Introduction
Nocardiosis is an rare, opportunistic bacterial
infection caused by Nocardia species that predominantly
affects the respiratory tract of immunocompromised patients
(1). Nocardia species are
Gram-positive, actinomycetes. The genus Nocardia includes
>80 species, of which >30 have been shown to cause disease in
humans. Lung nocardiosis is the most common type of nocardiosis,
although the infection can spread through the bloodstream to other
areas of the body (2). Due to the
rise in invasive surgical techniques, immunosuppressive therapies,
and acute respiratory distress syndrome, the incidence of
nocardiosis has been increasing (3–5).
The common clinical manifestation of pulmonary
nocardiosis include a cough and fever (6). In addition, 50% of pulmonary
nocardiosis cases are complicated by skin or intracranial
dissemination (6). Chest X-ray or
computed tomography (CT) imaging of the lungs typically show
pleural effusion, masses, infiltrates, cavities and nodules
(6). However, since its clinical
manifestations lack specificity, it is easily misdiagnosed, and
isolation and identification of Nocardia strains is
considered the only reliable diagnostic method. Treatment of
nocardiosis typically involves antibiotics: A previous study
demonstrated that Nocardia species were sensitive to
sulfonamide, amikacin, cefotaxime, ceftriaxone, minocycline,
fluoroquinolones and linezolid (6).
The present study aimed to improve the understanding
of lung nocardiosis by assessing two cases of lung nocardiosis in
patients admitted to the Beijing Shijitan Hospital (Beijing,
China), and by carrying out a review of the literature on infection
with Nocardia. The present study was approved by the Medical
Ethics Commitee of Beijing Shijitan Hospital, Capital Medical
University (Beijing, China). Written informed consent was obtained
from the patients.
Case reports
Case 1
The first patient was a 52-year-old male who
presented with paleness, a feeling of tiredness for 6 months and a
fever for 7 days. The patient was admitted to the Beijing Shijitan
Hospital on July 5th, 2011 with no history of medical illnesses.
Blood tests showed that the white blood cell (WBC) count was
4.09×109/l (normal range, 3.5–9.5×109/l), the
blood platelet (PLT) count was 68×109/l (normal range,
125–350×109/l), and the hemoglobin (Hb) levels were 73
g/l (normal level, 130–175 g/l). Results of an indirect
immunofluorescence assay (EUROBlotMaster II; EUROIMMUN Medical
Diagnostics (China) Co., Ltd., Beijing, China) were positive for
anti-nuclear antibodies (1:160; speckled pattern) and negative for
anti-extractable nuclear antigen antibodies. The bone marrow biopsy
was normal. Considering the high probability of immune-associated
hematocytopenia, the patient was treated with 40 mg/day oral
prednisone (Zhejiang Xianju Pharmaceutical Co., Ltd., Xianju,
China) once daily and discharged from the hospital on August 25th,
2011.
After 2 months, the routine blood tests of the
patient showed no improvement in WBC count, and the patient was
subsequently treated with prednisone combined with 400 mg/day
ciclosporin A (North China Pharmaceutical Co., Ltd., Shijiazhuang,
China). After 4 months, no improvement in WBC counts were observed.
The patient was admitted again to the hospital presenting with
fever (maximum temperature of 40°C) that had lasted for several
days. There was no rigor, cough, expectoration, dyspnea or
diarrhea. The patient felt progressively more tired and had a poor
appetite. When no changes were observed following treatment with
anti-infective therapy (0.5 g oral levofloxacin once daily; Daiichi
Sankyo Pharmaceutical Co., Ltd., Shanghai, China) for 7 days, the
patient was discharged and visited the hospital as an
outpatient.
On visiting the hospital as an outpatient, the
temperature of the patient was 38.6°C. The patient was malnourished
and his mucocutaneous zone was slightly pale, with no yellowing or
cyanosis. The patient's breathing sounded harsh, but there were no
rhonchus, rales or pleural friction sounds. The heart and abdomen
were normal.
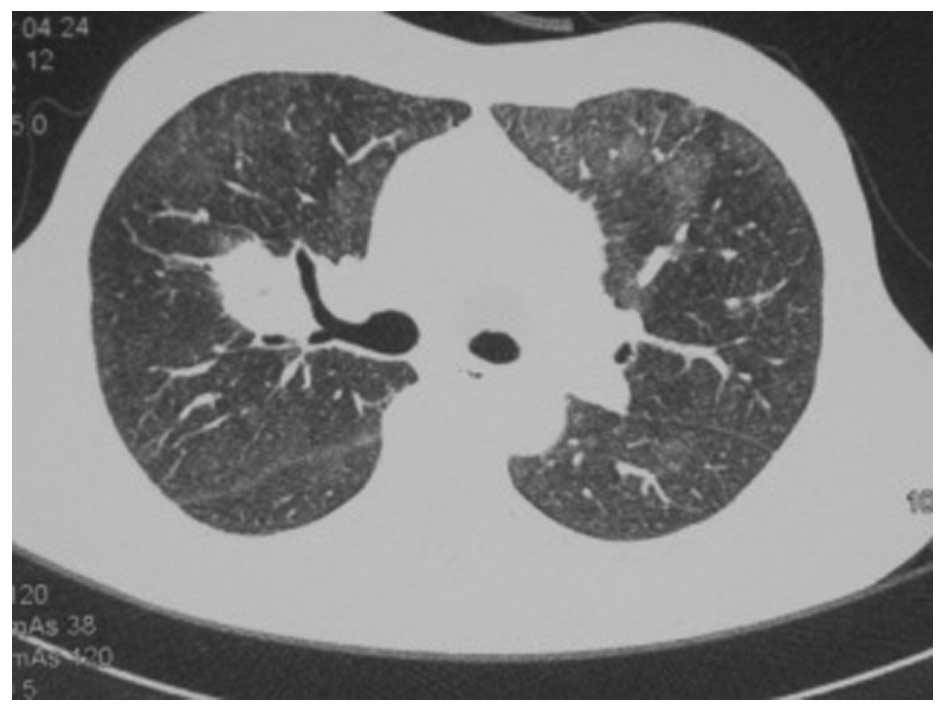
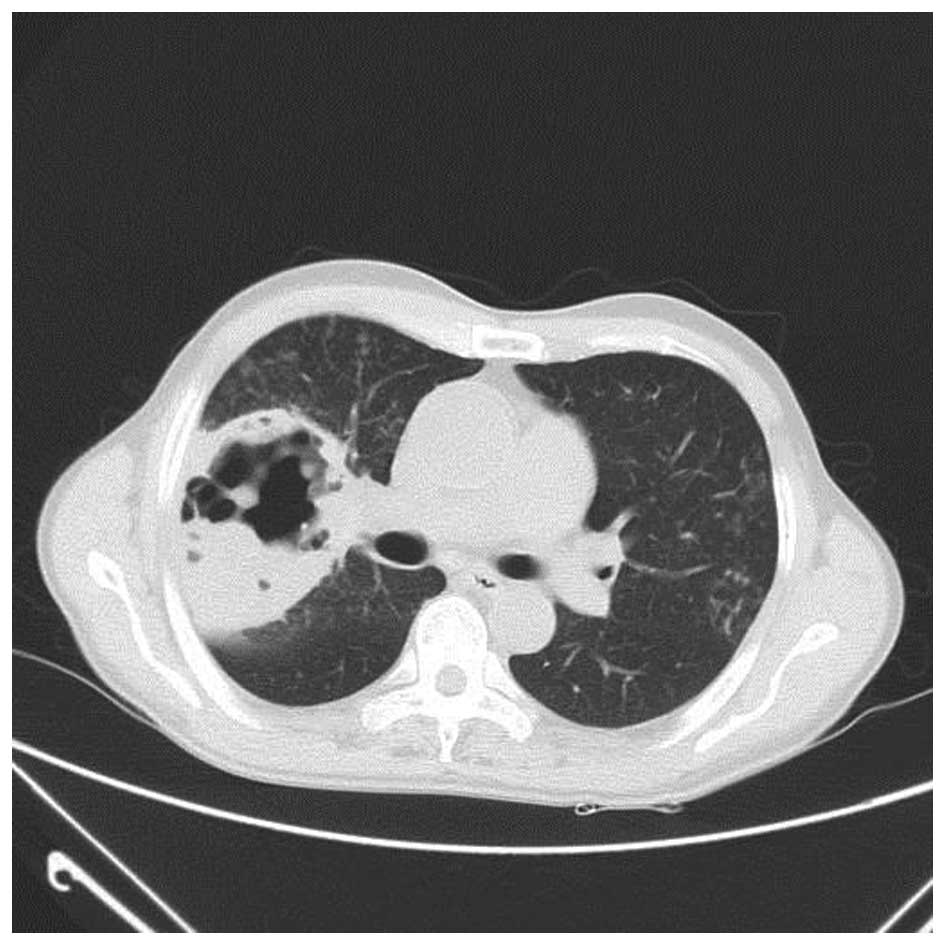
A chest CT scan (Fig.
1) showed a shadow of effusion and consolidation. Following
admission, routine blood tests showed that the WBC count was
6.3×109/l, red blood cell (RBC) count was
1.64×1012/l (normal range, 4.3–5.8×1012/l),
Hb levels were 50 g/l, PLT count was 45×109/l, and serum
tests showed that albumin (ALB) levels were 31.1 g/l (normal range,
40–55 g/l), lactate dehydrogenase levels were 896 U/l (normal
range, 40–240 U/l) and serum C-reactive protein (CRP) levels were
137 g/l (normal range, 0–5 g/l). Results of the tumor marker test,
hepatitis B virus (HBV) test (Abbott Trading Shanghai Co., Ltd.,
Shanghai, China), tubercle bacillus antibody test (Mp Biomedicals
Asia Pacific Pte., Ltd., Singapore, purified protein derivative
(PPD; used to diagnose latent tuberculosis) test (5 TU; Chengdu
Institute of Biology, Chinese Academy of Sciences, Sichuan, China)
and blood and sputum culture tests were negative.
Considering that the patient most likely suffered
from a bacterial and fungal infection as a result of long-term use
of glucocorticoids and immunosuppressive agents, the patient
received once daily (q.d.) intravenous (IV) administration of 400
mg fluconazole (Beit Lunan Pharmaceutical, Co., Ltd., Shandong,
China), 20 mg amphotericin B (Shanghai Asia Pioneer Pharmaceuticals
Co., Ltd., Shanghai, China) and 0.4 g moxifloxacin (Bayer,
Shanghai, China), and twice daily (b.i.d.) IV injection with
ceftizoxime (Shenzhen Zhijun Pharmaceutical, Co., Ltd., Guangzhou,
China). The temperature of the patient fluctuated between 37.5 and
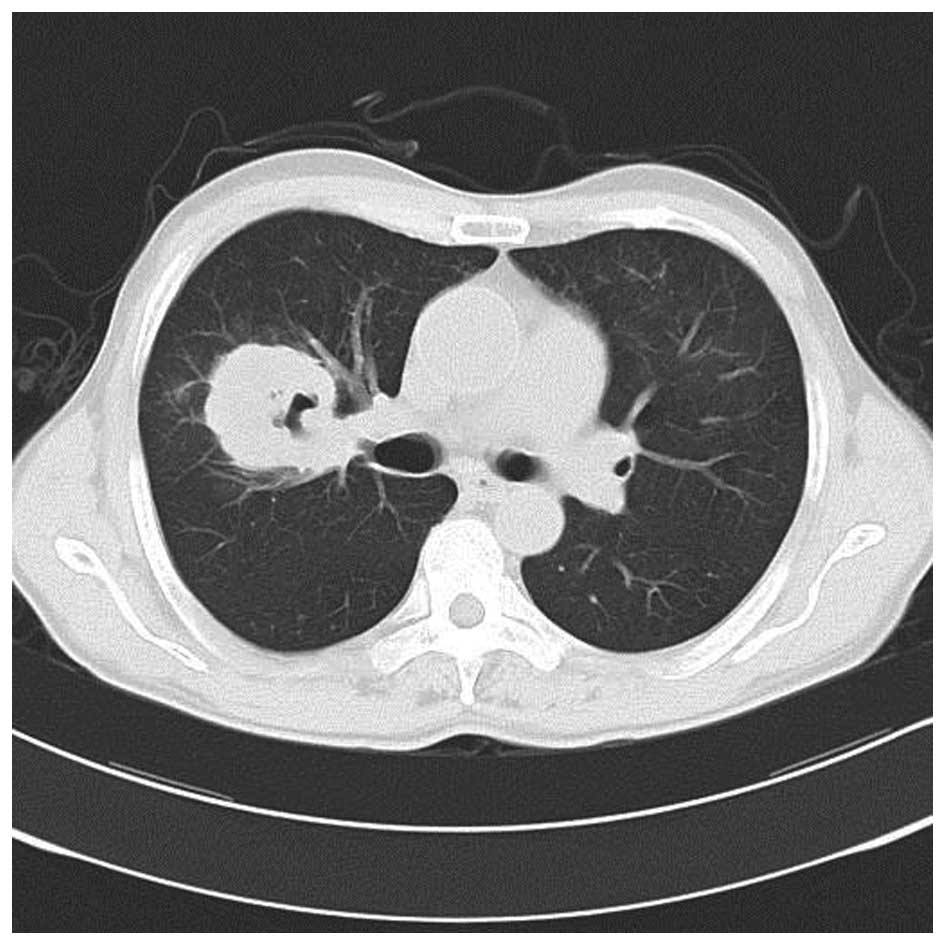
38.5°C. A subsequent chest CT scan revealed that the sheet shadow
in the upper right lung had enlarged and contained cavitation
(Fig. 2). Routine blood tests
demonstrated that the WBC count was 5.3×109/l,
neutrophils were at 75.7% (normal range, 40–75%), RBC count was
2.63×1012/l, Hb levels were 83 g/l and PLT count was
43×109/l; therefore, the treatment was changed to IV
injection with 4.5 g piperacillin (Shijiazhuang Pharmaceutical
Group, Co., Ltd., Shijiazhuang, China) and tazobactam (CSPC
Zhongnuo Pharmaceutical, Co., Ltd., Shijiazhuang, China) b.i.d. The
patient presented with a high fever (maximum temperature of 40.2°C)
on July 22nd, 2011. The patient also presented with dual ankle pain
and an occasional cough with expectoration, in the absence of any
rigor. The sputum culture showed Nocardia asteroides and the
treatment was adjusted as follows: Piperacillin and tazobactam
combined with 0.96 g b.i.d. oral sulfamethoxazole (Beijing Shuguang
Pharmaceutical Factory, Xian, China) and 200 mg b.i.d. oral
voriconazole (Pfizer Deutschland GmbH, Berlin, Germany).
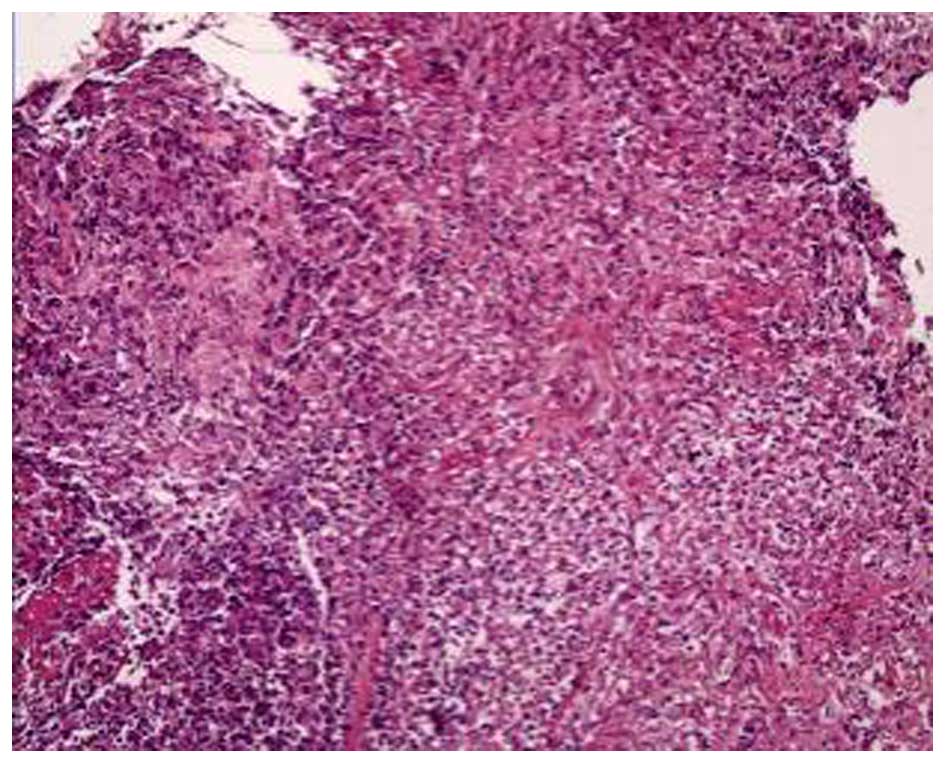
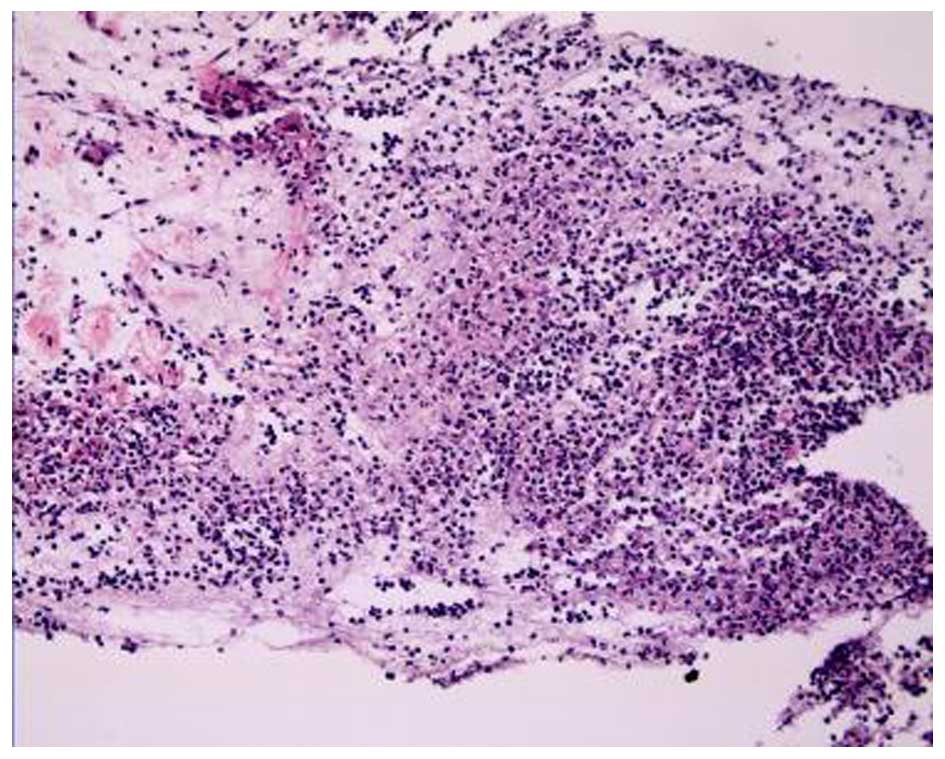
A bronchoscopy revealed that the bronchial mucosa in
the apicoposterior segment of the upper right lung lobe was
slightly congested and edematous, and contained yellow purulent
secretions. Following irrigation, there was no hemorrhage or
neoplasm in the bronchial lumen. The biopsy results indicated some
epithelioid cell granuloma, small-foci infarction, and nuclear
fragmentation in the tissue (Fig.
3). A subsequent chest CT scan showed progressive pneumonia and
that the shadow of consolidation had markedly enlarged and a cavity
had formed (Fig. 4).
After reviewing the results of the drug sensitivity
tests, treatment was changed to IV injection with 3.0 g
cefoperazone and sulbactam (Pfizer Deutschland GmbH) once every 12
h (q12h) and 0.96 g four times a day (q.i.d.) oral sulfamethoxazole
for 4 days, followed by 500 mg q12h imipenem and cilastatin
(Hangzhou MSD Pharmaceutical Co., Ltd., Hangzhou, China) combined
with 0.96 g q.i.d. oral sulfamethoxazole. The temperature of the
patient fluctuated between 36.2 and 39.2°C, and his cough and
expectoration did not improve. The patient received repeated
bronchoscopy examinations and bronchoalveolar lavage, after which
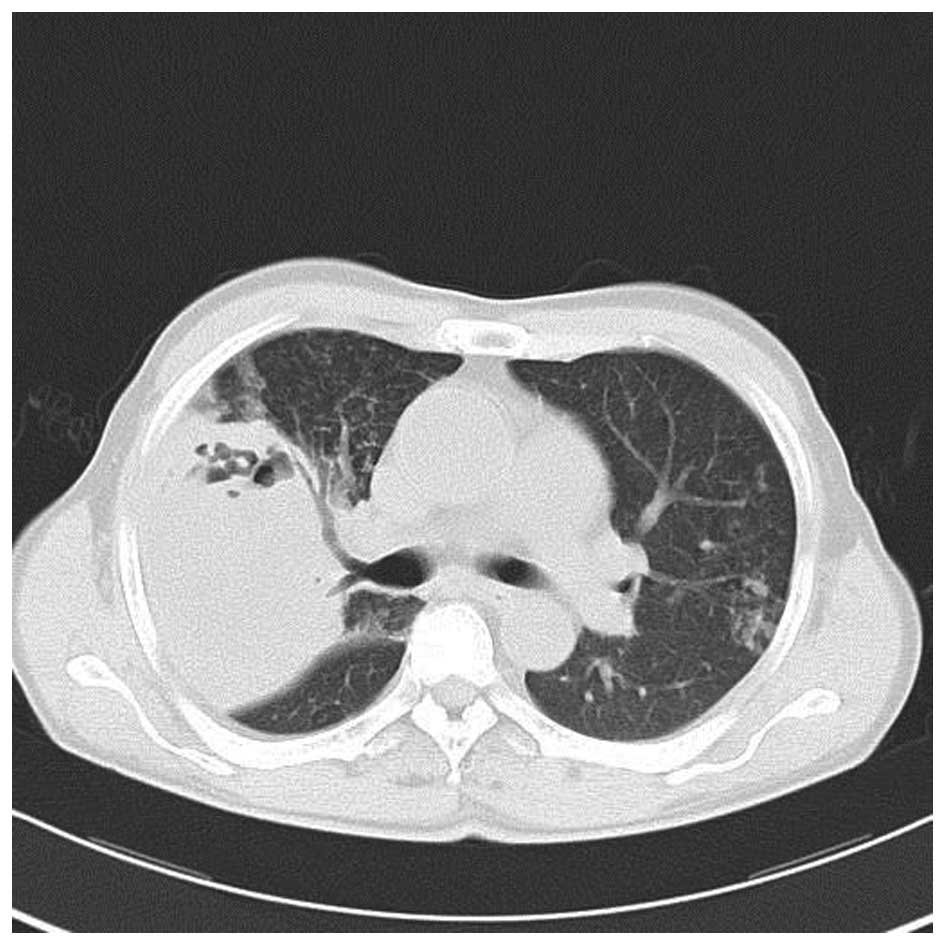
his temperature gradually returned to normal. After 11 days of
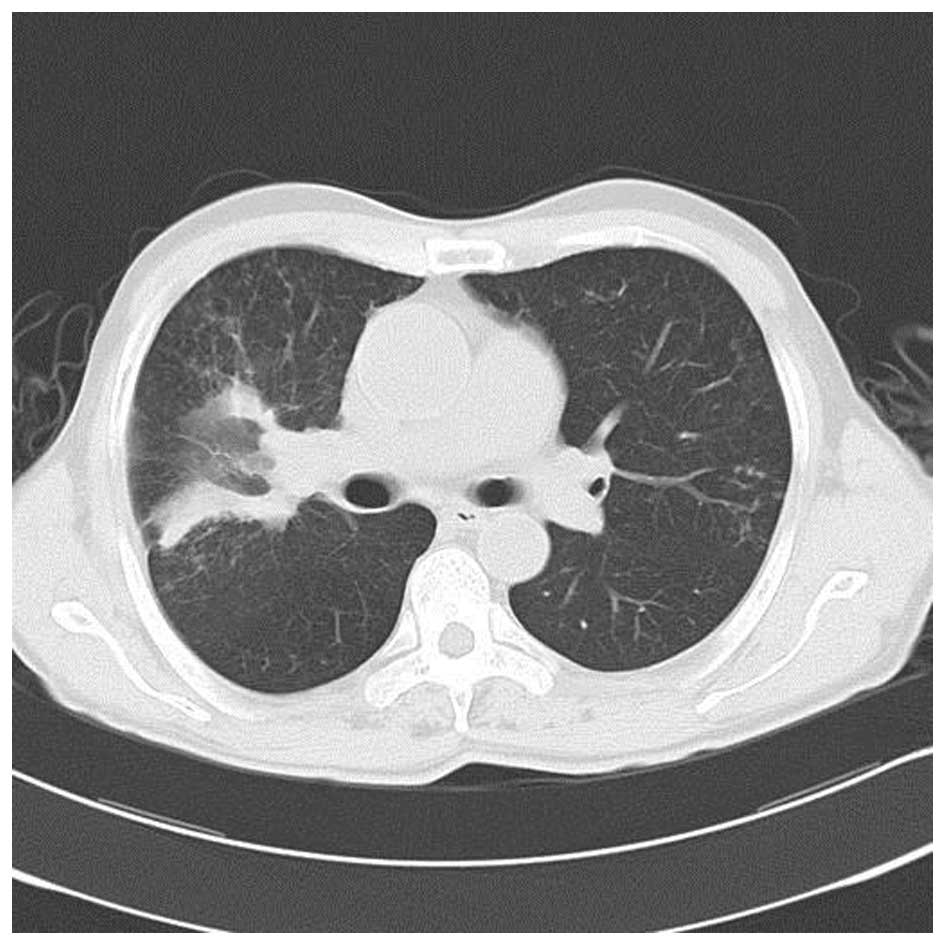
treatment, the chest CT scan revealed the large mass in the upper
right lung lobe had decreased with fewer cavities in it and that
the pneumonia had improved (Fig. 5).
The patient continued treatment with 0.96 g three times a day
(t.i.d.) oral sulfamethoxazole and 0.1 g t.i.d. oral cefdinir
(Jinkang Pharmaceuticals, Co., Ltd., Tianjin, China) following
discharge from the hospital on August 25, 2011. One month follow-up
following discharge, the patient had no fever, cough, or
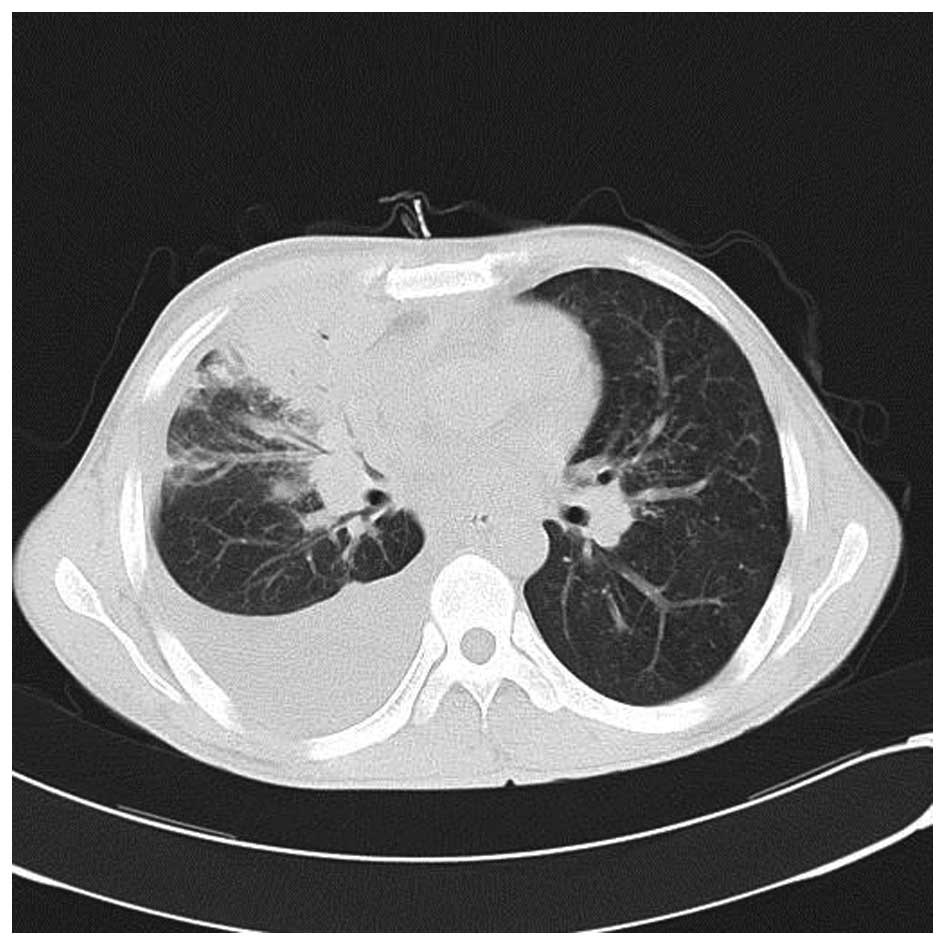
expectoration. Another chest CT scan showed that the lesion in the
right lung had been markedly resorbed (Fig. 6).
Case 2
The second patient was a 37-year-old male who
complained of having an intermittent cough and expectoration for
>1 month. The patient also located a cervical neck mass during
that time. The patient was admitted to the Beijing Shijitan
Hospital on July 3rd, 2014 with no history of previous medical
illnesses, although he had been exposed to occupational dust as a
carpenter for 3 years.
The patient suffered from a cough and had a small
amount of white sputum without obvious inducement prior to the
intermittent cough and heavier expectoration 1 month later. There
was no fever, night sweats, fatigue, chest tightness or chest pain.
The patient subsequently located a 4×6 cm mass on the right side of
his neck. A neck ultrasound revealed a hyperechoic mass with no
echo in the right side of the neck. Within a few days, the patient
felt a progressive increase in the size of the mass and noticed
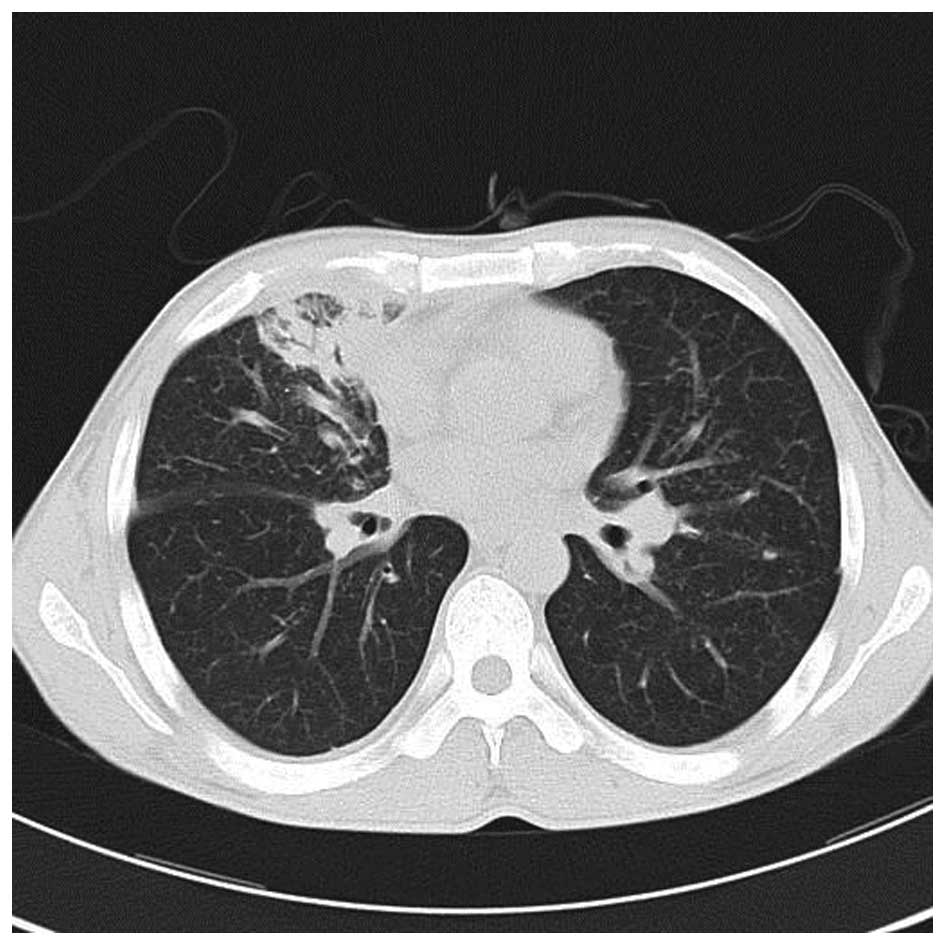
yellow phlegm in his sputum. A chest CT scan showed central
occupying lesions in the upper and the middle right lung with
obstructive pneumonia. The lesions were considered to be malignant
and had metastasized into the bilateral lungs. The scan showed
cancerous lymphangitis, multiple lymph node metastasis and large
amounts of pleural effusion in the right lobe (Fig. 7). Thoracentesis was performed and a
biopsy of the neck mass was conducted, guided by ultrasound. The
fluid culture showed growth of Nocardia, and the biopsy
showed inflammatory granuloma and abscesses (Fig. 8). Bronchoscopy revealed only a few
ciliated pseudostratified epithelial cells, RBCs, and individual
segmented cells. The patient received treatment as follows: A total
of 2 g b.i.d. IV drip cefminox (Meiji Seika Kaisha, Ltd., Tokyo,
Japan) for 10 days and 2 tablets b.i.d. sulfamethoxazole
trimethoprim (Beijing Double-Crane Pharmaceutical, Co., Ltd.,
Beijing, China) orally for 2 days. Following treatment, the neck
mass was only marginally reduced.
The temperature of the patient was 36.5°C, pulse was
75 bpm, respiration was 20 bpm, and blood pressure was 125/75 mmHg.
The skin and mucous membranes were not pale or yellow. The patient
had a 4×6 cm mass on the right side of the neck, abnormal skin
color, and elevated skin temperature. The left lung sounded clear,
whereas the right breath sound was lower than the left, although
the bilateral lung sounds indicated no rhonchus, bubbles or pleural
friction. The heart rate was 75 bpm, with a normal heart rhythm and
no cardiac murmur. The abdomen of the patient was soft but not
tender.
Following admission to the Beijing Shijitan
Hospital, routine blood tests revealed that the WBC count was
8.91×109/l, neutrophil count was 80.4%, Hb levels were
88 g/l, and PLT count was 45×109/l. Serum tests
demonstrated that the ALB levels were 32.6 g/l, the alkaline
phosphatase levels were 288 U/l (normal range, 45–125 U/l), and
γ-glutamyl transferase levels were 90 U/l (normal range, 10–60
U/l). Serum CRP levels were 98 g/l, the erythrocyte sedimentation
rate was 77 mm/h (normal, <20 mm/h) and D-dimer levels were
2,276.0 ng/ml (normal, <243 ng/ml). The 1,3-β-D glucan detection
test (Beijing Jin Shanchuan Technology Development, Co., Ltd.,
Beijing, China), HBV test, tubercle bacillus antibody test, PPD
test (5 TU) and sputum culture were negative. Pleural fluid
examination showed that the Reye's reaction was positive, specific
density was 1.025, WBC count was 1.8×109/l, multinucleated cells
were at 9% and monocytes were at 91%. The patient then received an
abdominal CT enhanced scan that showed multiple metastases in the
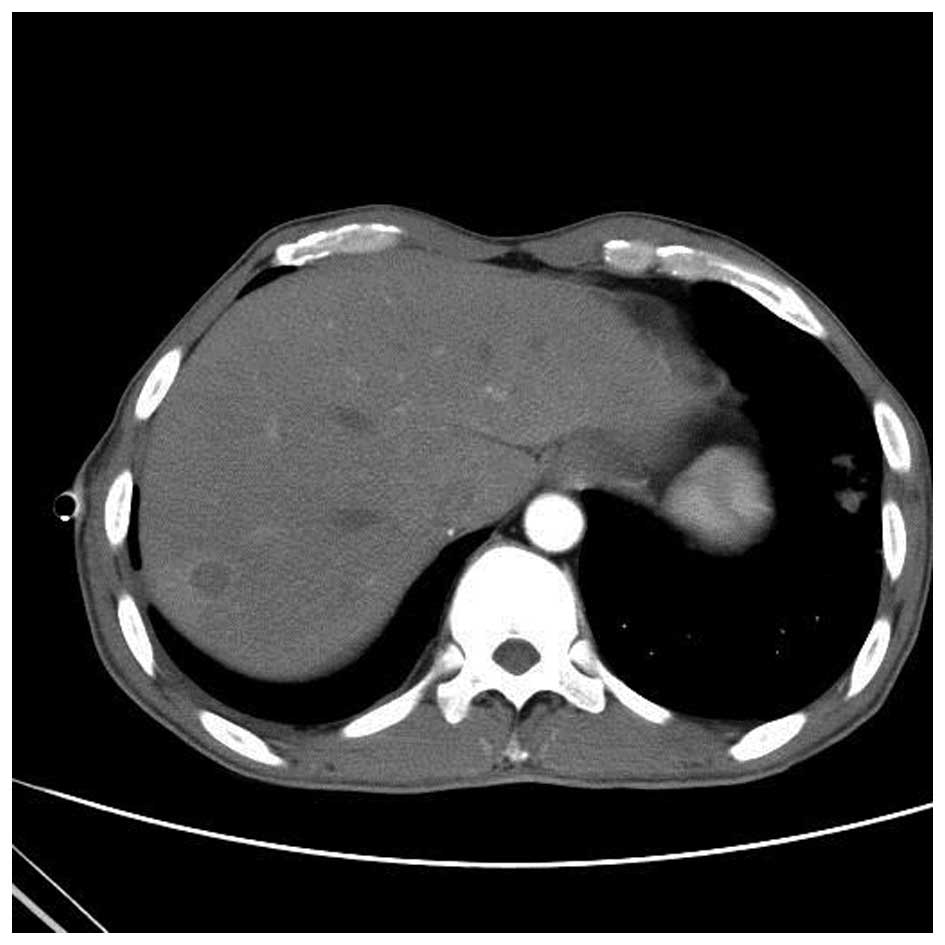
right lobe of the liver (Fig. 9),
indicating that the liver was involved in the disease. The final
diagnosis was disseminated Nocardia infection (involving the
skin, lung, liver and mediastinum).
Following second admission to the Beijing Shijitan
Hospital, the patient was administered two tablets b.i.d. oral
sulfamethoxazole trimethoprim combined with 2 g q.i.d. IV drip
ceftriaxone (Bristol-Myers Squibb Pharmaceutical, Co., Ltd., New
York, NY, USA). The neck mass was reduced and the patient's
temperature returned to normal. Another chest CT scan revealed that
the lesions in the upper and the middle right lung had markedly
resorbed (Fig. 10).
Discussion
Nocardia are Gram-positive, slow growing,
aerobic filamentous bacteria that are widely distributed in the
soil. Nocardia belong to the Actinobacteria class (2). The morphology of Nocardia is
similar to that of Actinomyces israelii; however, the end of
the hyphae do not show coliform dilatation, and Nocardia are
weakly positive for modified Kinyoun's acid-fast staining. The
hyphae is able to agglomerate to form actinomycetic grains. To
date, no Nocardia infections have been reported to spread
between humans; therefore, nocardiosis is considered an acquired
infection that spreads predominantly through the respiratory tract
(7). Occasionally, nocardiosis can
cause hematogenous dissemination (7), but it is rare that soft tissue
infection is caused directly by cutaneous injury (2). The prevalence of nocardiosis has
specific seasonal variations and geographical distribution
(8).
Of the cases of nocardiosis, 30% are caused by
opportunistic bacteria, which demonstrates that nocardiosis is an
exogenous and opportunistic infection (9). The most common bacterial species
associated with human nocardiosis are N. asteroides
and Nocardia brasiliensis (2), and N. asteroides in particular
(10). Impaired cell-mediated
immunity is an important risk factor for nocardiosis, and the
majority of patients with the infection have chronic diseases or
abnormal immune function (11). A
previous study reported that >50% of patients with nocardiosis
were immunocompromised from various conditions (12). Common causes of nocardiosis were
chronic pulmonary diseases, including chronic obstructive pulmonary
diseases, human immunodeficiency virus (HIV) infections (2), neoplastic disease, diabetes mellitus
(10), alcohol abuse, use of
systemic corticosteroids and use of immunosuppressive agents
(13,14). Case 1 in the present study had a long
history of systemic corticosteroid and immunosuppressive drug use,
both of which are risk factors for nocardiosis.
Clinical manifestations of Nocardia lack
specificity (15). In China, 68.8%
of patients with nocardiosis have pneumonia in the early stages
(9). Lung nocardiosis often
manifests as chest pain, coughing, sputum, dyspnea, fatigue and
loss of appetite (9). Some patients
present with hemoptysis (16) or
subacute pneumonia (7). In a
previous study, non-HIV-infected patients with lung nocardiosis
presented with elevated WBC counts and neutrophil ratios (17). Endobronchial nocardiosis is less
common, and it is difficult to distinguish lung Nocardia in
patients with normal immune function but with pneumonia and lung
abscesses (17). According to a
previous report, Nocardia in the lungs of patients with
normal immune function is associated with bronchial stone disease
(18). A previous study demonstrated
that 50% of lung nocardiosis infections were able to spread to
extrapulmonary tissues, predominantly via the bloodstream but also
through the lymphatic system (19).
When hematogenous dissemination occurs, patients may present with
brain abscesses, subcutaneous abscesses, pericarditis or other
symptoms (10,16). Case 2 in the present study lacked
typical respiratory symptoms upon examination, and presented with
only a mild cough and a small amount of white phlegm.
The imaging characteristics of lung nocardiosis in
both cases lacked specificity, which means the disease is
pleomorphic. The most common imaging characteristics are pulmonary
opacities, nodules and/or a mass, and some patients may have
symptoms combined with pleural effusion (9). The pathological manifestations of
nocardiosis are pyogenic or necrotic changes, and the typical
pathological change is liquefaction necrosis with abscess
formation; therefore, low-density areas or cavities often appear in
the pulmonary opacities and nodules, which is an important
manifestation that is also characteristic of bacterial pneumonia
(20). Consistent with a previous
report (13), the most common CT
manifestations of pulmonary nocardiosis in the present study were
air-space consolidation and nodules. The patients complained of
only a fever without a cough and sputum during the course of the
disease, although chest radiographic imaging showed pulmonary
opacities, predominately present in the right lung, that gradually
progressed into large areas of consolidation with cavity formation
to a diameter of 15 cm. These characteristics were not consistent
with the typical pathological manifestations. The initial treatment
strategy had no positive effect on the patient and his condition
worsened. Following treatment with an effective antibiotic therapy
together with physical therapy, the patient did not show any signs
of the disease being disseminated throughout the body via the
bloodstream.
The clinical manifestations of lung nocardiosis lack
specificity, which leads to misdiagnosis. Testing for pathogens in
order to positively identify Nocardia is the only method for
accurate diagnosis of this disease (21). Any of the following protocols can be
used as test specimens: Sputum, pus, pleural effusion, puncture
fluid, bronchoalveolar lavage fluid or drainage from a pulmonary
abscess (9). Nocardia species
are aerobic bacteria that are able to grow into visible colonies
within 2–6 days in common medium at 37°C and under aerobic
conditions using CO2 (9).
Nocardia bacteria grow slowly; therefore, if the required
time frame of 4 weeks is not provided to culture the bacteria, it
can remain undetected. Use of the correct medium, prolonged culture
time, and multiple cultures may improve the rate of positive
results (22). In case 1, when there
was no improvement following anti-infection treatment, the patient
was given several bronchoscopy tests. The results from the sputum
cultures revealed an astro-nocardiosis infection; these cultures
had an important role in timely clinical treatment.
The treatment of nocardiosis should include the use
of specific antibiotics, incision and drainage, surgical excision
of the lesion and protocols to improve the immune system of the
host (23,24). The selection of a specific treatment
depends on numerous factors, including the host, severity of
disease, lesion site, immune status and toxicity of the drug to
organs (9). Sulfa drugs are the best
choice for treating nocardiosis (2).
The advantage of sulfa drugs is their high oral bioavailability,
improved penetration and improved clustering in the lungs and
central nervous system (6). Patients
who are allergic to sulfa drugs, cannot tolerate them, or suffer
toxic reactions from them, can choose amoxicillin-clavulanate
potassium or minocycline as alternatives (25). A previous study demonstrated that
Nocardia are sensitive not only to the above-mentioned
drugs, but also to amikacin, ceftriaxone, and imipenem (2). For patients with pulmonary or skin
nocardiosis, sulfa drug monotherapy is highly effective; however,
for patients with severe immune suppression, such as organ
transplant patients or patients with systemic nocardiosis,
combination therapy with a polyantibiotic is suggested, such as a
combination of imipenem with third-generation cephalosporins or
amikacin (7,16). In vitro drug-sensitivity tests
have demonstrated that Nocardia are also sensitive to
linezolid (26), although clinical
data on this is lacking.
Total mortality rates from Nocardia
infections are high (31%) (27), and
the in-hospital mortality rate is ~20% (16). The prognosis is closely associated
with the diagnosis and treatment time frame, the severity of the
underlying condition of the patient, and whether there is
dissemination through the bloodstream. The important predictive
factors of a poor prognosis are the early and frequent use of
systemic corticosteroids and systemic infection (5). The majority of patient mortalities
occur as a result of disseminated systemic infections, brain
abscesses, or infections resulting from bacterial strains resistant
to sulfa drugs (16). The early
isolation and identification of Nocardia strains, and the
timely and effective treatment of nocardiosis with sulfa drugs, may
help to reduce patient mortality.
In summary, nocardiosis is an exogenous and
opportunistic bacterial infection. The lungs are the most common
target of nocardiosis and patients with hypoimmunity are more
susceptible to infection (1). The
clinical manifestations and image characteristics of pulmonary
nocardiosis lack specificity (15),
such that the disease may be misdiagnosed as other infections,
including tuberculosis, bacterial pneumonia, lung abscesses, and
pulmonary aspergillosis. The following patients are highly
susceptible to nocardiosis and should be regularly tested: i)
Patients with compromised immunity, such as HIV infection; ii)
organ transplant recipients; iii) patients with long-term history
of systemic corticosteroids or immunosuppressive agent use; iv)
patients with tumors following chemotherapy; v) patients with
chronic lung disease, diabetes or other chronic diseases; and vi)
patients with a pulmonary infection following conventional
anti-infective therapy, anti-tuberculosis treatment or anti-fungal
therapy, which is complicated by lesions in the central nervous
system, soft tissue or skin (28).
Antibiotic therapy combined with aspiration and drainage by
bronchoscopy is an effective treatment strategy for pulmonary
nocardiosis. It is important to improve the recognition and
understanding of Nocardia infection to reduce misdiagnosis,
implement an effective and timely anti-infection therapy and
decrease the mortality rates of nocardiosis.
References
|
1
|
BrownElliott BA, Brown JM, Conville PS and
Wallace RJ Jr: Clinical and laboratory features of the Nocardia
spp. based on current molecular taxonomy. Clin Microbiol Rev.
19:259–282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
King AS, Castro JG and Dow GC: Nocardia
farcinica lung abscess presenting in the context of advanced HIV
infection: Spontaneous resolution in response to highly active
antiretroviral therapy alone. Can J Infect Dis Med Microbiol.
20:e103–e106. 2009.PubMed/NCBI
|
|
3
|
Tuo MH, Tsai YH, Tseng HK, Wang WS, Liu CP
and Lee CM: Clinical experiences of pulmonary and bloodstream
nocardiosis in two tertiary care hospitals in northern Taiwan,
2000-2004. J Microbiol Immunol Infect. 41:130–136. 2008.PubMed/NCBI
|
|
4
|
Abreu C, RochaPereira N, Sarmento A and
Magro F: Nocardia infections among immunomodulated inflammatory
bowel disease patients: A review. World J Gastroenterol.
21:6491–6498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu BQ, Zhang TT, Zhu JX, Liu H, Huang J
and Zhang WX: Pulmonary nocardiosis in immunocompromised host:
Report of 2 cases and review of the literature. Zhonghua Jie He He
Hu Xi Za Zhi. 32:593–597. 2009.(In Chinese). PubMed/NCBI
|
|
6
|
Mari B, Montón C, Mariscal D, Luján M,
Sala M and Domingo C: Pulmonary nocardiosis: Clinical experience in
ten cases. Respiration. 68:382–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Márquez-Diaz F, Soto-Ramirez LE and
Sifuentes-Osornio J: Nocardiasis in patients with HIV infection.
AIDS Patient Care STDS. 12:825–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HE and Wang Yan: Analysis of Nocardia
infections 16 cases. Yi Yao Lun Tan Za Zhi. 16:71–73. 2011.(In
Chinese).
|
|
9
|
Kageyama A, Yazawa K, Ishikawa J, Hotta K,
Nishimura K and Mikami Y: Nocardial infections in Japan from 1992
to 2001, including the first report of infection by Nocardia
transvalensis. Eur J Epidemiol. 19:383–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holtz HA, Lavery DP and Kapila R:
Actinomycetales infection in the acquired immunodeficiency
syndrome. Ann Intern Med. 102:203–205. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farina C, Boiron P, Goglio A and Provost
F: Human nocardiosis in northern Italy from 1982 to 1992. Northern
Italy Collaborative Group on Nocardiosis. Scand J Infect Dis.
27:23–27. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geiseler PJ and Andersen BR: Results of
therapy in systemic nocardiosis. Am J Med Sci. 278:188–194. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez R, Reyes S and Menéndez R:
Pulmonary nocardiosis: Risk factors, clinical features, diagnosis
and prognosis. Curr Opin Pulm Med. 14:219–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurahara Y, Tachibana K, Tsuyuguchi K,
Akira M, Suzuki K and Hayashi S: Pulmonary nocardiosis: A clinical
analysis of 59 cases. Respir Investig. 52:160–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mootsikapun P, Intarapoka B and
Liawnoraset W: Nocardiosis in Srinagarind Hospital, Thailand:
Review of 70 cases from 1996-2001. Int J Infect Dis. 9:154–158.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z: Clinical Microbiology and
Microorganism Analysis. Third edition. People Health Publishing
House; Beijing: pp. pp226–pp229. 2003, (In Chinese).
|
|
17
|
Tam WO, Wong CF and Wong PC: Endobronchial
nocardiosis associated with broncholithiasis. Monaldi Arch Chest
Dis. 69:183–185. 2008.PubMed/NCBI
|
|
18
|
Menéndez R, Cordero PJ, Santos M,
Gobernado M and Marco V: Pulmonary infection with Nocardia species:
A report of 10 cases and review. Eur Respir J. 10:1542–1546. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liping Wu, Yang Yi and Ting Liu: One case
of pulmonary nocardiosis. Zhong Guo Kang Gan Ran Hua Liao Za Zhi.
5:117–118. 2005.(In Chinese).
|
|
20
|
Smego RA Jr and Gallis HA: The clinical
spectrum of Nocardia brasiliensis infection in the United States.
Rev Infect Dis. 6:164–180. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar N and Ayinla R: Endobronchial
pulmonary nocardiosis. Mt Sinai J Med. 73:617–619. 2006.PubMed/NCBI
|
|
22
|
Chedid MB, Chedid MF, Porto NS, Severo CB
and Severo LC: Nocardial infections: Report of 22 cases. Rev Inst
Med Trop Sao Paulo. 49:239–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuhua Wang, Qingjun Wu and Xiaofeng Zeng:
Systemic lupus erythematosus complicating Nocardiosis: Two case
reports and review of the literature. Beijing Yixue. 28:2652006.(In
Chinese).
|
|
24
|
Munksgaard B: Nocardia infections. Am J
Transplant. 4(Suppl 10): 47–50. 2004.PubMed/NCBI
|
|
25
|
BrownElliott BA, Ward SC, Crist CJ, Mann
LB, Wilson RW and Wallace RJ Jr: In vitro activities of linezolid
against multiple Nocardia species. Antimicrob Agents Chemother.
45:1295–1297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torres OH, Domingo P, Pericas R, Boiron P,
Montiel JA and Vázquez G: Infection caused by Nocardia farcinica:
Case report and review. Eur J Clin Microbiol Infect Dis.
19:205–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martínez Tomás R, Menéndez Villanueva R,
Reyes Calzada S, Santos Durantez M, Vallés Tarazona JM, Modesto
Alapont M and Gobernado Serrano M: Pulmonary nocardiosis: Risk
factors and outcomes. Respirology. 12:394–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kandi V: Human Nocardia Infections: A
Review of Pulmonary Nocardiosis. Cureus. 7:e3042015.PubMed/NCBI
|