Introduction
During intestinal ischemia-reperfusion (IR), mucosal
tissue damage is associated with cytokine release, increase in
intestinal mucosal permeability, translocation of endotoxins and
intestinal microflora. These can lead to multiple organ dysfunction
syndrome (MODS) (1,2). Inflammatory response of lower
intestinal mucosa is a crucial factor defining mucosal damage after
IR caused by major surgery or severe trauma (3).
Glutamine (Gln) is a conditionally essential amino
acid with a variety of biological functions. It is the key energy
substrate for intestinal epithelial cells and lymphocytes. It is
required to maintain proper mucosal barrier function as it promotes
cell differentiation and proliferation (4–6). To the
best of our knowledge, few studies have focused on the effects of
Gln on inflammatory cytokine production in intestinal mucosa after
IR.
In the present study, we established an animal model
of intestinal IR injury and tested the effects of Gln on mucosal
function as well as the high mobility group box 1 (HMGB1), tumor
necrosis factor-α (TNF-α), interleukin-1 (IL-1). The results showed
that Gln repaired the intestinal mucosal injury in IR by reducing
the expression of HMGB1 and inflammatory cytokines, and reduce the
permeability of intestinal mucosa.
Materials and methods
Animals
Animal model of IR
Forty-eight healthy male Sprague-Dawley rats were
obtained from the Experimental Animal Center of Tongji University
(Shanghai, China). The animals had an average weight of 150±12 g.
After feeding for 3 days, the animals were anesthesized and fixed
on a wooden board, and a ventral midline incision (3–4 cm long)
into the abdomen was performed. The mesenteric arteries were
identified and isolated, the initial part of the upper mesentery
artery was clamped with a non-damagebulldog clamp (Jinzhong Co.,
Shanghai, China) for 35 min, prior to the clamp being loosened to
re-perfuse for 2 h. This established a small intestinal IR
injury.
Animal groups
Animals were randomly divided into the control and
Gln groups (n=24 per group). The rats were fed separately in a
clean animal house with stainless steel cage. The control group
rats were fed vegan chow (Nutricia) supplemented with 3% soy
protein, whereas the Gln group animals were fed vegan chow
(Nutriciacompany, Paris, France) supplemented with 3% Gln. The
enteral nutrition of the two groups had the following calorie and
nitrogen supply: calorie 125.4 kJ/kg/day and nitrogen 0.2 g/kg/day.
Enteral nutrition supplemented with 3% Gln and 3% soybean protein
was given once. Prior to feeding enteral nutrition to rats, daily
disposable lavage, change of food intake and body weight were
measured.
Prior and after induction of IR injury (days 3 and 7
of the experiment), 6 rats were selected to obtain 5 ml of arterial
blood from the femoral artery. Blood was centrifuged for 10 min at
2,000 × g to collect serum, and then stored at −80°C. In addition,
small intestine 10 cm away from the ileocecal intestinal tissue was
also obtained to evaluate the levels of HMGB1, TNF-α and IL-1, and
for pathological analysis.
HMGB1 expression
HMGB1 expression was assessed using western blot
analysis, as previously described (7). The homogenates of intestinal mucosa
were prepared and supernatants were obtained and separated with
SDS-PAGE. The primary antibody, anti-HMGB1 antibody (catalog no.:
sc-191583; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
secondary antibody (Santa Cruz Biotechnology, CA, USA; catalog
no.:sc-395763) were subsequently applied. The gel image was
captured and bands were analyzed with quantity one image analysis
software (Bio-Rad, Berkeley, CA, USA).
TNF-α and IL-1 expression
A 2 cm specimen of small intestine proximal jejunum
was obtained. The specimen was rinsed with normal saline and
200–300 mg of intestinal mucosa were scraped off the jejunum. The
tissue homogenate were diluted with nine volumes of normal saline.
The specimen was centrifuged (800 × g for 10 min) and the
supernatant was obtained. The concentrations of TNF-α and IL-1 were
quantified with respective ELISAs (R&D Systems, Inc.,
Minneapolis, MN, USA). The results were expressed as pg cytokine/g
wet weight.
Plasma Gln TNF-α, IL-1, diamine oxidase (DAO) and
D-lactic acid
TNF-α and IL-1 were quantified by respective ELISAs
(R&D Systems, Inc.). The concentration of plasma D-lactic acid
was also calculated by ELISA (Sigma, St. Louis, MO, USA) whereas
the concentration of DAO was quantified spectrophotometrically
(Hitachi 7600 automatic biochemical analyzer; Hitachi, Tokyo,
Japan). Gln concentration was quantified by HPLC (7) (Waters, Milford, MA, USA).
Morphological changes
A 2-cm specimen of the proximal jejunum was
obtained. After paraffin embedding, tissue slices were prepared and
stained with hematoxylin and eosin. The slides were viewed under
magnification (×100), electron microscopy observation of small
intestinal villus and crypt structure organization allowed the
assessment of morphological changes.
Statistical analysis
SPSS 13.0 statististical software (SPSS, Inc.,
Chicago, IL, USA) was used to analyze the data. Quantitative data
were presented as mean ± SD and analyzed by the paired t-test.
Qualitative data were analyzed by the Chi-square test. P<0.05
indicated statistical significance.
Results
Expression of HMGB1 in intestinal
mucosa
Compared with basal values, the expression of HMGB1
intestinal mucosa was significantly (p<0.05) increased
immediately after, and on days 3 and 7 after IR (Table I). Animals treated with Gln showed a
significantly faster recovery of this parameter on day 7 after IR
(Table I).
 | Table I.Expression of HMGB1 protein was
examined in rat intestinal mucosal. |
Table I.
Expression of HMGB1 protein was
examined in rat intestinal mucosal.
| Group | n | Before modeling | After modeling | 3rd day | 7th day | P1 | P2 | P3 |
|---|
| Control | 24 | 0.13±0.01 | 0.93±0.08 | 0.79±0.05 | 0.85±0.06 | 0.034 | 0.026 | 0.035 |
| Gln | 24 | 0.13±0.01 | 0.87±0.07 | 0.72±0.04 |
0.58±0.03a,b | 0.023 | 0.032 | 0.029 |
Expression of nuclear factor-κB
(NF-κB) in intestinal mucosa
Compared with basal values, the percentage of
NF-κB-positive cells was markedly upregulated immediately after,
and on days 3 and 7 after IR injury (Table II). Similar to HMGB1 expression, the
animals treated with Gln showed a significantly (p<0.05 vs.
control group; Table II) faster
decrease in the number of NF-κB-positive cells on day 7.
 | Table II.Expression of NF-κB were examined by
immunohistochemitry in rat intestinal mucosal [n (%)]. |
Table II.
Expression of NF-κB were examined by
immunohistochemitry in rat intestinal mucosal [n (%)].
| Group | n | Before modeling | After modeling | 3rd day | 7th day | P1 | P2 | P3 |
|---|
| Control | 24 | 2 (8.3) | 18 (75.0) | 16 (66.7) | 15 (62.5) | 0.013 | 0.027 | 0.002 |
| Gln | 24 | 2 (8.3) | 17 (70.8) | 14 (58.3) | 9 (37.5)a,b | 0.019 | 0.038 | 0.032 |
Levels of IL-1 and TNF-α in plasma and
intestinal mucosa
Compared with before modeling, two groups after
modeling of blood plasma and intestinal mucosal IL-1 and TNF-α
level increased significantly (p<0.05). On the 3rd and 7th day
of the experiment, the control group of intestinal mucosa and serum
IL-1 and TNF-α level was significantly higher than the former
(p<0.05), while there was no statistical significance compared
with the after modeling (p<0.05). On the 3rd day of the
experiment, the Gln group of plasma and intestinal mucosal level of
IL-1 and TNF-α level was significantly higher than before
(p<0.05). Compared with the after modeling and control group, on
the 7th day of experiment, the Gln group of plasma and intestinal
mucosal IL-1, and TNF-α levels were significantly decreased
(p<0.05). There was no statistical significance compared with
other data (Table III).
 | Table III.Levels of TNF-α and IL-1 in rat plasma
and intestinal mucosa. |
Table III.
Levels of TNF-α and IL-1 in rat plasma
and intestinal mucosa.
|
|
| Plasma (ng/l) | Intestinal mucosa
(pg/g) |
|---|
|
|
|
|
|
|---|
| Group | n | TNF-α | IL-1 | TNF-α | IL-1 |
|---|
| Control | 24 |
|
|
| Before
modeling | 6 | 85.18±8.84 | 112.75±17.48 | 172.45±33.76 | 230.15±55.74 |
| After
modeling | 6 |
127.62±14.24a |
148.55±21.56a |
241.28±65.29a |
315.86±74.36a |
| The 3rd
day | 6 |
121.75±13.72a |
140.79±18.36a |
226.23±55.35a |
301.24±69.45a |
| The 7th
day | 6 |
113.83±11.69a |
141.78±20.65a |
214.76±54.82a |
298.37±65.33a |
|
P1 |
| 0.018 | 0.027 | 0.036 | 0.031 |
|
P2 |
| 0.025 | 0.021 | 0.042 | 0.028 |
|
P3 |
| 0.041 | 0.029 | 0.038 | 0.022 |
| Gln group | 24 |
|
|
|
|
| Before
modeling | 6 | 85.18±8.84 | 113.82±17.28 | 172.45±33.76 | 230.39±54.66 |
| After
modeling | 6 |
123.86±13.75a |
145.76±21.38a |
240.35±64.86a |
317.48±70.64a |
| The 3rd
day | 6 |
117.35±11.29a |
138.22±17.38a |
213.78±43.76a |
301.65±64.57a |
| The 7th
day | 6 |
92.76±9.42b |
131.65±14.57b |
184.53±42.16b |
237.26±56.81b |
|
P1 |
| 0.035 | 0.027 | 0.011 | 0.031 |
|
P2 |
| 0.036 | 0.039 | 0.032 | 0.030 |
|
P4 |
| 0.032 | 0.031 | 0.026 | 0.017 |
|
P5 |
| 0.025 | 0.028 | 0.039 | 0.022 |
Levels of Gln, D-lactic acid and DAO
in rat plasma
Compared with before modeling, the two groups after
modeling of rats plasma D-lactic acid and DAO levels were increased
significantly (p<0.05), while the plasma level of Gln was
decreased. On the 3rd day and the 7th day of the experiment, the
control group rats plasma D-lactic acid and DAO levels were
significantly higher than the before modeling, while the plasma
level of Gln below the before modeling was increased significantly
(p<0.05). There was no statistical significance compared with
other data (Table IV).
 | Table IV.Levels of Gln, D-lactic acid and DAO
in rat plasma. |
Table IV.
Levels of Gln, D-lactic acid and DAO
in rat plasma.
| Group | n | D-lactic acid
(mmol/l) | DAO (U/ml) | Gln (g/l) |
|---|
| Control | 24 |
|
|
|
| Before
modeling | 6 | 0.27±0.02 | 1.52±0.24 | 0.39±0.03 |
| After
modeling | 6 |
0.46±0.03a |
2.76±0.57a |
0.18±0.01a |
| The 3rd
day | 6 |
0.41±0.03a |
2.51±0.52a |
0.22±0.01a |
| The 7th
day | 6 |
0.53±0.05a |
2.47±0.55a |
0.21±0.03a |
|
P1 |
| 0.041 | 0.015 | 0.026 |
|
P2 |
| 0.029 | 0.034 | 0.042 |
|
P3 |
| 0.030 | 0.016 | 0.035 |
| Gln group | 24 |
|
|
|
| Before
modeling | 6 | 0.27±0.02 | 1.52±0.24 | 0.39±0.03 |
| After
modeling | 6 |
0.51±0.04a |
2.58±0.51a |
0.21±0.01a |
| The 3rd
day | 6 |
0.45±0.03a |
2.26±0.43a |
0.32±0.02b |
| The 7th
day | 6 |
0.31±0.02b |
1.76±0.34b |
0.40±0.03b |
|
P1 |
| 0.018 | 0.037 | 0.031 |
|
P2 |
| 0.032 | 0.025 | 0.032 |
|
P4 |
| 0.024 | 0.022 | 0.028 |
|
P5 |
| 0.037 | 0.036 | 0.032 |
Morphology of small intestinal
mucosa
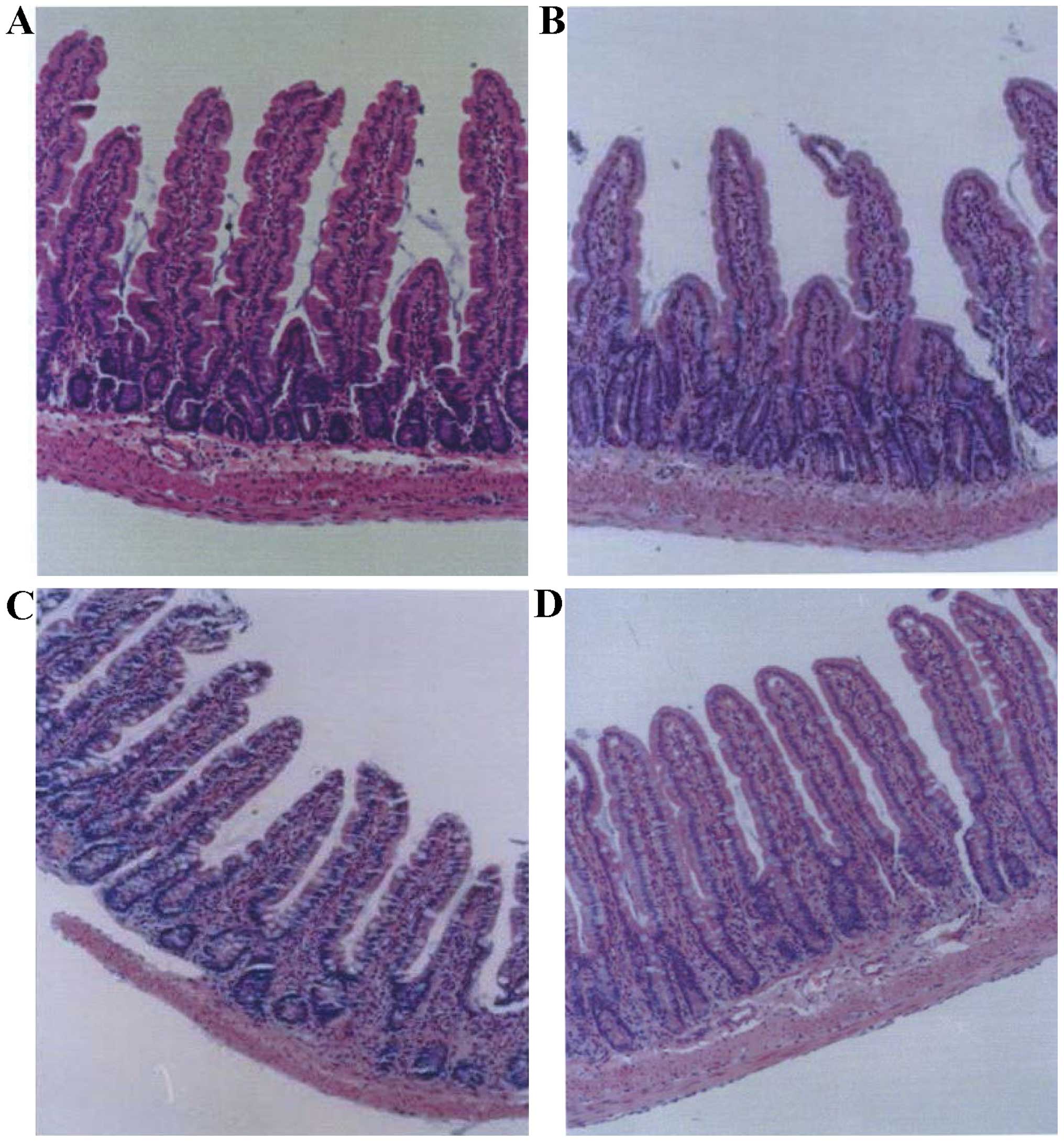
Basal small intestinal mucosa and crypt structure
was normal, with no obvious infiltration of inflammatory cells in
the lamina propria (Fig. 1A).
However, IR led to damage to the villi and crypt structure, and
shorter, thinner hairs (Fig. 1B).
Furthermore, the lamina propria exhibited a large number of
invading inflammatory cells, lymphangiectasia and edema (Fig. 1B). On day 7 after IR, the animals
treated with Gln showed a marked recovery of the structure of the
intestine structure and crypts, such that they became comparable to
the small intestinal mucosa at basal condition, and lamina propria
had a small amount of invading inflammatory cells (Fig. 1C). However, the control group showed
substantial morphological changes and marked inflammatory cell
infiltration on day 7 (Fig. 1D).
Discussion
Visceral blood flow reduction following surgical
trauma or shock is a common clinical phenomenon (8). It causes intestinal IR injury and
activates neutrophils to release inflammatory cytokines and free
radicals which cause tissue damage and affect intestinal mucosal
permeability (9). Gln is the main
energy material for intestinal mucosal rapidly dividing cells.
During IR, deficiency of Gln can occur (10). In the present study, we demonstrate
that animal IR is associated with lower levels of plasma Gln and
the upregulation of inflammatory. However, supplementation with Gln
led to increased recovery.
Gln is an important precursor of adenosine
triphosphate and adenosine monophosphate which are used to power
the intestinal mucosal cell metabolic oxidation (11,12).
Furthermore, Gln is the precursor to glutathione, an important
antioxidant that protects intestinal epithelial cells from oxidant
damage and inhibits intestinal mucosal apoptosis (13,14). Gln
can modulate inflammatory responses (15,16).
Additionally, Gln increases the cell oxygen uptake rate, enterocyte
mitochondrial respiratory function, and improves the intestinal
blood supply (17).
HMGB1 is a nucleoprotein present in almost all
eukaryotic nuclei and involved in the regulation of inflammatory
responses (18). When cells are
stimulated with microbial pathogens or inflammatory factors,
intracellular HMGB1 can be released into the extracellular
compartment to stimulate immune responses (19). This stimulation increases intestinal
mucosa monolayer permeability, facilitates bacterial translocation,
and allows the endotoxin from the gut lumen to reach inside the
body. We have demonstrated that Gln supplementation decreases the
expression of HMGB1 in the rat intestinal mucosa.
The same beneficial effect of Gln supplementation
was observed with respect to NF-κB. This transcription factor is
crucial for inciting and prolonging the inflammatory response
(20). Activation of this
transcription factor leads to upregulation of the production of
inflammatory cytokines, such as TNF-α and IL-1, which were
investigated in the present study. Downregulation of NF-κB
expression by Gln supplementation, as observed in the present
results, may be associated with reduced degradation of IκB, as
demonstrated in Gln-supplemented cells (21). IκB normally prevents NF-κB from
activation, thus, Gln beneficially modulated NF-κB in the present
study.
Supporting the involvement of NF-κB in
anti-inflammatory effects of Gln supplementation, TNF-α and IL-1,
initially upregulated by IR injury, were downregulated in
Gln-supplemented animals. Similar studies in the literature have
shown that, Gln exerts a protective effect on the barrier function
of intestinal mucosa during inflammatory insults (22). This can decrease exposure to gut
microflora or their virulence factors.
We also studied the kinetics of plasma D-lactate and
DAO levels as markers of intestinal membrane injury (23,10).
Notably, plasma D-lactate levels have been found to reflect the
permeability and barrier function of intestinal mucosa (24), whereas plasma DAO levels are
associated with intestinal mucosal epithelial cell injury and
repair (25,26). We observed that Gln supplementation
markedly decreased the levels of the aforementioned markers.
These beneficial changes in Gln-supplemented animals
were documented at day 7 after induction of the IR injury and were
supported by less pronounced morphological changes of the
intestinal mucosa. This is likely to indicate that inflammatory
responses during IR strongly contribute to the damage to the
intestinal mucosa, and that reducing the inflammatory response, as
in the case of Gln-supplemented animals, is a prerequisite to
preventing this damage to the intestinal mucosa.
Since the aforementioned beneficial changes of Gln
supplementation occurred after 7 days post-IR injury, we believe
that Gln facilitates cell recovery from the injury. In conclusion,
we have demonstrated that Gln supplementation exerts beneficial
anti-inflammatory effects in a rat model of IR injury and reduces
morphological changes in the intestinal mucosa after this injury.
This provides experimental evidence for the utilization of Gln
supplementation to facilitate recovery of patients with intestinal
IR injury.
Acknowledgements
The present study was supported by the Key Specialty
Construction Project of the Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZz2013-17).
References
|
1
|
Mura M, Andrade CF, Han B, Seth R, Zhang
Y, Bai XH, Waddell TK, Hwang D, Keshavjee S and Liu M: Intestinal
ischemia-reperfusion-induced acute lung injury and oncotic cell
death in multiple organs. Shock. 28:227–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He GZ, Dong LG, Chen XF, Zhou KG and Shu
H: Lymph duct ligation during ischemia/reperfusion prevents
pulmonary dysfunction in a rat model with ω-3 polyunsaturated fatty
acid and glutamine. Nutrition. 27:604–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collange O, Tamion F, Chanel S, Hue G,
Richard V, Thuilliez C, Dureuil B and Plissonnier D: D-lactate is
not a reliable marker of gut ischemia-reperfusion in a rat model of
supraceliac aortic clamping. Crit Care Med. 34:1415–1419. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wischmeyer PE: Glutamine: role in gut
protection in critical illness. Curr Opin Clin Nutr Metab Care.
9:607–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Songsasen N, Wesselowski S, Carpenter JW
and Wildt DE: The ability to achieve meiotic maturation in the dog
oocyte is linked to glycolysis and glutamine oxidation. Mol Reprod
Dev. 79:186–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnes JL, Hartmann B, Holst JJ and
Tappenden KA: Intestinal adaptation is stimulated by partial
enteral nutrition supplemented with the prebiotic short-chain
fructooligosaccharide in a neonatal intestinal failure piglet
model. JPEN J Parenter Enteral Nutr. 36:524–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padda RS, Gkouvatsos K, Guido M, Mui J,
Vali H and Pantopoulos K: A high-fat diet modulates iron metabolism
but does not promote liver fibrosis in hemochromatotic
Hjv−/− mice. Am J Physiol Gastrointest Liver Physiol.
308:G251–G261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen AD, Jiang YP and Fan YX: Using HPLC
fluorescence method for rapid detection of glutamine in human
plasma and muscle. Chromatography. 406–407. 1995.
|
|
9
|
Kim KH, Kuh SU, Park JY, Kim KS, Chin DK
and Cho YE: What is the importance of ‘halo’ phenomenon around bone
cement following vertebral augmentation for osteoporotic
compression fracture? Osteoporos Int. 23:2559–2565. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian R, Tan JT, Wang RL, Xie H, Qian YB
and Yu KL: The role of intestinal mucosa oxidative stress in gut
barrier dysfunction of severe acute pancreatitis. Eur Rev Med
Pharmacol Sci. 17:349–355. 2013.PubMed/NCBI
|
|
11
|
Chen X, Guan T, Li C, Shang H, Cui L, Li
XM and Kong J: SOD1 aggregation in astrocytes following
ischemia/reperfusion injury: a role of NO-mediated S-nitrosylation
of protein disulfide isomerase (PDI). J Neuroinflammation.
9:2372012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alves MA, Guimarães SB, Dias DA and
Vasconcelos PR, Coelho VP and Vasconcelos PR: Effects of
L-alanyl-glutamine upon the blood and kidney biochemical parameters
in the rat hind limb model of ischemia/reperfusion. Acta Cir Bras.
20:445–449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De-Souza DA and Greene LJ: Intestinal
permeability and systemic infections in critically ill patients:
effect of glutamine. Crit Care Med. 33:1125–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sözen S, Topuz O, Uzun AS, Cetinkünar S
and Das K: Prevention of bacterial translocation using glutamine
and melatonin in small bowel ischemia and reperfusion in rats. Ann
Ital Chir. 83:143–148. 2012.PubMed/NCBI
|
|
15
|
Deniel N, Marion-Letellier R, Charlionet
R, Tron F, Leprince J, Vaudry H, Ducrotté P, Déchelotte P and
Thébault S: Glutamine regulates the human epithelial intestinal
HCT-8 cell proteome under apoptotic conditions. Mol Cell
Proteomics. 6:1671–1679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh SL, Lai YN, Shang HF, Lin MT, Chiu WC
and Chen WJ: Effects of glutamine supplementation on splenocyte
cytokine mRNA expression in rats with septic peritonitis. World J
Gastroenterol. 11:1742–1746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kessel A, Toubi E, Pavlotzky E, Mogilner
J, Coran AG, Lurie M, Karry R and Sukhotnik I: Treatment with
glutamine is associated with down-regulation of Toll-like
receptor-4 and myeloid differentiation factor 88 expression and
decrease in intestinal mucosal injury caused by lipopolysaccharide
endotoxaemia in a rat. Clin Exp Immunol. 151:341–347. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filipp FV, Ratnikov B, De Ingeniis J,
Smith JW, Osterman AL and Scott DA: Glutamine-fueled mitochondrial
metabolism is decoupled from glycolysis in melanoma. Pigment Cell
Melanoma Res. 25:732–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naruse K, Sado T, Noguchi T, Tsunemi T,
Yoshida S, Akasaka J, Koike N, Oi H and Kobayashi H: Peripheral
RAGE (receptor for advanced glycation endproducts)-ligands in
normal pregnancy and preeclampsia: novel markers of inflammatory
response. J Reprod Immunol. 93:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hedl M and Abraham C: Nod2-induced
autocrine interleukin-1 alters signaling by ERK and p38 to
differentially regulate secretion of inflammatory cytokines.
Gastroenterology. 143:1530–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang J, Tae N, Min BS, Choe J and Lee JH:
Malabaricone C suppresses lipopolysaccharide-induced inflammatory
responses via inhibiting ROS-mediated Akt/IKK/NF-κB signaling in
murine macrophages. Int Immunopharmacol. 14:302–310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karatepe O, Acet E, Battal M, Adas G,
Kemik A, Altiok M, Kamali G, Koculu S, Catay A, Kamali S, et al:
Effects of glutamine and curcumin on bacterial translocation in
jaundiced rats. World J Gastroenterol. 16:4313–4320. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Websky M, Fujishiro J, Ohsawa I,
Praktiknjo M, Wehner S, Abu-Elmagd K, Kitamura K, Kalff JC,
Schaefer N and Pech T: The novel guanylhydrazone CPSI-2364
ameliorates ischemia reperfusion injury after experimental small
bowel transplantation. Transplantation. 95:1315–1323. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan ZY, Long CL and Wang H: Functional and
morphological structure changes in the gut barrier during
cholinesterase inhibitor intoxication and therapeutic effect of
benthiactzine in rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
22:197–200. 2010.(In Chinese). PubMed/NCBI
|
|
25
|
Cai C, Li W, Chen J, Li X and Chen S:
Diamine oxidase as a marker for diagnosis of superior mesenteric
arterial occlusion. Hepatogastroenterology. 59:155–158.
2012.PubMed/NCBI
|
|
26
|
Santos RG, Quirino IE, Viana ML, Generoso
SV, Nicoli JR, Martins FS, Nogueira-Machado JA, Arantes RM, Correia
MI and Cardoso VN: Effects of nitric oxide synthase inhibition on
glutamine action in a bacterial translocation model. Br J Nutr.
111:93–100. 2014. View Article : Google Scholar : PubMed/NCBI
|