.7±0.8 | 1.5 | 0.6–4.8 |
| Previous treatment
prior to study |
|
|
|
|
|
Radioactive iodine
therapy | 4 (6.9) |
|
|
|
| Oral
glucocorticoid | 11 (19.0) |
|
|
|
|
Retrobulbar injection of
triamcinolone acetonide | 2 (3.4) |
|
|
|
| Comorbidities |
|
|
|
|
|
Diabetes | 4 (6.9) |
|
|
|
|
Impaired glucose
tolerance | 6 (10.3) |
|
|
|
|
Hypertension | 11 (19.0) |
|
|
|
| Dormant
tuberculosis | 3 (5.2) |
|
|
|
| Duration of
follow-up (months) |
| 28.4±13.8 | 26.0 | 12–57 |
| 12≤
duration <24 | 26 (44.8) |
|
|
|
| 24≤
duration <48 | 23 (39.7) |
|
|
|
|
Duration ≥48 | 9 (15.5) |
|
|
|
| Patients tested for
serum TRAb and sICAM-1 | 23 (39.7) |
|
|
|
|
Female | 14 (24.1) |
|
|
|
|
Male | 9 (15.5) |
|
|
|
| Graves
disease | 21 (36.2) |
|
|
|
|
Hashimoto's thyroiditis | 2 (3.4) |
|
|
|
| Cycles of high-dose
IVMP therapy |
|
|
|
|
| 2 | 8 (13.8) |
|
|
|
| 3 | 33 (56.9) |
|
|
|
| 4 | 11 (19.0) |
|
|
|
| 5 | 3 (5.2) |
|
|
|
| 6 | 2 (3.4) |
|
|
|
| 7 | 1 (1.7) |
|
|
|
| Cumulative doses of
intravenous methylprednisolone |
|
| 9.0 | 3.0–19.5 |
|
3.0 | 2 (3.4) |
|
|
|
|
4.5 | 10 (17.2) |
|
|
|
|
6.0 | 6 (10.3) |
|
|
|
|
7.5 | 1 (1.7) |
|
|
|
|
8.0 | 2 (3.4) |
|
|
|
|
9.0 | 25 (43.1) |
|
|
|
|
10.5 | 2 (3.4) |
|
|
|
|
12.0 | 7 (12.1) |
|
|
|
|
13.0 | 1 (1.7) |
|
|
|
|
15.0 | 1 (1.7) |
|
|
|
|
19.5 | 1 (1.7) |
|
|
|
Treatment
According to age, weight, degree of illness and the
presence of comorbidities, high-dose IVMP therapy was administered.
IVMP therapy composed of 0.5–1 g IV methylprednisolone three times
every other day, repeated with intervals of 20 days for a total of
three cycles. Additional cycles were administered in specific
special and severe patients under the clinician's judgment
(Table I). During the course of
treatment, proton pump inhibitors or H2 receptor antagonists and
calcium supplements were prescribed to every patient. Low-salt
diet, high pillow lying and sunglasses were also recommended,
whereas eye drops were only used when required in 42 patients. A
total of 47 patients were administered thyroxine or antithyroid
agents, depending on their thyroid function. Five patients received
anti-tuberculosis drugs for at least three months due to a positive
reaction to a purified protein derivative skin test; although no
other evidence of infection was detected. All patients were
informed of the risk of smoking for TAO and all cigarette smokers
were advised to stop smoking.
Follow-up
Patients were followed-up by via face-to-face
interviews, telephone calls and mail. At the end of follow-up, an
appointment was made with the patients for a thorough examination
of eyelid swelling, ophthalmalgia, photophobia, lacrimation,
diplopia, ocular motility, visual acuity, proptosis, intraocular
pressure and CAS. The first four symptoms were subjectively
categorized into four grades: 0, no; 1, mild; 2, moderate; and 3,
severe. According to the EUGOGO criteria, the severity measures of
diplopia/ocular motility were as follows, 0, no diplopia/free; 1,
intermittent (diplopia in primary position of gaze, when tired or
when first awakening)/restriction of eye movement at <4/6
directions (upper right, lateral right, lower right, upper left,
lateral left and lower left); 2, inconstant (diplopia at extremes
of gaze)/restriction of eye movement at ≥4/6 directions; and 3,
constant (continuous diplopia in primary or reading
position)/fixation (6). Individual
symptom responses were defined as a complete response if the
symptom was completely resolved or as a partial response if there
was an improvement in the symptom without complete resolution.
Examinations of visual acuity, proptosis and intraocular pressure
were conducted by an ophthalmologist and endocrinologist using the
international standard vision chart, and a statometer and
noncontact tonometer. Mean values were taken. Furthermore, adverse
effects of glucocorticoid therapy were also recorded, including:
Low bone mineral density, assessed by bone density test or
symptoms; liver damage, assessed via blood test; weight gain;
cushingoid features, assessed by symptoms; hypertension, assessed
via blood pressure; hyperglycemia, assessed via blood glucose;
peptic ulcer, assessed by symptoms; mental disorder, assessed by
symptoms and serious infection, assessed by symptoms.
Levels of serum TRAb (human TRAb RIA kit, Medipan
GmbH, Dahlewitz, Germany) and sICAM-1 (ELISA kit, Bender Company,
Vienna, Austria) were evaluated by a competitive radioimmunoassay
and enzyme-1inked immunosorbent assay, respectively, prior to
treatment and on the second day following each IVMP therapy in 23
patients who received three cycles of pulse therapy.
Statistical analysis
Symptoms and the results of ophthalmological
examination were compared before treatment, after treatment (one
month after IVMP) and at the end of follow-up using
repeated-measures analysis of variance (ANOVA). To determine if the
values differed with gender, age or smoking status, one-way
repeated-measures ANOVA was used. TRAb and sICAM-l levels were
analyzed prior to and following each treatment cycle using the
least significant difference t-test and analysis of covariance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Remission of symptoms was achieved
following treatment
Symptoms and clinical outcomes of the 58 patients
are shown in Table II. The grades
of the five symptoms (eyelid swelling, ophthalmodynia, photophobia,
lacrimation and diplopia) were all significantly reduced one month
after treatment, as compared with before treatment (P<0.001). No
significant differences in the grades of eyelid swelling,
photophobia and lacrimation were detected between one month after
therapy and the end of follow-up; whereas a significant increase in
the grades of ophthalmodynia (P=0.046) and diplopia (P<0.001)
were detected between these time points. Dividing the 58 patients
into two groups according to gender, age (≤40 years vs. >40
years old) and smoking status, demonstrated that these groupings
did not make a significant difference to the interaction effect in
all five symptoms.
 | Table II.Response rate to IVMP over time, as
determined by five clinical symptoms. |
Table II.
Response rate to IVMP over time, as
determined by five clinical symptoms.
|
|
| 1 month after
treatment | End of
follow-up |
|---|
|
|
|
|
|
|---|
| Symptom | Baseline No
(%) | CR No (%) | PR No (%) | CR No (%) | PR No (%) |
|---|
| Eye swelling | 50 (86.2) | 15 (30.0) | 23 (46.0) | 22 (44.0) | 19 (38.0) |
| Ophthalmodynia | 36 (62.1) | 29 (80.6) | 6
(16.7) | 32 (88.9) | 1 (2.8) |
| Photophobia | 54 (93.1) | 16 (29.6) | 33 (61.1) | 28 (51.9) | 19 (35.2) |
| Lacrimation | 52 (89.7) | 24 (46.2) | 23 (44.2) | 36 (69.2) | 8 (15.4) |
| Diplopia | 43 (74.1) | 7
(16.3) | 12 (27.9) | 21 (48.8) | 16 (37.2) |
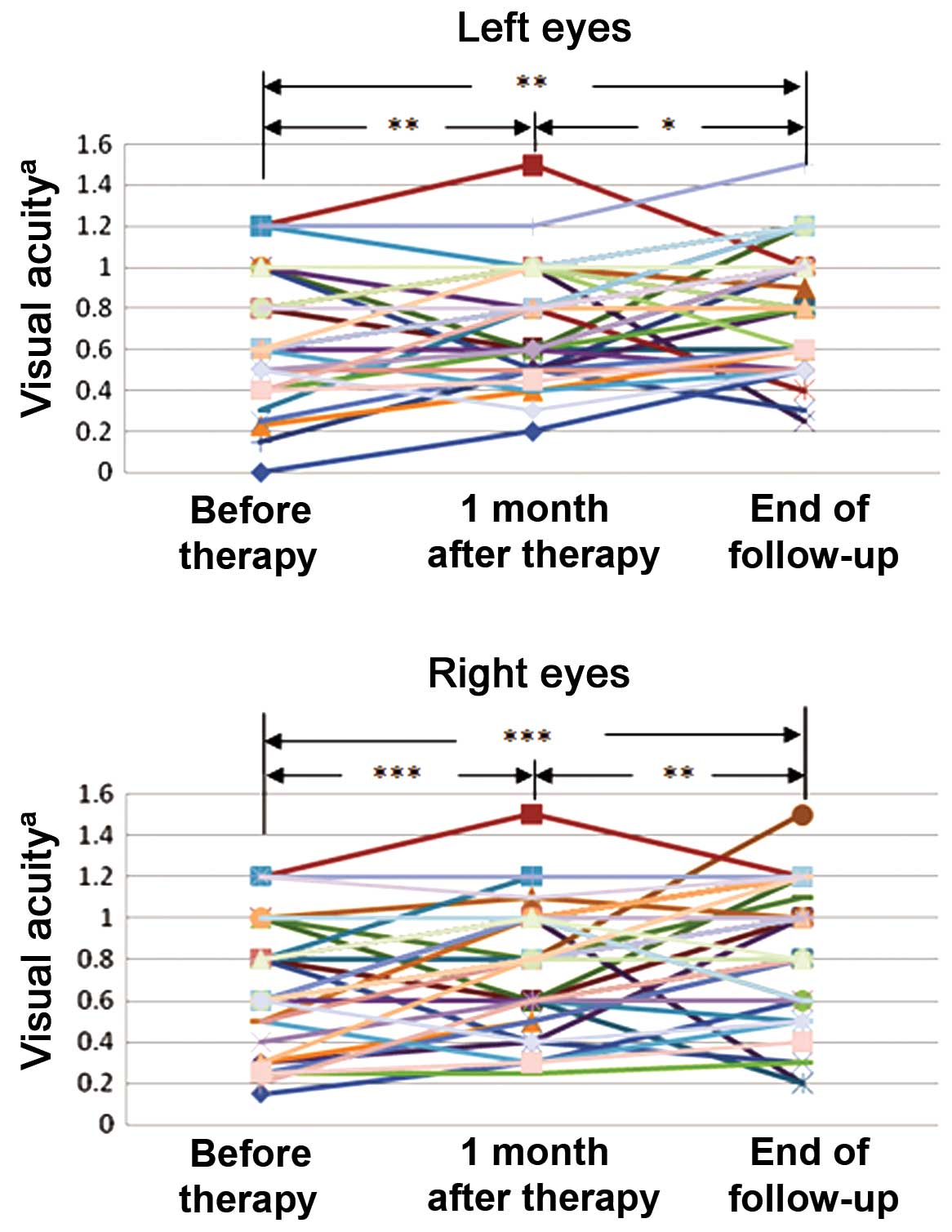
Visual acuity was improved following
treatment
Among the 58 patients examined, 52 patients
exhibited reduced visual acuity due to TAO prior to treatment.
Visual acuity testing with the international standard vision chart
(Fig. 1) revealed that visual acuity
was significantly improved one month after therapy in the left eye
(P=0.006) and right eye (P<0.001) and at the end of follow-up in
the left eye (P=0.001) and right eye (P<0.001), as compared with
before treatment. Significant improvements in visual acuity were
also detected between one month after therapy and the end of
follow-up in both the left (P=0.020) and right (P=0.004) eyes.
Ocular motility was improved after
treatment
Prior to treatment, 51 patients exhibited disordered
ocular motility in both eyes. Grading of ocular motility was
significantly reduced in their binoculus (left eye, P<0.001;
right eye, P<0.001) one month after treatment, as compared with
before treatment, and were also significantly reduced between one
month after therapy and the end of follow-up (left eye, P<0.001;
right eye, P<0.001). Moreover, the enrolled patients were
divided into two groups according to gender, age and smoking
status, and no significant differences were detected in the
interaction effect of treatment with any grouping in ocular
motility.
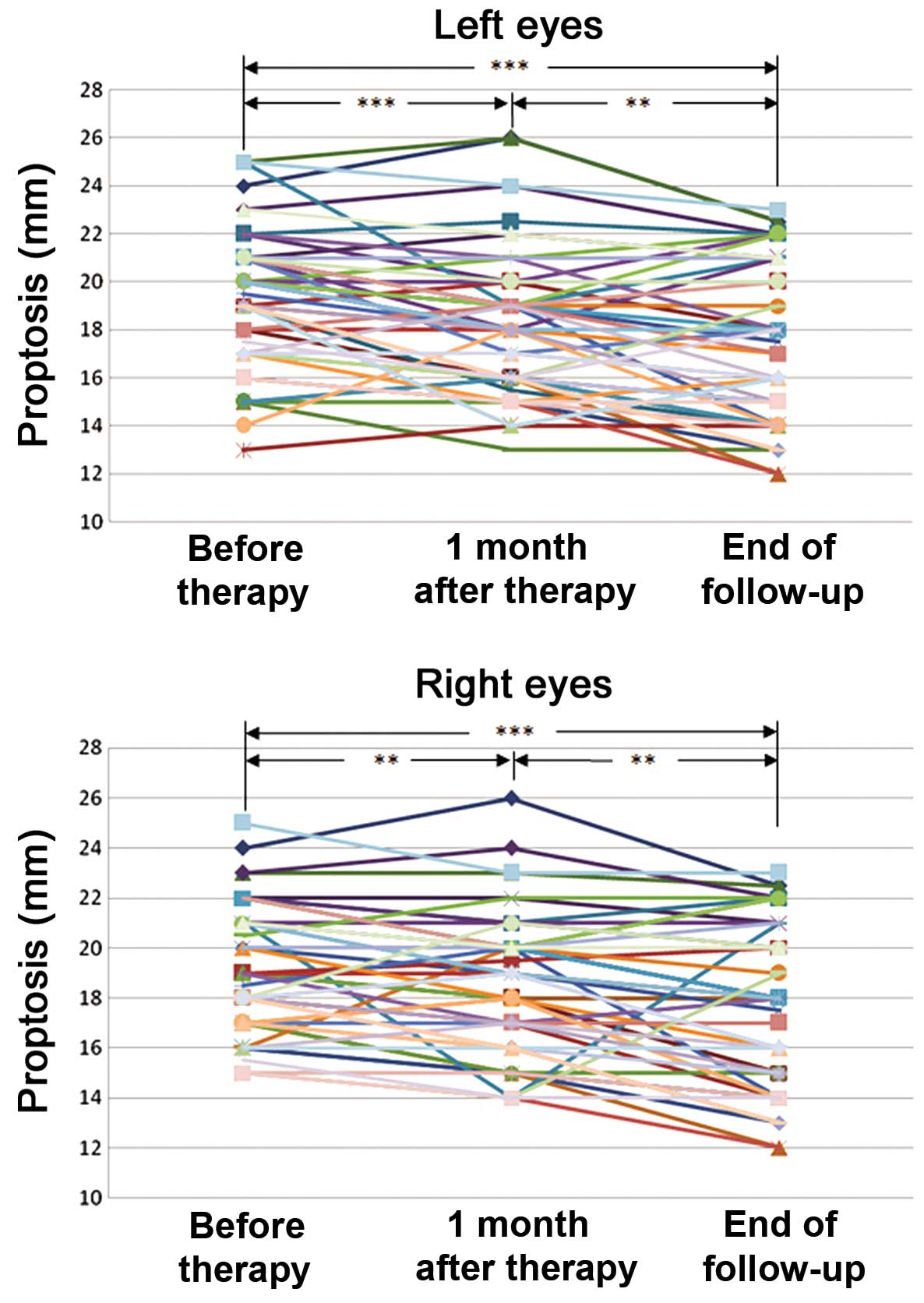
Proptosis was reduced after
treatment
Compared to baseline, the number of patients with
reduced proptosis (≥2 mm) was 19 (33%) one month after therapy and
30 (52%) at the end of follow up in the left eye, and 13 (22%) one
month after therapy and 27 (47%) at the end of follow up in the
right eye. Proptosis data are presented in Table III. It was demonstrated that
proptosis (Fig. 2) was significantly
reduced one month after therapy in the left (P<0.001) and right
(P=0.006) eyes and at the end of follow-up in the left (P<0.001)
and right (P<0.001) eyes. Moreover, proptosis was significantly
decreased between one month after therapy and the end of follow-up
in the left (P=0.005) and the right (P=0.001) eyes. No significant
difference was detected in the interaction effect of treatment
following grouping (gender, age and smoking status) in either
eye.
 | Table III.Comparisons of laboratory indicators
of proptosis over time. |
Table III.
Comparisons of laboratory indicators
of proptosis over time.
| Eye | Baseline | 1 month after
treatment | End of
follow-up | Reference
range |
|---|
| Left | 19.14±2.74 | 18.16±2.90 | 17.44±3.11 | 12.00–14.00 |
| Right | 18.82±2.56 | 18.23±2.78 | 17.32±3.14 | 12.00–14.00 |
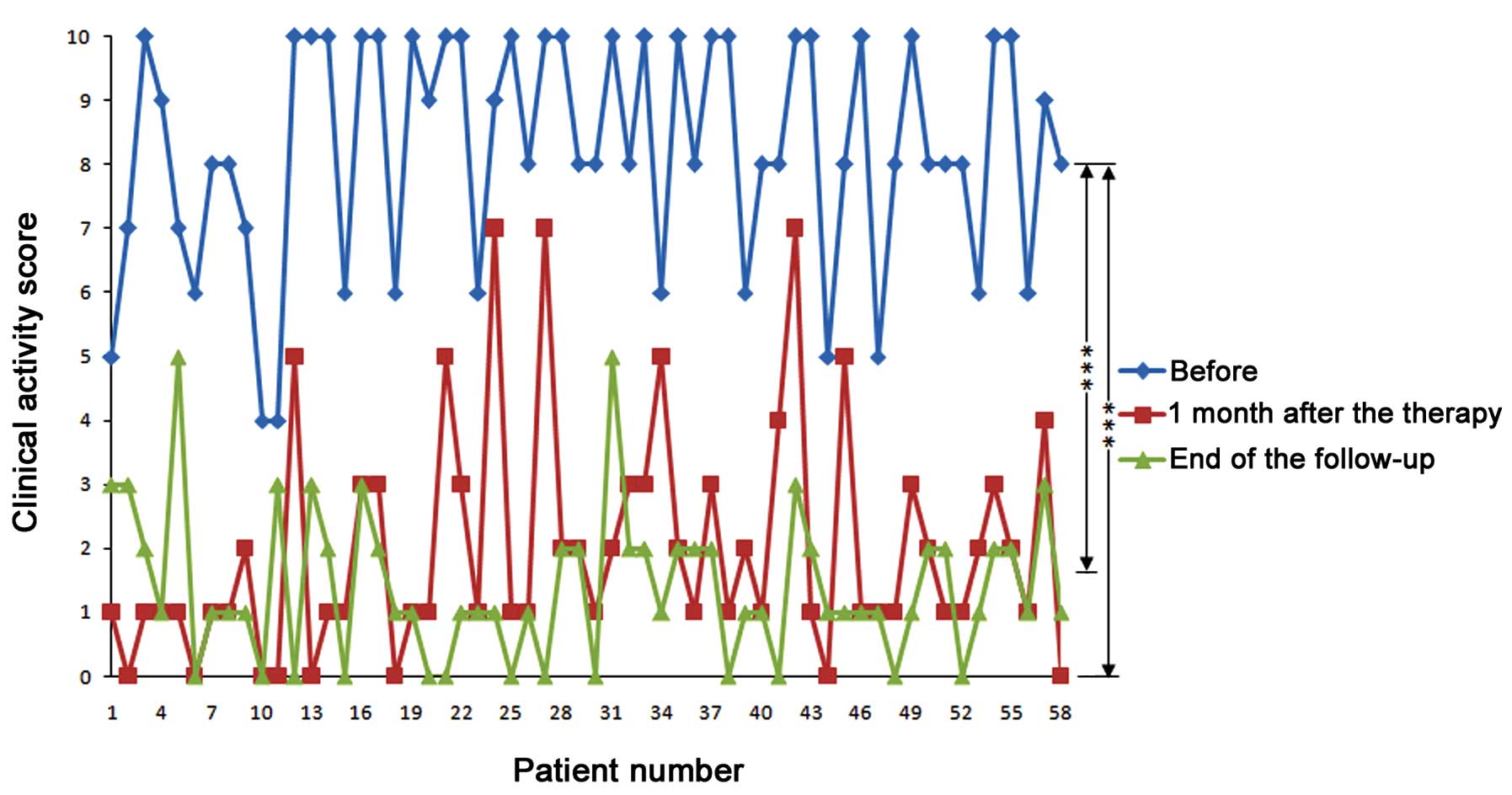
CAS was reduced after treatment
CAS values were 8.24±1.81 (n=58) prior to therapy,
1.98±1.79 one month after therapy and 1.41±1.17 at the end of
follow-up. CAS was significantly reduced one month after the
therapy (P<0.001) and at the end of follow-up (P<0.001), as
compared with the baseline (Fig. 3).
No significant differences in CAS values were detected between the
one month after therapy and end of follow-up time points.
Intraocular pressure was improved
after treatment
Complete intraocular pressure could not be obtained
for four patients. Data from the remaining 54 patients demonstrated
that the intraocular pressure changed significantly with treatment
in both the left (P=0.012) and right (P=0.046) eyes.
TRAb was correlated with CAS
Analysis of covariance demonstrated that the
difference in serum TRAb was predominantly correlated with CAS
(P=0.020) and was not correlated with smoking (P=0.722) or age
(P=0.789). Significant differences were detected between active TAO
and the baseline and after 3 cycles of treatment (P=0.033), but no
significant difference was found after 1 or 2 cycles of treatment
(P=0.722 and P=0.155, respectively), as compared with the
baseline.
sICAM-1 levels were correlated with
duration of eye disease
Analysis of covariance demonstrated that the change
in sICAM-1 levels over time was correlated with the duration of eye
disease (P=0.015) but not to CAS (P=0.912). Compared with the
sICAM-1 level before treatment, no significant difference was
detected after 1, 2 or 3 cycles of treatment (P=0.074, P=0.230 and
P=0.072, respectively).
Little adverse effects were identified
after treatment
Following IVMP therapy, a female patient who was
simultaneously administered fenofibrate-loaded
polylactide/polyethylene glycol microspheres, suffered from severe
liver injury. The patient recovered after hospitalization, during
which she received liver-protecting treatment and ceased
fenofibrate treatment. A male patient suffered from mildly abnormal
liver function one month after IVMP therapy and fully recovered
after dynamic observation for three months without any treatment.
Compared with their pretreatment body weight, three females (60, 68
and 47 years old, respectively) and one male (42 years old) gained
10, 6, 5 and 10 kg, respectively, by the end of follow-up, with no
moon face or buffalo hump symptoms. Three of these patients
exhibited normal body fat distribution, and one female had a large
waist circumference. During the therapeutic process, blood glucose
and blood pressure were monitored, and if necessary, appropriate
interventions were employed for the 11 cases of hypertension and 10
cases of diabetes or impaired glucose tolerance to reduce
fluctuations. No interventions for blood glucose or blood pressure
were employed in the other patients, even if a rising trend was
detected. Within a week the increased blood glucose and pressure
were resolved in these cases. No osteoporosis, fractures,
tuberculosis, serious infections, peptic ulcers or mental disorders
were detected in any of the participants during the observation
period.
Discussion
TAO is a chronic autoimmune disease that affects the
retrobulbar tissue and has strong etiological links with autoimmune
thyroid disease, which leads to blurring of vision, proptosis,
extraocular muscle dysfunction, redness of the conjunctiva and pain
(13). Although there are other
therapies, including orbital radiotherapy and oral glucocorticoids,
IVMP therapy has superior efficiency and few adverse effects in the
treatment of patients with moderately to severely active TAO
(13–15). In a published consensus statement
(6,16), the EUGOGO recommend the use of IV
glucocorticoids as first-line treatment for patients with active or
severe TAO.
Numerous randomized and nonrandomized trials of
intravenous glucocorticosteroid have demonstrated the beneficial
effects of glucocorticoids in TAO (17,18),
including 13 nonrandomized trials with a total of 346 patients and
10 randomized trials with a total of 234 patients. The treatment
protocols for each of these studies were 0.5–1.0 g or 12.5–15.0
mg/kg IV methylprednisolone with or without oral prednisolone and
orbital irradiation (17). Treatment
with IV glucocorticoids was safe and effective in the nonrandomized
(response rate, 82.6%) and randomized (response rate, 79.9%) trials
and was associated with a lower recurrence rate and less frequent
adverse effects, as compared with oral regimens (17). Ohtsuka et al (19) conducted a case-control study with 39
Japanese patients in which the first 20 patients underwent
high-dose IVMP therapy followed by 24-Gy orbital radiotherapy and
the remaining 19 patients only received high-dose IVMP therapy. No
significant difference was detected between the two groups;
suggesting that the addition of 24 Gy irradiation to IV
prednisolone had no extra therapeutic benefit. In addition, the
radiation may have caused radiation optic neuropathy if the
cumulative dose of radiation exceeded 50 Gy or if radiation
fractions of >2 Gy were used (20). In a survey, 91% of the responding
members of the European Thyroid Association indicated that they
would treat an index patient who had active and severe TAO with
glucocorticoids, and 71% would immediately start with IV
glucocorticoids (21). Similarly,
58% of the responding Latin-American thyroidologists would
administer IV glucocorticoids (22).
The short-and long-term effects of IVMP therapy were
investigated in the present report, indicating the superiority of
the present study over previous studies (17,18). The
findings of the present study suggested that the symptoms of
patients with moderately to severely active TAO may be improved or
cured through IVMP therapy and that the curative effect is stable
over time. It was also demonstrated that some symptoms, including
diplopia, visual acuity, ocular motility and proptosis, may
continue to improve with time after the withdrawal of therapy. No
serious adverse events occurred in this carefully monitored and
closely followed-up study. Serious liver injury occurred in one
female patient who was taking fenofibrate-loaded
polylactide/polyethylene glycol microspheres at the same time as
the IVMP therapy. Her liver injury may be due to the joint impact
of high-dose methylprednisolone and micronized fenofibrate. Liver
injury is a serious adverse effect of high dose methylprednisolone
and should be careful monitored during the course of treatment
(17). Moreover, high-dose
methylprednisolone should never be used with any agent that is
potentially harmful to the liver. Four patients who exhibited mild
weight gain over the observation period had taken oral
corticosteroids for six months or more prior to treatment with
IVMP. A systematic review and meta-analysis of randomized trials
for TAO (23) determined that
patients in the oral administration group had a high rate of
steroid-related adverse events, including weight gain (26%),
hypertension (8%) and cushingoid features (7%), whereas patients in
the IV administration group exhibited a lower rate of
steroid-related events (3–4%). Considering both the positive and
adverse effects of IVMP, Zang et al (17) suggested that single doses of IV
glucocorticoids should not be administered on consecutive days. An
every-other-day regimen was employed in the present study.
The majority of previous studies have demonstrated
that smoking is associated with an increased risk of TAO
progression in Western countries and that smoking cessation may
minimize the progression of TAO and improve the response to
treatment (24–28). Meanwhile, a previous study in Taiwan
demonstrated that no significant association with cigarette smoking
was detected in male patients (29).
Exploratory grouping demonstrated that smoking did not play a role
in the outcomes of the present study. Smoking may not have had an
effect in the present study as patients were divided according to
their pretreatment status as smokers or non-smokers, and the
majority of smokers stopped smoking or severely reduced their
intake under the guidance of their doctors. Gender may have been a
confounding factor as all of the smokers in the present study,
except for one, were male, and the sample size was relatively
small.
Stimulation by TRAb in the synthesis and secretion
of thyroid hormone is a direct cause of Graves' disease, and TRAb
is also a serum marker of Graves' disease. In the present study,
the TRAb level was correlated with the activity of eye disease and
may serve as a predictor of the response to methylprednisolone
therapy. Treatment had no significant effect on sICAM-1 levels in
the present study and sICAM-1 levels may increase during acute
stress, infection and other diseases besides TAO (30–34),
therefore sICAM-1 is not specific for TAO and cannot effectively
reflect the changes in TAO.
In conclusion, high-dose IVMP therapy is an
effective, safe and well-tolerated treatment for patients with TAO,
and is associated with few adverse effects and relapses. The
effects of IVMP therapy are stable, and some symptoms, including
diplopia, visual acuity, ocular motility and proptosis, may
continue to improve with time following treatment withdrawal.
Furthermore, it was demonstrated that serum TRAb levels are
correlated with the activity of TAO and may serve as a predictor of
the response to methylprednisolone therapy, whereas sICAM-1 levels
are not correlated. This research could provide some evidence for
the treatment of moderately-to-severely active TAO and selection of
IVMP schedules.
Acknowledgements
The present study was supported by the Special
Project of Health Industry Research of China (grant no.
201002002).
References
|
1
|
Wall JR and Lahooti H: Pathogenesis of
thyroid eye disease - does autoimmunity against the TSH receptor
explain all cases? Endokrynol Pol. 61:222–227. 2010.PubMed/NCBI
|
|
2
|
Bartley GB: The epidemiologic
characteristics and clinical course of ophthalmopathy associated
with autoimmune thyroid disease in Olmsted County, Minnesota. Trans
Am Ophthalmol Soc. 92:477–588. 1994.PubMed/NCBI
|
|
3
|
Brent GA: Clinical practice. Graves'
disease. N Engl J Med. 358:2594–2605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinsell LW, Partridge JW and Foreman N:
The use of ACTH and cortisone in the treatment and in the
differential diagnosis of malignant exophthalmos. Ann Intern Med.
38:913–917. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartalena L and Tanda ML: Clinical
practice. Graves' ophthalmopathy. N Engl J Med. 360:994–1001. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartalena L, Baldeschi L, Dickinson A,
Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P,
Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas GE,
Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce
S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G and
Wiersinga WM: European Group on Graves' Orbitopathy (EUGOGO).
Consensus statement of the European Group on Graves' orbitopathy
(EUGOGO) on management of GO. Eur J Endocrinol. 158:273–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khoo DH, Eng PH, Ho SC, Tai ES,
Morgenthaler NG, Seah LL, Fong KS, Chee SP, Choo CT and Aw SE:
Graves' ophthalmopathy in the absence of elevated free thyroxine
and triiodothyronine levels: Prevalence, natural history and
thyrotropin receptor antibody levels. Thyroid. 10:1093–1100. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khoo DH, Ho SC, Seah LL, Fong KS, Tai ES,
Chee SP, Eng PH, Aw SE and Fok AC: The combination of absent
thyroid peroxidase antibodies and high thyroid-stimulating
immunoglobulin levels in Graves' disease identifies a group at
markedly increased risk of ophthalmopathy. Thyroid. 9:1175–1180.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Bellis A, Di Martino S, Fiordelisi F,
Muccitelli VI, Sinisi AA, Abbate GF, Gargano D, Bellastella A and
Bizzarro A: Soluble intercellular adhesion molecule-1 (sICAM-1)
concentrations in Graves' disease patients followed up for
development of ophthalmopathy. J Clin Endocrinol Metab.
83:1222–1225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heufelder AE and Bahn RS: Soluble
intercellular adhesion molecule-1 (sICAM-1) in sera of patients
with Graves' ophthalmopathy and thyroid diseases. Clin Exp Immunol.
92:296–302. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dickinson AJ and Perros P: Controversies
in the clinical evaluation of active thyroid-associated
orbitopathy: Use of a detailed protocol with comparative
photographs for objective assessment. Clin Endocrinol (Oxf).
55:283–303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mourits MP, Koornneef L, Wiersinga WM,
Prummel MF, Berghout A and van der Gaag R: Clinical criteria for
the assessment of disease activity in Graves' ophthalmopathy: A
novel approach. Br J Ophthalmol. 73:639–644. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang DD, Gonzalez MO and Durairaj VD:
Medical management of thyroid eye disease. Saudi J Ophthalmol.
25:3–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wakelkamp IM, Baldeschi L, Saeed P,
Mourits MP, Prummel MF and Wiersinga WM: Surgical or medical
decompression as a first-line treatment of optic neuropathy in
Graves' ophthalmopathy? A randomized controlled trial. Clin
Endocrinol (Oxf). 63:323–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tambe K, Bhargava J, Tripathi A, Gregory
M, Burns J and Sampath R: The role of intravenous
methylprednisolone immunosuppression in the management of active
thyroid eye disease. Orbit. 29:227–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartalena L, Baldeschi L, Dickinson A,
Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P,
Boboridis K, Boschi A, et al: Consensus statement of the European
Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J
Endocrinol. 158:273–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zang S, Ponto KA and Kahaly GJ: Clinical
review: Intravenous glucocorticoids for Graves' orbitopathy:
Efficacy and morbidity. J Clin Endocrinol Metab. 96:320–332. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang TC, Kao SC, Hsiao YL, Lu CP, Huang
KM and Tzeng SS: Therapeutic responses to corticosteroids in
Graves' ophthalmopathy. J Formos Med Assoc. 95:833–838.
1996.PubMed/NCBI
|
|
19
|
Ohtsuka K, Sato A, Kawaguchi S, Hashimoto
M and Suzuki Y: Effect of steroid pulse therapy with and without
orbital radiotherapy on Graves' ophthalmopathy. Am J Ophthalmol.
135:285–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sánchez-Orgaz M, Grabowska A, Royo-Oreja
A, Asencio-Durán M, Romero-Martín R and Arbizu-Duralde A: Optic
neuropathy following orbital irradiation for Graves'
ophthalmopathy: A case report and literature review. Orbit.
31:30–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
European Group of Graves' Orbitopathy.
Perros P, Baldeschi L, Boboridis K, Dickinson AJ, Hullo A, Kahaly
GJ, Kendall-Taylor P, Krassas GE, Lane CM, Lazarus JH, et al: A
questionnaire survey on the management of Graves' orbitopathy in
Europe. Eur J Endocrinol. 155:207–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos HE, Diehl LA, Camacho CP, Perros P
and Graf H: Latin American Thyroid Society: Management of Graves'
orbitopathy in Latin America: An international questionnaire study
compared with Europe. Clin Endocrinol (Oxf). 69:951–956. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stiebel-Kalish H, Robenshtok E,
Hasanreisoglu M, Ezrachi D, Shimon I and Leibovici L: Treatment
modalities for Graves' ophthalmopathy: Systematic review and
metaanalysis. J Clin Endocrinol Metab. 94:2708–2716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gillespie EF, Smith TJ and Douglas RS:
Thyroid eye disease: Towards an evidence base for treatment in the
21st century. Curr Neurol Neurosci Rep. 12:318–324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hägg E and Asplund K: Is endocrine
ophthalmopathy related to smoking? Br Med J (Clin Res Ed).
295:634–635. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bartalena L, Martino E, Marcocci C,
Bogazzi F, Panicucci M, Velluzzi F, Loviselli A and Pinchera A:
More on smoking habits and Graves' ophthalmopathy. J Endocrinol
Invest. 12:733–737. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bahn RS: Graves' ophthalmopathy. N Engl J
Med. 362:726–738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prummel MF and Wiersinga WM: Smoking and
risk of Graves' disease. JAMA. 269:479–482. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YL, Chang TC and Chen CJ: Influence
of smoking on Graves' disease with or without ophthalmopathy and
nontoxic nodular goiter in Taiwan. J Formos Med Assoc. 93:40–44.
1994.PubMed/NCBI
|
|
30
|
Briassoulis G, Papassotiriou I, Mavrikiou
M, Lazaropoulou C and Margeli A: Longitudinal course and clinical
significance of TGF-beta1, sL- and sE-Selectins and sICAM-1 levels
during severe acute stress in children. Clin Biochem. 40:299–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou SL, Chen YL, Peng XZ, Zhao YX, Xie
PZ, Ling L, Chen XW and Dai KM: Expression of serum sICAM-1 in
patients with primary hepatocellular carcinoma and its relationship
with liver fibrosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
28:181–182. 2012.(In Chinese). PubMed/NCBI
|
|
32
|
de Pablo R, Monserrat J, Reyes E, Díaz D,
Rodríguez-Zapata M, de la Hera A, Prieto A and Álvarez-Mon M:
Circulating sICAM-1 and sE-Selectin as biomarker of infection and
prognosis in patients with systemic inflammatory response syndrome.
Eur J Intern Med. 24:132–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cha JJ, Hyun YY, Jee YH, Lee MJ, Han KH,
Kang YS, Han SY and Cha DR: Plasma concentration of soluble
intercellular adhesion molecule-1 (sICAM-1) is elevated in type 2
diabetic patients, and sICAM-1 synthesis is associated with
leptin-induced activation of the mitogen-activated protein kinase
(MAPK) pathway. Inflammation. 36:878–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dymicka-Piekarska V, Guzinska-Ustymowicz
K, Kuklinski A and Kemona H: Prognostic significance of adhesion
molecules (sICAM-1, sVCAM-1) and VEGF in colorectal cancer
patients. Thromb Res. 129:e47–e50. 2012. View Article : Google Scholar : PubMed/NCBI
|