Introduction
Despite several decades of research, peripheral
nerve injury (PNI) repair remains suboptimal, as full recovery is
difficult to achieve (1). Nerve
autografting remains the gold standard in PNI therapy (2); however, autografting may be a clinical
challenge due to the creation of a secondary injury site and the
limited availability of donor graft resources. Peripheral nerve
allografts have proved efficacious in the treatment of nerve
injury, and thus provide a viable alternative to the challenges of
autografting (3). Nerve allografts
have been demonstrated to preserve the extracellular environment of
the injury site (4,5), as well as promoting a favorable
environment for local neurotrophic factors to aid in axonal
regeneration (6), collectively
aiding the biocompatibility of the technique. Though promising,
over the previous few decades it has become clear that
microsurgical approaches themselves cannot fully reconcile the
complex nature of PNI. For this purpose, combinatorial strategies,
which employ microsurgical and biological manipulations in tandem
(such as neurotrophic factors, extracellular matrix components,
over-riding inhibitory cues and cell transplantation), have been
increasingly investigated (7,8).
Coupling allografts with growth-enhancing factors
has demonstrated improved outcomes compared with unassisted nerve
grafts. For example, allografts of the sciatic nerve, when combined
with brain-derived neurotrophic factor (BDNF) scaffolding,
increased neuronal regeneration in a model of rat spinal cord
injury (9). Similarly, stem cell
transplantation is also able to aid PNI recovery, likely due to the
multipotent differentiation potential and successful integration
into host systems of stem cells, without the need for
immunosuppression (10). Bone
mesenchymal stem cells (BMSCs) in particular have been demonstrated
to aid the regeneration of sciatic nerve injury (11). Previously, we demonstrated that BMSC
transplantation combined with chondroitinase ABC (an axonal growth
inhibitor-cleaving enzyme) during peripheral nerve allograft
enhanced axonal regeneration and functional recovery in a rat model
of sciatic nerve injury (12). In
general, combinatorial therapies that have been assessed appear to
supersede the efficacy of various isolated techniques (7). However, the mechanisms underlying these
intervention strategies at the molecular level have yet to be
elucidated. Uncovering such strategies is paramount to the
optimization of combinatorial therapies and the reduction of
adverse outcomes. Herein, we aimed to explore the mechanistic
underpinnings of BMSCs and Ch-ABC-assisted acellular allografts on
axonal regeneration in peripheral nerve injury. Using a rat model
of sciatic nerve gap repair [acellular nerve allograft (ANA)], the
cellular, molecular and functional profiles of spinal cord
motoneurons, donor nerve, and target tissues were evaluated in
order to comprehensively assess the biological outcomes of this
combinatorial therapy. The current study describes the direct
enhancement of axonal regeneration at the graft site, as well as
growth enhancement on spinal cord motoneurons and target organs.
These are likely the result of the retro- and anterograde
propagation of growth signals sourced from the donor nerve, working
in unison to improve observed functional outcomes. The current
study reveals a number of the molecular contributors to the
therapeutic success of BMSCs and Ch-ABC assisted ANA contributions
that may serve as targets for greater investigation and
optimization in the future.
Materials and methods
Animals
Healthy Wistar rats (n=48, including 24 male and 24
female) and Sprague-Dawley (SD) rats (n=24, including 12 male and
12 female) weighing 180–220 g were provided by the Experimental
Animal Center of China Medical University (Shenyang, China; cert
no. SCXK Liao 2008-0005). SD rats were used as nerve donors and
Wistar rats were used as graft recipients. The rats were housed in
a temperature- and humidity-controlled environment under a 12-h
light/dark cycle, with ad libitum access to food and water.
The animals were housed separately. The present study was approved
by the Experimental Animal Administration and Ethics Committee of
China Medical University.
Preparation and treatment of ANA
ANA were prepared by a hypotonic chemical detergent
method described previously (8). On
the day prior to surgery, ANA were incubated with either 100 µl
phosphate buffered saline (PBS; pH 7.4) containing 2 U/ml Ch-ABC
(Sigma-Aldrich, St. Louis, MO, USA) or with PBS alone (vehicle) for
16 h at 37°C. The grafts were rinsed twice with PBS and stored on
ice prior to use.
Preparation of BMSCs
Bone marrow was aspirated with a disposable needle
from the femurs of rats sacrificed by CO2 overdose for
preparation of ANA. The cells were collected in heparinized
syringes following a previously described protocol (13). Briefly, femurs were removed, cleaned
of muscle and connective tissue, and bone marrow was flushed out
with PBS. Following centrifugation at 1,000 × g for 5 min at 4°C,
the supernatant was discarded and the cells were re-suspended in
complete medium containing Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and
100 U/ml streptomycin at 37°C and 5% CO2. Following
incubation of the dissociated cells for 24 h at 37°C, the
non-adherent cells were removed. The adherent cells were
continuously cultured at 37°C in 5% CO2, and then used
for the subsequent experiments. In all experiments, cells were used
at passages II–IV.
Preparation of ANA seeded with
BMSCs
BMSCs were harvested with 0.25% trypsin (Hyclone; GE
Healthcare Life Sciences) and suspended in complete medium at a
density of 2×107 cells/ml. A total of 2×107
BMSCs in 100 µl complete medium were injected into four evenly
spaced points of the nerve section using a micro-injector under an
SXP-10 microscope (magnification, ×10; Shanghai Medical Equipment
Works Co., Ltd., Shanghai, China). The nerve grafts were then
incubated in complete medium in a humidified atmosphere containing
5% CO2 at 37°C for 48 h, after which they were collected
for in vivo experiments.
Scanning electron microscopy (SEM)
analysis
BMSC adhesion to donor ANA was assessed via SEM
analysis. Grafts were fixed for 24 h in 4% glutaraldehyde in 0.1 M
PBS. The scaffolds were further rinsed in deionized water, and
dehydrated with a graded series of ethanol solutions (50, 70, 90,
100%; 10 min each). Finally, the scaffold was treated with
hexamethyldisilazane (Sigma-Aldrich) and air-dried in a fume hood
overnight. The specimens were mounted on stubs and sputter coated
with gold, loaded into a scanning electron microscope (S3400N;
JEOL, Ltd., Tokyo, Japan), and viewed under an accelerating voltage
of 5 kV.
Additionally, some ANAs were embedded in 1.5% (w/v)
agarose (Sigma-Aldrich), fixed in 2% (v/v) glutaraldehyde
(Sigma-Aldrich) in 0.1 M phosphate buffer (pH 7.2), post-fixed with
1% (w/v) osmium tetroxide (Sigma-Aldrich), dehydrated in a series
of ascending ethanol concentrations, and embedded in epon 812
(Sigma-Aldrich). Ultrathin sections (60 nm) were collected on
formvar-coated grids, post-stained with lead citrate and uranyl
acetate, and subsequently imaged using electron microscopy (JEOL
1010; JEOL, Ltd.).
Surgical procedures
All Wistar rats were randomly divided into four
groups (n=12/group) as follows: Group I, DMEM control group (ANA
incubated in DMEM only); Group II, Ch-ABC group (ANA incubated in
Ch-ABC only); Group III, BMSCs group (ANA seeded with BMSCs only);
Group IV, Ch-ABC + BMSC group (Ch-ABC treated ANA then seeded with
BMSCs). In all experiments, the DMEM group served as a negative
control. Rats were anesthetized by intraperitoneal injection of 100
g/l chloral hydrate (350 mg/kg weight) and the right sciatic nerve
was exposed, the nerve segment was sharply transected, allowed to
retract, and then transected again to bridge a 10-mm defect. The
1-cm acellular nerve grafts were stitched (four pins end-to-end) to
bridge the two stumps of the nerve defects with 9-0 nylon sutures
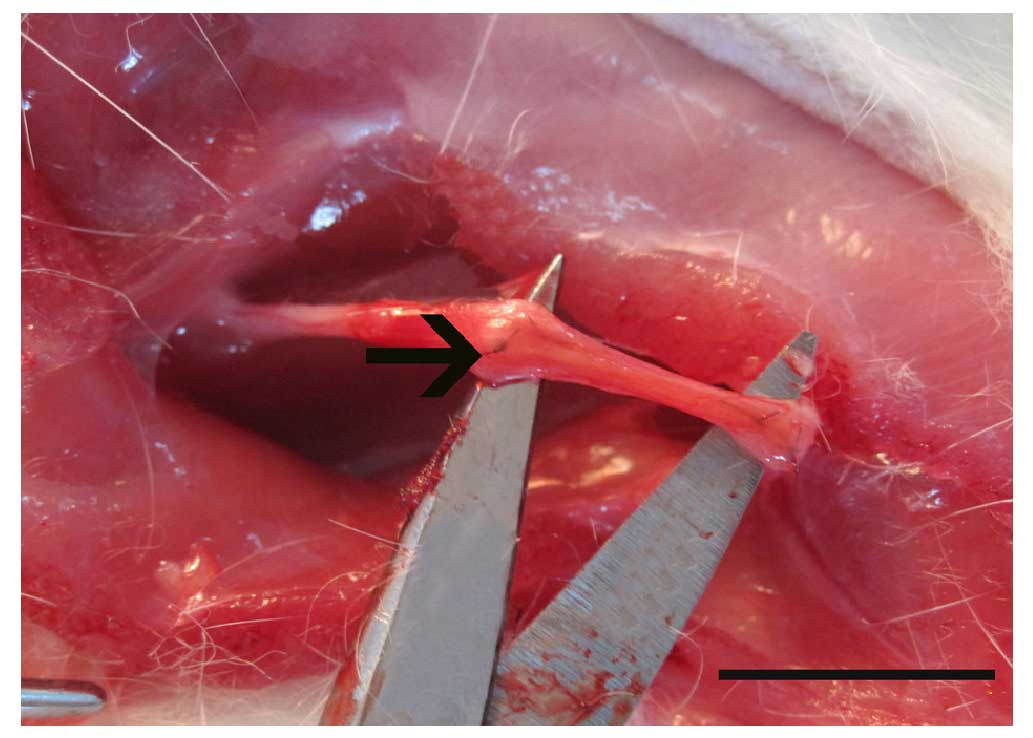
under an operation microscope (Fig.
1). The wounds of all rats were rinsed with 40,000 U gentamicin
sulfates and muscle and skin were sutured. Following surgery, each
rat was assigned an identification number and placed under a warm
light, allowed to recover from anesthesia, and then housed
separately with access to food and water in a colony room
maintained at constant temperature (19–22°C) and humidity (40–50%)
with a 12-h light:dark cycle. No animals were lost to surgical or
post-surgical complications.
Immunohistochemistry
Briefly, serial 25 µm-thick frozen sections of the
middle nerve graft were cut from each group (n=6) on a cryostat
(Leica CM 3000; Leica Microsystems, Inc., Buffalo Grove, IL, USA),
mounted on superfrost/plus slides (Menzel-Glaser, Braunschweig,
Germany) and incubated with the following primary antibodies for 12
h at 4°C: Goat anti-nerve growth factor (NGF) polyclonal antibody
(1:200 dilution; cat. no. N8773; Sigma-Aldrich), rabbit anti-BDNF
polyclonal antibody (1:150 dilution; cat. no. SAB2108004;
Sigma-Aldrich), rabbit anti-vascular endothelial growth factor
(VEGF) monoclonal antibody (1:150 dilution; cat. no. ab51745;
Abcam, Cambridge, MA, USA), rabbit-anti neuronal marker (NeuN)
monoclonal antibody (1:200 dilution; cat. no. ab177487; Abcam),
mouse anti-choline acetyltransferase (ChAT) monoclonal antibody
(1:200 dilution; cat. no. AMAB91129; Sigma-Aldrich) and mouse-anti
synaptophysin (SYP) monoclonal antibody (1:200 dilution; cat. no.
S5768; Sigma-Aldrich). The immunoreactivity was visualized by
incubation with fluorescein isothiocyanate-conjugated goat
anti-mouse immunoglobulin (Ig)G (1:200 dilution; cat. no. F0257;
Sigma-Aldrich), Texas Red-conjugated goat anti-rabbit IgG (1:200
dilution, cat. no. SAB3700888; Sigma-Aldrich) and CY5-conjugated
goat anti-mouse IgG (1:200 dilution; cat. no. SAB4600397;
Sigma-Aldrich) for 1 h at room temperature. A MetaMorph/DP10/BX41
analytical imaging system (Olympus Soft Imaging Solutions GmbH,
Münster, Germany) was used to scan and generate figures. The
integrated optical density (IOD) of positive immunological
reactions was also analyzed using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA). For each section,
the positive IOD of five representative visual fields without
overlap were observed under a high-power microscope.
The L4 spinal cord was positioned and removed by
backward tracking of the repaired nerve. Homolateral tibialis
anterior muscles were additionally removed from each group
(n=6/group). The tissues were cut as 25 µm-thick frozen serial
cross-sections. The sections were incubated in 3%
H2O2 for 10 min at room temperature then
washed carefully with PBS three times (10 min each). The sections
were blocked with 5% bovine serum albumin (M B-Chem Corporation,
Mumbai, India) for 30 min at 37°C, then incubated at 4°C overnight
with rabbit anti-NF-200 polyclonal antibody (only for nerve graft;
1:200 dilution; cat. no. N4142; Sigma-Aldrich), goat anti-NGF
polyclonal antibody (1:150 dilution), rabbit anti-BDNF polyclonal
antibody (1:100 dilution) or rabbit anti-VEGF monoclonal antibody
(1:100 dilution). Subsequently, the tissue sections were washed
three times in PBS, and then incubated with biotin-labeled goat
anti-rabbit IgG (1:200 dilution; cat. no. ZDR-5118; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30
min at 37°C, followed by incubation with streptavidin peroxidase
complex (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 30
min at 37°C. Following washing with PBS and staining with
3,3-diaminobenzidine (Sigma-Aldrich) for 10 min, the tissue
sections were observed under a light microscope (DM3000; Leica
Microsystems GmbH, Wetzlar, Germany). The positively stained
motoneurons in the spinal cord anterior horn were counted, and the
integrated optical density of positively stained nerves and muscles
was analyzed using an analytical imaging system (Olympus Soft
Imaging Solutions GmbH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the nerve graft, spinal
cord and muscles using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol (n=6/group). Purified RNA was diluted to 500 ng/µl and 3
µl RNA was used to synthesize cDNA with a Prime Script RT reagent
kit (Takara Biotechnology Co., Ltd., Dalian, China). Primers were
designed and synthesized by Takara Biotechnology Co., Ltd., and
their sequences are displayed in Table
I. RT-qPCR was performed using a SYBR Premix Ex Taq kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol for qPCR systems (ABI Prism 7000; Applied Biosystems;
Thermo Fisher Scientific, Inc.). The amplification conditions were
as follows: 30 sec at 95°C (one cycle), 5 sec at 95°C and 30 sec at
60°C (40 cycles) followed by 72°C for 5 min (one cycle). RT-qPCR
was performed three times in triplicate to evaluate the
reproducibility of the data. The cycle number at which the
amplification curve crossed the threshold line was noted as the
critical quantification (Cq) and gene expression was calculated by
the comparative Cq (2−ΔΔCq) method, using β-actin as the
housekeeping gene for normalization.
 | Table I.Primer sequences for quantitation of
mRNA. |
Table I.
Primer sequences for quantitation of
mRNA.
| Gene | Sequence | Product size |
|---|
| NGF | Forward:
ACCTCTTCGGACACTCTGGA | 168 bp |
|
| Reverse:
GTCCGTGGCTGTGGTCTTAT |
|
| BDNF | Forward:
GGTCACAGTCCTGGAGAAAG | 214 bp |
|
| Reverse:
GTCTATCCTTATGAACCGCC |
|
| VEGF | Forward:
GGACATCTTCCAGGAGTACC | 147 bp |
|
| Reverse:
CGCATGATCTGCATAGTGAC |
|
Motoneurons, dendritic number and
morphology
Eight weeks after injury, the rats were
re-anesthetized and the left vastus lateralis muscle of the
quadriceps was exposed and injected with horseradish peroxidase
(HRP) conjugated to the cholera toxin B subunit (BHRP; 2 µl; 0.2%;
List Biological Laboratories, Inc., Campbell, CA, USA). BHRP
labeling permits population level quantitative analysis of
motoneuron soma and dendritic morphology. Following BHRP injection
[(48 h after, in order to ensure optimal labeling of motoneurons
(14,15)], the rats were weighed and received a
lethal dose of Nembutal (45 mg/kg, i.p.; Sigma-Aldrich). The rats
were subsequently perfused intracardially with saline followed by
cold fixative solution (1% paraformaldehyde/1.25% glutaraldehyde),
and then cut on a cryostat (40-µm-thick transverse sections for
spinal cords).
Motoneurons and dendritic lengths in a single
representative set of alternate L4 spinal cord sections were
measured under darkfield illumination initiated with the first
section in which BHRP-labeled motoneurons and fibers were present.
Labeling throughout the entire rostrocaudal extent of the
quadriceps motoneuron dendritic field was assessed in each section
using a computerized morphometry system (Neurolucida; MBF
Bioscience, Inc., Williston, VT, USA) at a final magnification
×250. No attempt was made to distinguish BHRP-labeled fibers as
either dendrites or axons. Average dendritic length per labeled
motoneuron was estimated by totaling the measured dendritic lengths
of the serial sections, multiplying by three to correct for
sampling error, and then dividing by the total number of labeled
motoneurons that was detected in that series (16). The length of labeled dendrites of
motoneurons was assessed in sections 320 µm apart through the
length of the lumbar spinal cord. A total of six rats were analyzed
per group.
Electrophysiological analysis
Electrophysiological analysis was performed on the
rats prior to sacrifice by CO2 overdose, using a Haishen
NDI-200P1 electroneurogram device (Shanghai, China). The stimulus
intensity was 1–20 mA to ensure a maximum waveform and to prevent
independent muscle contractions. The stimulus duration was 0.1–0.2
msec, and the stimulation frequency was 1 Hz. Subsequently, with
each animal under general anesthesia (pentobarbital; 60 mg/ml i.p.
injection; Sigma-Aldrich) the right sciatic nerve was exposed. A
crook-shaped silver needle electrode was placed on the proximal and
distal ends of the grafts, which were then stimulated at the distal
end and recorded at the proximal end. The distance between the two
electrodes was measured with a sliding caliper with 0.2 mm
precision. Nerve conduction velocity, latency period and wave
amplitude were then recorded in eight rats from each group.
Tibialis anterior muscle weights
The tibialis anterior muscles were dissected from
both sides and detached from the bone at their origin and terminal
point following electrophysiological analysis, and weighed
immediately with an electron scale to 0.0001 precision. The weight
on the operated side was expressed as a percentage of the muscle
weight on the unoperated side.
Statistical analysis
All quantitative data were expressed as means ±
standard deviation and analyzed with SPSS statistical software
(version 13.0; SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance followed by Dunnett's test was used for the comparison
among experimental groups. Non-parametric data were compared using
the Mann-Whitney U test among experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
BMSC cultures and secreted growth
factor
BMSCs appeared suspended in culture medium on day 1
and exhibited a small, spindle-like or fibroblast-like morphology
on day 3. Cells reached 90% confluence on day 7. By the 3rd
passage, BMSCs began to exhibit a whirlpool-like arrangement
(Fig. 2A-D). After 5 days of
co-culture of BMSCs with DMEM or Ch-ABC-treated ANA, SEM
observation revealed that BMSCs attach tightly to ANA irrespective
of pretreatment with ANA (Fig.
2E-F). Subsequent to 5 days of co-culture, the growth factors
NGF, BDNF and VEGF were investigated in pre-grafted ANA. Growth
factors were detected at higher levels in the BMSC and Ch-ABC +
BMSC groups compared with the DMEM group and Ch-ABC groups. No
significant difference was observed between the Ch-ABC group and
the DMEM group, nor did the addition of Ch-ABC to BMSC seeded ANA
appear to enhance the growth factor response (Fig. 2G), indicating that, at least
pre-operatively, BMSCs are primarily responsible for the
stimulation of growth on the donor nerve.
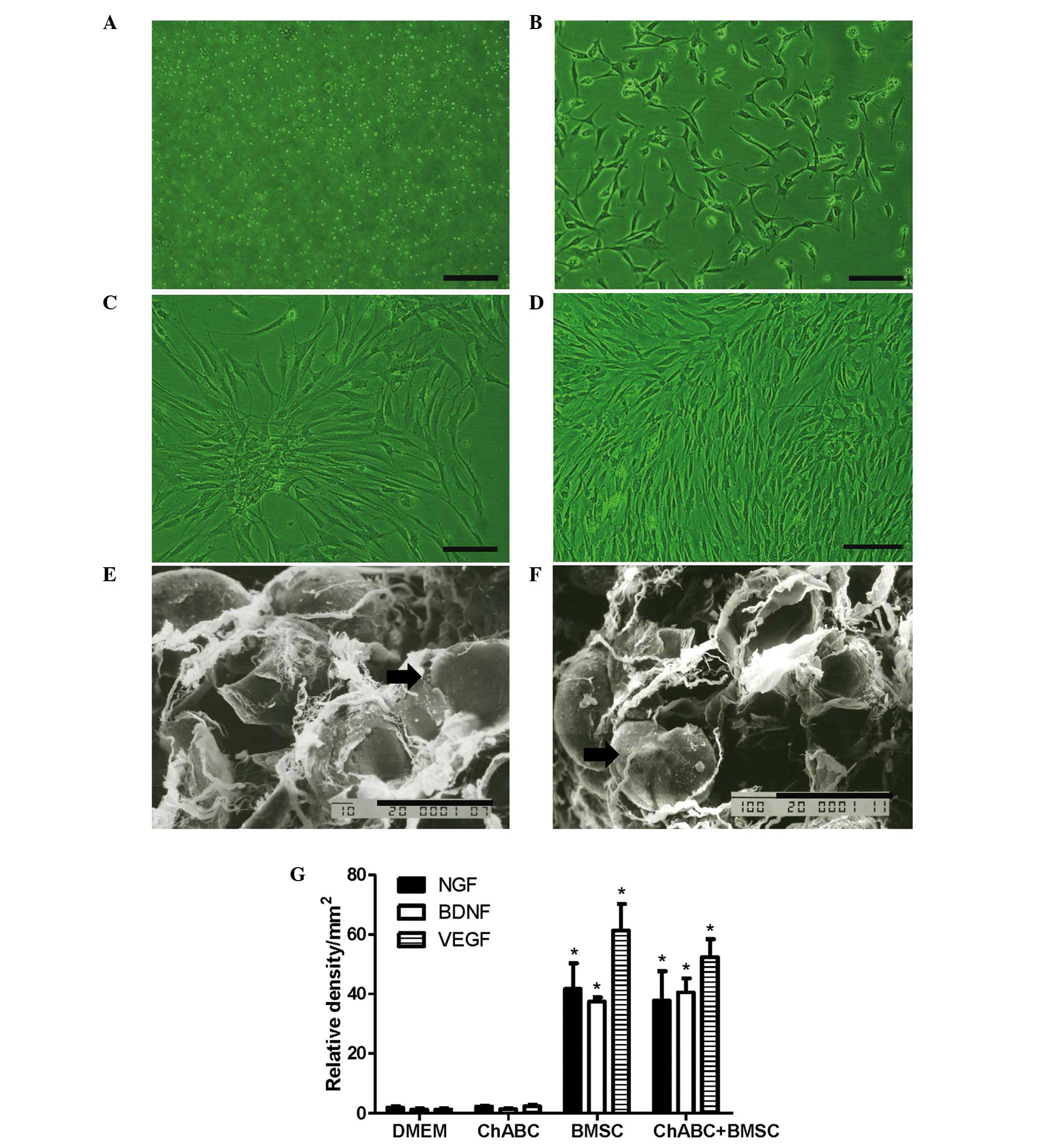 | Figure 2.BMSC culture and adherence to ANA.
BMSCs were cultured at (A) day 1, (B) day 3, (C) day 7 and (D) at
passage III (Scale bar=20 µm). SEM evaluation of BMSCs (black
arrow) revealed (E) adhesion to the ANA and (F) Ch-ABC incubated
ANA in vitro (Scale bar=10 µm). Analysis of NGF, BDNF and
VEGF immunoreactivity in ANA sections was determined by integrated
optical density. (G) NGF, BDNF and VEGF relative densities. n=6 per
treatment condition. All data are expressed as means ± standard
deviation. *P<0.05, vs. the DMEM group. ANA, acellular nerve
allografts; SEM, scanning electron microscope; BMSC, bone marrow
stromal cell; Ch-ABC, chondroitinase ABC; DMEM, Dulbecco's modified
Eagle's medium; NGF, nerve growth factor; BDNF, brain-derived
neurotrophic factor; VEGF, vascular endothelial growth factor. |
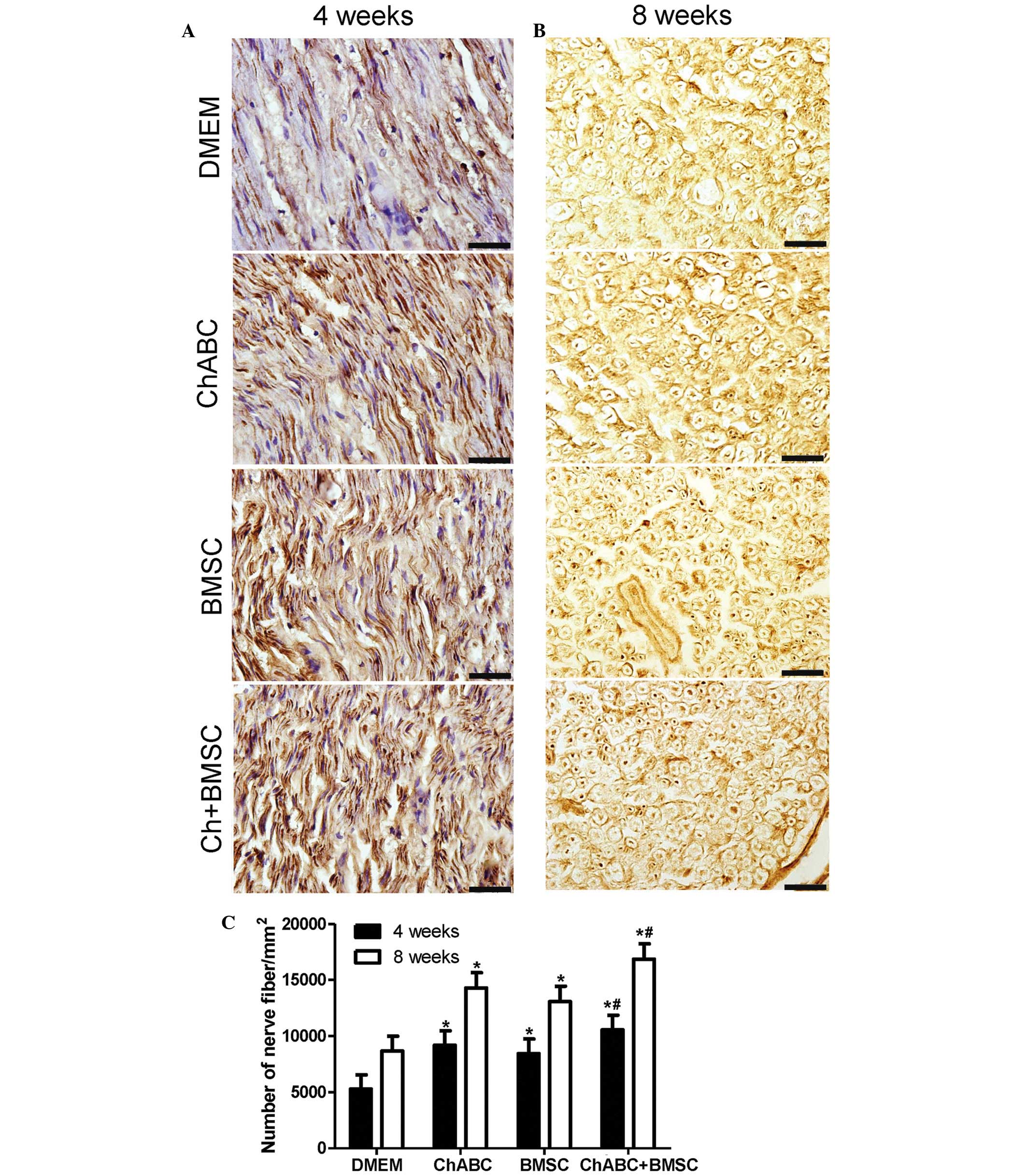
Combination treatment promotes axon
regeneration
Immunohistochemical staining was performed for a
nerve fiber marker (NF-200) in ANA in 4 and 8 week post-operative
nerve recipients. It was revealed that the number of nerve fibers
was significantly increased in all treatment groups compared with
the DMEM negative control at 4 and 8 weeks (P<0.05; Fig. 3). Notably, the number of nerve fibers
in the Ch-ABC + BMSCs group was greater compared with the Ch-ABC
and BMSC groups (P<0.05; Fig. 3).
The aforementioned results indicate that combined BMSCs and Ch-ABC
treatment promotes axonal regeneration at a greater capacity than
any single treatment of the ANA 4–8 weeks after grafting.
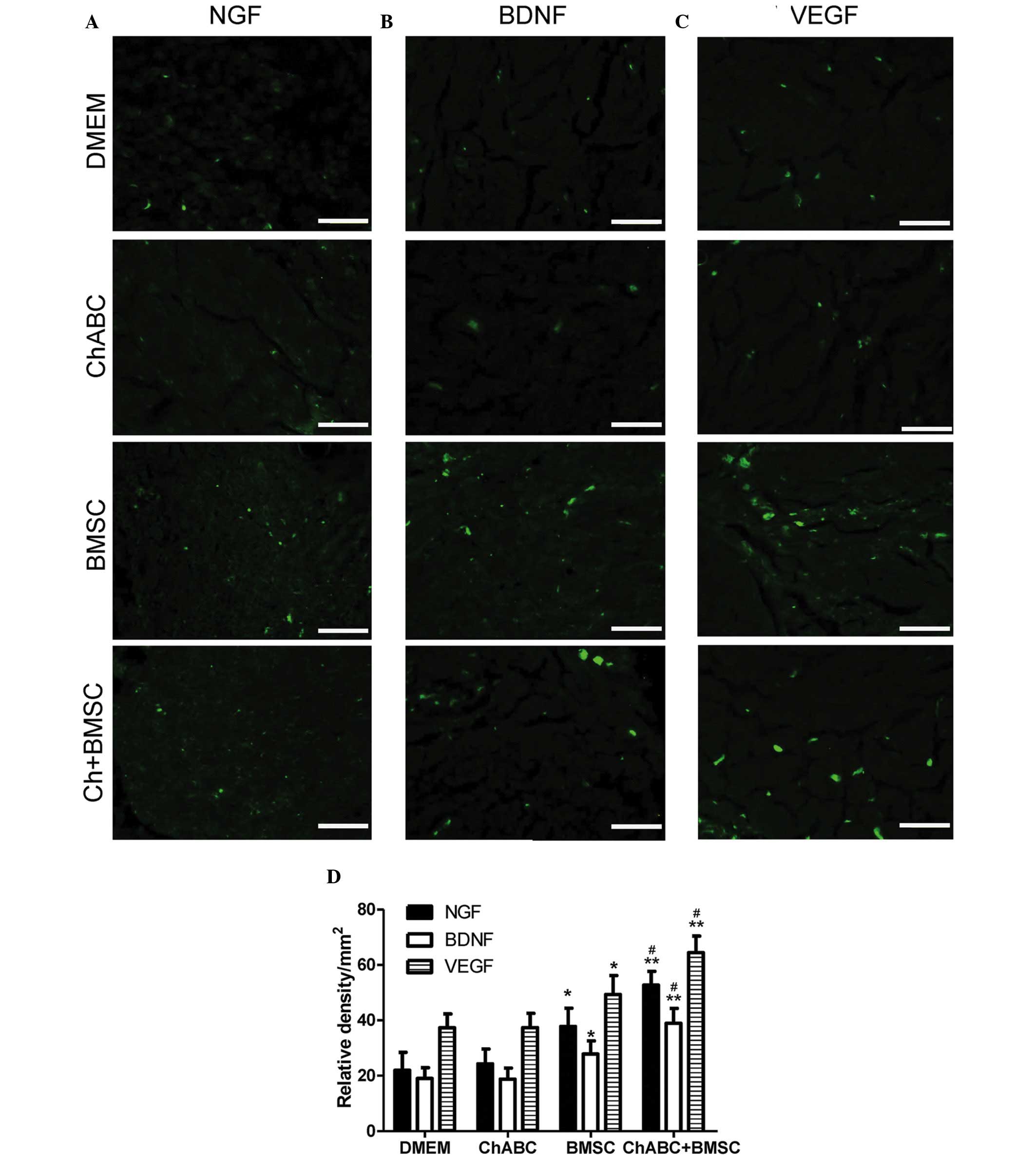
Combination therapy promotes NGF, BDNF
and VEGF expression in the regenerated tissues
Immunofluorescence analysis revealed that in the
centre of the ANA, NGF, BDNF and VEGF protein expression levels
were increased in the BMSC and ChABC + BMSC groups compared with
the DMEM and Ch-ABC groups (P<0.05; Fig. 4). Across all growth factors, the
addition of Ch-ABC pre-treatment further increased the expression
levels of the growth factors, compared with the BMSC group
(P<0.05). Motoneurons of the L4 spinal cord anterior horn
(Fig. 5) and target muscle tissue
(Fig. 6) were similarly examined and
resulted in similar growth factor expression patterns as the ANA
(P<0.05). The immunohistochemical data demonstrates the local
and distal generation of growth factor responses primarily to BMSC
seeding of the graft, with a level of additional enhancement due to
Ch-ABC pre-treatment of the ANA.
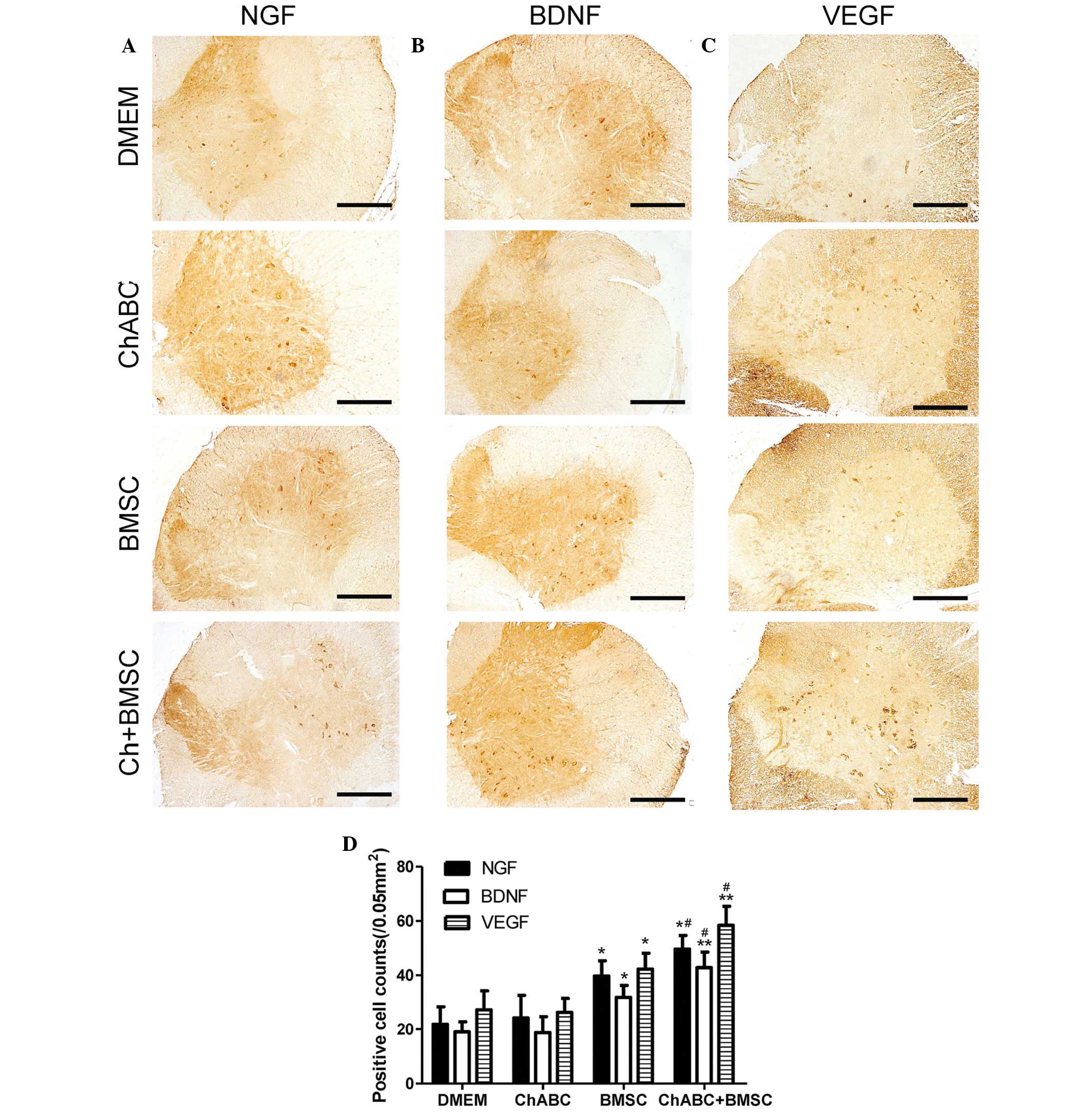 | Figure 5.Immunohistochemical staining of (A)
NGF, (B) BDNF and (C) VEGF in the L4 spinal cord. 8 weeks after
grafting (Scale bar=100 µm). (D) The numbers of positively stained
neurons counted across sections of the spinal cord. All data are
expressed as means ± standard deviation. *P<0.05, **P<0.05
vs. the DMEM group, #P<0.05, vs. the BMSC group. n=6
per group. BMSC, bone marrow stromal cell; Ch-ABC, chondroitinase
ABC; DMEM, Dulbecco's modified Eagle's medium; NGF, nerve growth
factor; BDNF, brain-derived neurotrophic factor; VEGF, vascular
endothelial growth factor. |
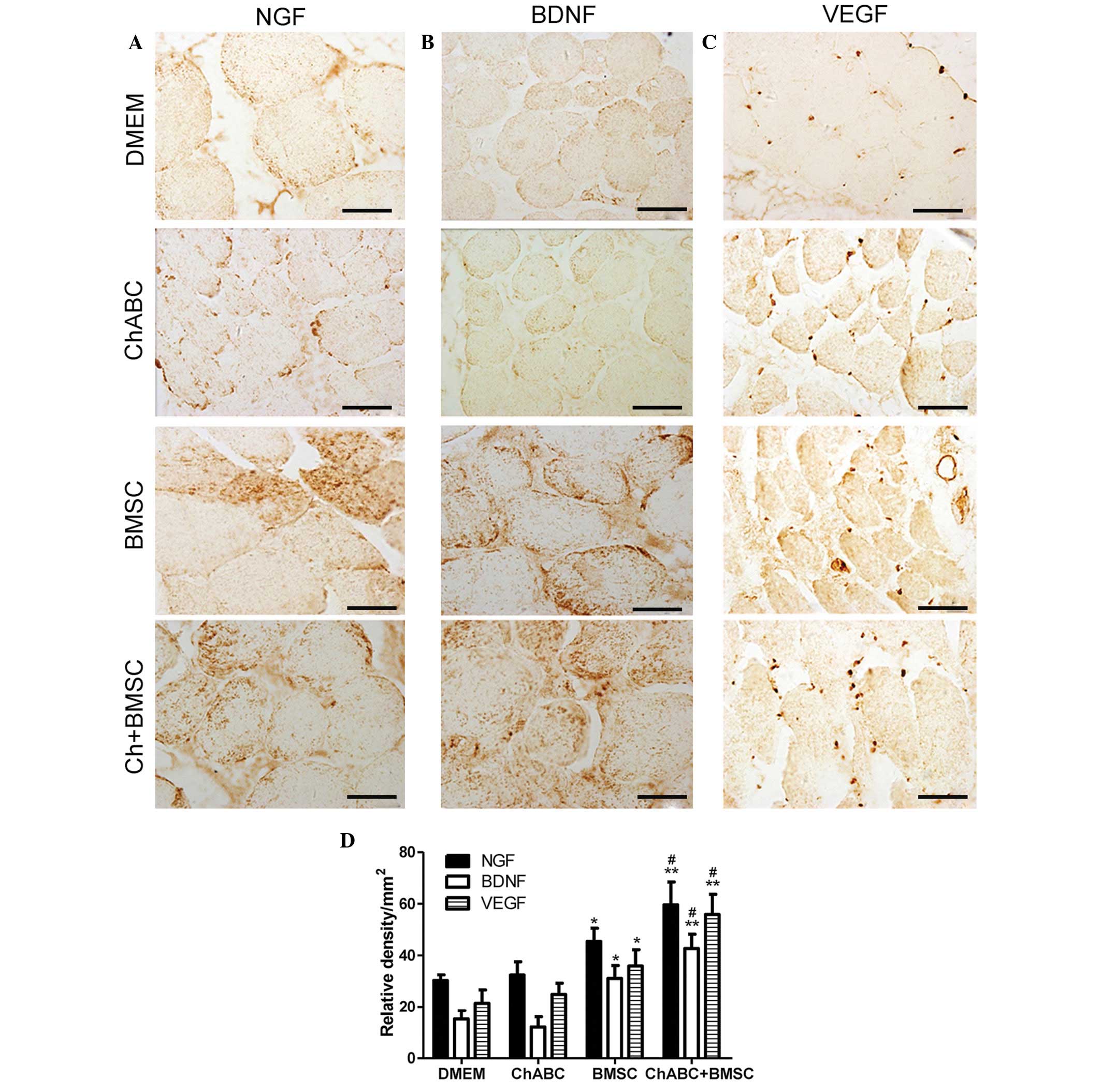 | Figure 6.Immunohistochemical analysis of NGF,
BDNF and VEGF in the anterior tibial muscles. 8 weeks after
grafting, target muscle organ sections from each group were
assessed for (A) NGF, (B) BDNF and (C) VEGF immunoreactivity (Scale
bar=20 µm). (D) Relative density of positive signals. All data are
expressed as means ± standard deviation. *P<0.05, **P<0.01
vs. the DMEM group; #P<0.05 vs. the BMSC group. n=6
per group. BMSC, bone marrow stromal cell; Ch-ABC, chondroitinase
ABC; DMEM, Dulbecco's modified Eagle's medium; NGF, nerve growth
factor; BDNF, brain-derived neurotrophic factor; VEGF, vascular
endothelial growth factor. |
RT-qPCR analysis indicated that the comparative
patterns of NGF, BDNF and VEGF mRNA expression levels in the ANA,
L2-4 spinal cord and distal muscles were generally consistent with
their comparative protein expression patterns. NGF, BDNF and VEGF
mRNA expression levels in the BMSC group and Ch-ABC + BMSC group
were higher compared with the DMEM and Ch-ABC groups (P<0.05)
when ANA mRNA expression levels were analyzed (Fig. 7A). Spinal cord and muscle mRNA
analysis indicated similar patterns of expression for BDNF, VEGF
and NGF (Fig. 7B and C). Detected
across all tissues examined was the enhancement of the mRNA
expression levels of NGF, BDNF and VEGF for the combined group BMSC
+ Ch-ABC compared with the BMSC group (P<0.05). Conclusively,
the aforementioned results indicate that combination therapy is
able to increase growth factor protein and mRNA expression levels
in the ANA, and across the distal spinal cord and target tissue
sites.
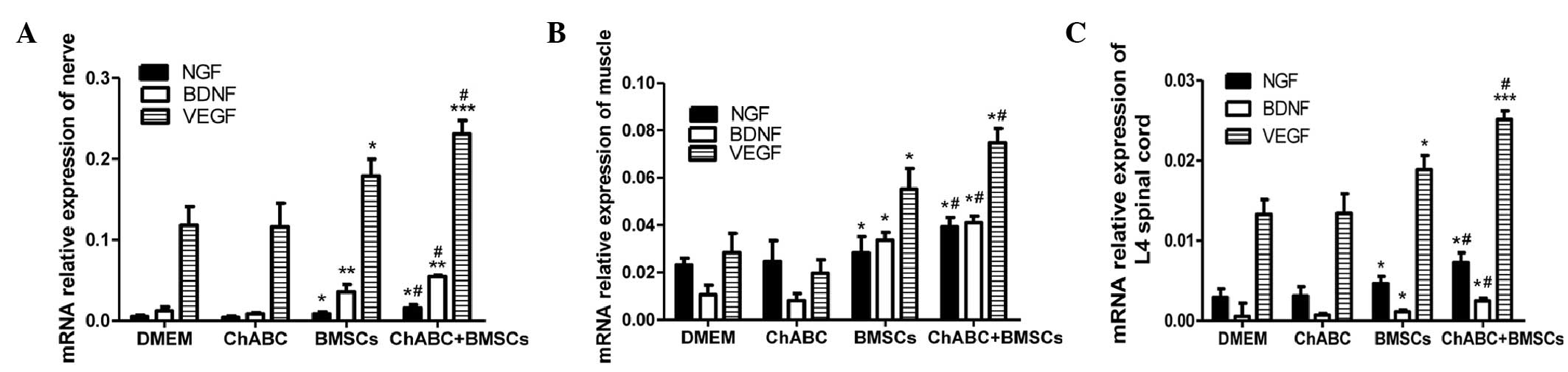 | Figure 7.mRNA expression of NGF, BDNF and VEGF.
8 weeks after grafting, (A) ANA, (B) spinal cord and (C) anterior
tibial muscles were extracted from all treatment groups for
quantitation of NGF, BDNF and VEGF relative expression. mRNA
expression was determined by reverse transcription-quantitative
polymerase chain reaction. All data are expressed as means ±
standard deviation. *P<0.05, **P<0.01, ***P<0.001, vs. the
DMEM group; #P<0.05, vs. the BMSC group; (n=6). BMSC,
bone marrow stromal cell; Ch-ABC, chondroitinase ABC; DMEM,
Dulbecco's modified Eagle's medium; NGF, nerve growth factor; BDNF,
brain-derived neurotrophic factor; VEGF, vascular endothelial
growth factor. |
Combination therapy protects motoneurons.
Immunohistochemical staining was performed to detect the NeuN,
motoneuron marker (ChAT), SYP, and HRP retrograde labeled
motoneurons and dendrites in the L5 spinal cord. It was revealed
that the number of NeuN and ChAT-positive neurons and synaptophysin
protein expression was significantly increased in the Ch-ABC, BMSC
and Ch-ABC + BMSC groups compared with the DMEM group at 8 weeks
(P<0.05). Notably, the number of NeuN and ChAT-positive neurons,
and synaptophysin-positive relative density in the Ch-ABC + BMSC
group were much greater compared with the Ch-ABC group or the BMSC
group (P<0.05; Fig. 8).
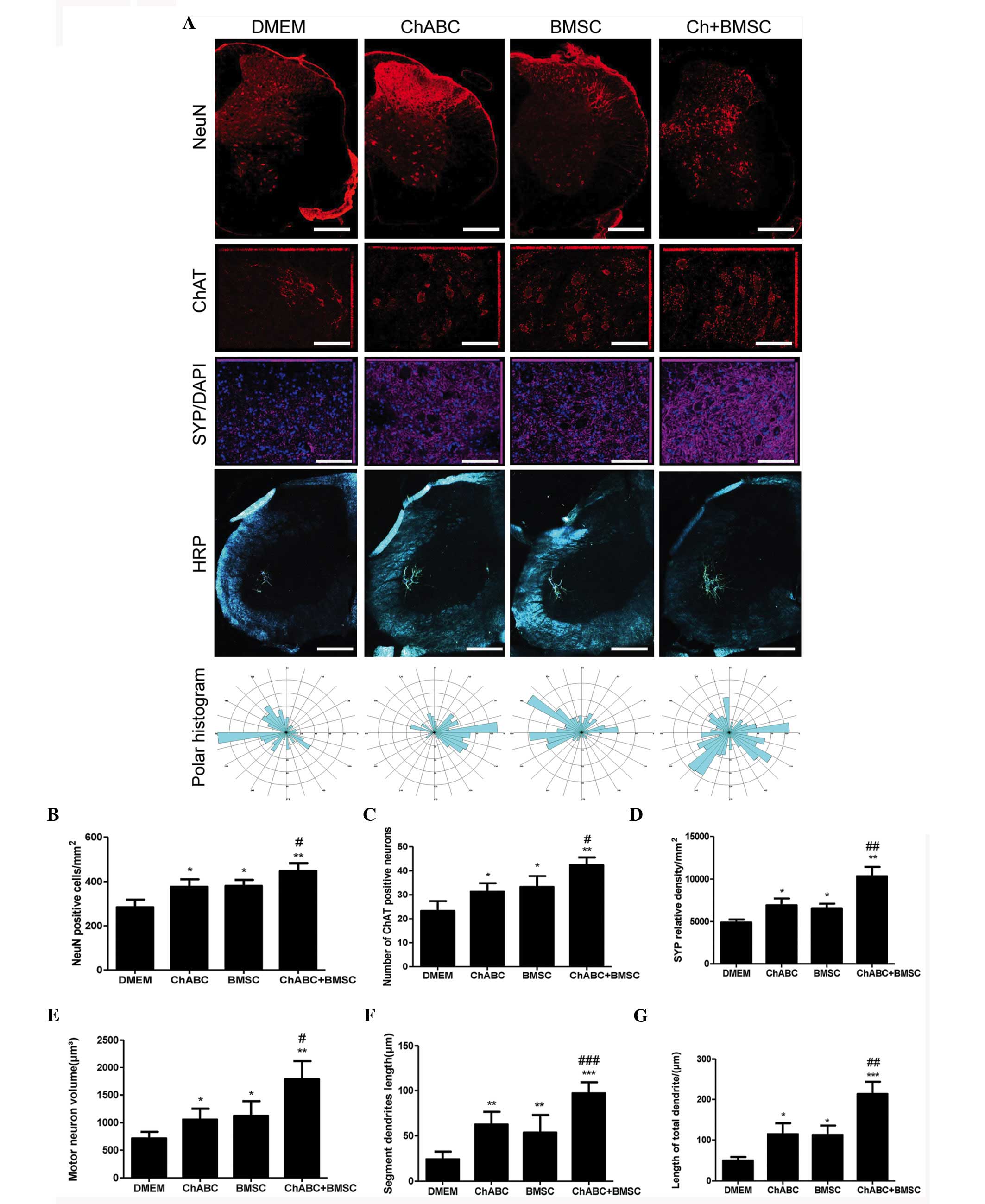 | Figure 8.Motoneuron survival and
dentritogenesis in the L4 spinal cord 8 weeks after graft: (A)
Immunohistochemical staining of NeuN (Scale bar=100 µm), ChAT
(Scale bar=20 µm) and synaptophysin (Scale bar=20 µm). DAPI nuclear
counterstain (blue) was performed on L4 spinal cord sections across
treatment groups using fluorescent reporters. Below, horseradish
peroxidase retrograde tracing of motoneuron dendrites in the L4
spinal cord (Scale bar=100 µm) were generated by injection with
B-horseradish peroxidase 4 weeks after injury (analyzed 48 h after
injection). Polar histograms of dendritic fields were generated by
computer morphometry system across the various groups. Numbers of
(B) NeuN and (C) ChAT-positively stained neurons are counted and in
(D) synaptophysin relative density of the spinal cord is displayed.
(E) Area of motoneurons is displayed, and (F) the segment lengths
of the motoneuron dendrites as well as total dendritic lengths (G)
are also presented. Data are expressed as means ± standard
deviation; *P<0.05, **P<0.01, ***P<0.001, vs. the DMEM
group. #P<0.05, ##P<0.01,
###P<0.001, vs. the BMSC group. n=6 per group). BMSC,
bone marrow stromal cell; Ch-ABC, chondroitinase ABC; DMEM,
Dulbecco's modified Eagle's medium; ChAT, choline
acetyltransferase; NeuN, neuronal marker. |
Molecular indicators of neuronal growth and
protection can be used to demonstrate the functional recovery of an
artificial nerve alongside morphological indicators of axonal
growth. Specifically, the motoneuron area, dendritic segment length
and total dendritic length exhibited greater values in the BMSC,
Ch-ABC and BMSC + Ch-ABC treated ANA compared with DMEM negative
control. Thus, this further confirms previous results indicating
that the BMSC + Ch-ABC group displayed significantly higher values
compared with all other groups (P<0.05; Fig. 8). The results suggest that combined
BMSCs and Ch-ABC treatment optimally protects motoneurons and
enhances dendritic profiles following injury.
Combined treatment promotes the
recovery of sciatic nerve function
At 8 weeks post-surgery, the conduction velocity
(Fig. 9A), latency period (Fig. 9B) and wave amplitude (Fig. 9C) of the nerve graft were assessed by
electrophysiology. The conduction velocity (Fig. 9A) and wave amplitude (Fig. 9C) were higher in all treatment groups
compared with DMEM negative controls. Ch-ABC + BMSC combined
treatment increased the values above BMSC or Ch-ABC alone
(P<0.05). Conversely, the latency period (Fig. 9B) was decreased significantly in all
treatment groups compared with the DMEM control group (P<0.05).
Furthermore, the latency period was decreased to a greater extent
in the Ch-ABC + BMSC group compared with the Ch-ABC and BMSC group
(P<0.05). A final investigation of the recovery of tibialis
anterior muscle weights (Fig. 9D)
corroborated the effectiveness of either Ch-ABC or BMSC treatment
while reinforcing that the combinatorial treatment supersedes the
muscle recovery of either (P<0.05). These results support the
hypothesis that while BMSC and Ch-ABC alone contributed to
functional recovery of the nerve graft in vivo, the
combination of the two therapies enhances the recovery rate
significantly.
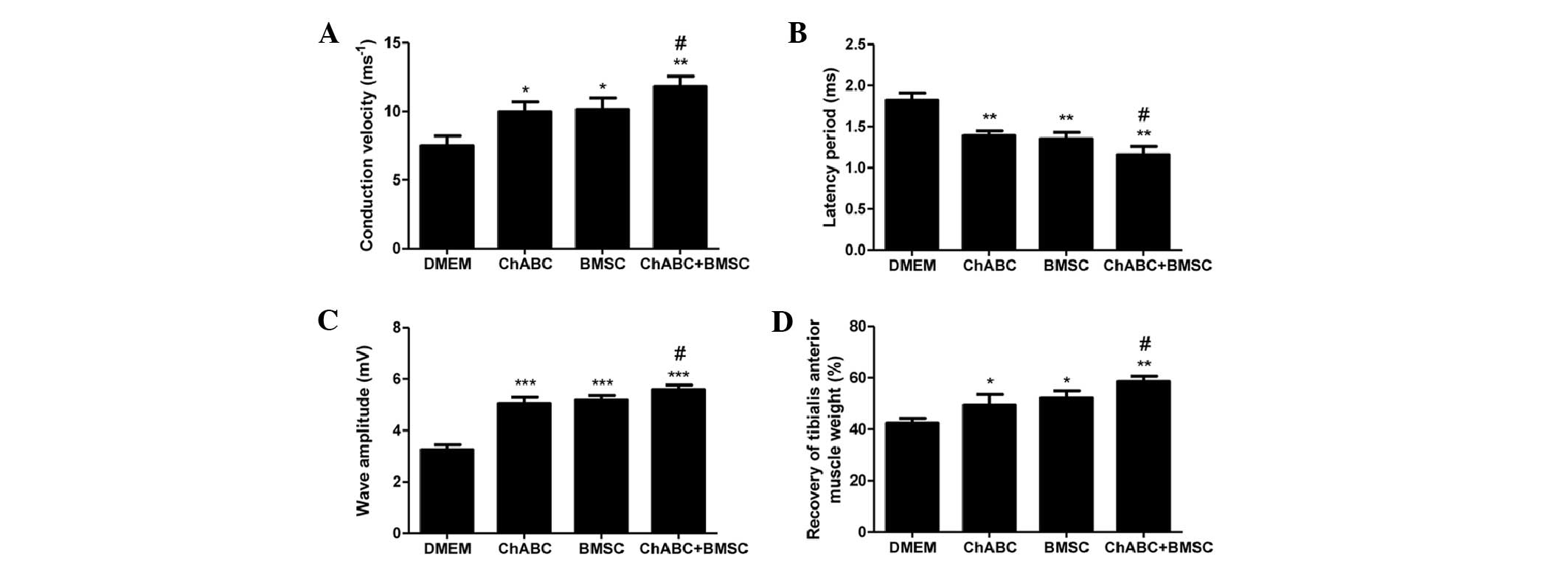 | Figure 9.Electrophysiological index and
tibialis anterior muscle recovery. (A) Conduction velocity, (B)
latency period, (C) wave amplitude of the nerve graft were assessed
by electrophysiology prior to sacrifice (8 weeks after graft). (D)
Recovery assessment of tibialis anterior muscle was also indicated
by the weight ratio of muscle from operated side to the muscle
weight of the un-operated (contralateral) side. All data expressed
as mean ± standard deviation. *P<0.05, **P<0.01,
***P<0.001, vs. the DMEM group; #P<0.05, vs. the
BMSC group; (n=8 per group). BMSC, bone marrow stromal cell;
Ch-ABC, chondroitinase ABC; DMEM, Dulbecco's modified Eagle's
medium; NGF, nerve growth factor; BDNF, brain-derived neurotrophic
factor; VEGF, vascular endothelial growth factor. |
Discussion
There is an increasing necessity in the field of PNI
for therapies with improved long-term outcomes. Biologically, this
equates to the requirement of novel and more efficacious methods of
axonal regeneration. Recently, it has been demonstrated that the
complex aberrations of PNI are better treated with a combination of
peripheral nerve grafting and growth-promoting factors (such as
stem cell transplantation and growth factor enhancement) (17). Despite improvements in functional and
anatomical parameters of recovery, little is understood about the
molecular drivers of combinatorial therapy such as Ch-ABC and
BMSC-assisted ANA. Identifying the molecular growth cues of
peripheral nerve regeneration, and how these may be enhanced by
therapies such as stem cell transplantation and axonal growth
factors may assist with the stratification of the adverse outcomes
of nerve grafting, and guide future therapeutic intervention.
Molecular, anatomical and functional parameters of peripheral nerve
injury recovery were evaluated in the present study, subsequent to
the establishment of a model of ANA pretreated with Ch-ABC and
seeded with BMSCs.
BMSCs adhere tightly to donor ANA and stimulate the
expression of NGF and BDNF growth factors in vitro. This
signifies that even prior to surgery, the presence of multipotent
stem cells is already enhancing the ability of the donor nerve to
secrete growth-permissive factors (12). This ‘priming’ may ultimately
contribute to the biocompatibility of the donor graft to the
recipient. BMSC seeding of the ANA serves to continually improve
growth factor secretion in vivo with Ch-ABC significantly
enhancing NGF, VEGF and BDNF secretion post-transplantation. The
latter is likely due to the function of Ch-ABC as a remover of
proteoglycan obstructions impeding axonal growth, and is a probable
explanation for the absence of a Ch-ABC growth enhancement in
vitro.
To the best of our knowledge, the present study
reports for the first time that BMSC and BMSC + Ch-ABC treatments
enhance the growth factor responses at distal sites of the ANA, at
the spinal cord and target muscle, in a rat model of sciatic nerve
gap. The patterns observed at distal sites reflect growth factor
expression at the graft site, indicating that the propagation of
growth cues occurs bidirectionally. Notably, retrograde axonal
transport of growth factors, such as BDNF and NT-3, has previously
been observed in spinal cord motoneurons with a distinct
selectivity (18). Anterograde
axonal transport of the neurotrophic factor, leukemia inhibitory
factor, to denervated muscle was previously observed in a model of
sciatic nerve transection (19),
thus suggesting that neurotrophins such as BDNF, NGF and VEGF may
also accumulate in target organs such as muscle. In 2001, von
Bartheld et al (20) proposed
several intricate, multi-step transfers of neurotrophic factors
across neural circuits, a number of which may fit within the
observed axonal anterograde transport and even reach dendritic
fields. Ultimately, this bidirectional propagation of growth
signals is likely an important contributor to the cellular and
functional recovery observed after injury in previous BMSC + Ch-ABC
treatment models (12).
Combining the aforementioned data, the growth signal
enhancement of the ANA, spinal cord and target muscle converge to
guide axonal regeneration, neuronal protection and functional
recovery.
The synergistic effect of local and distal growth
signaling manifest therapeutic benefits, such as the observed nerve
fiber growth that occurs on the ANA, up to 8 weeks after grafting
(Fig. 3). Additionally, spinal cord
motoneurons benefit through increased expression of mature neuronal
markers and motoneuron specifiers (Fig.
5). The indicated increase in motoneuron survival has
previously been observed in intramuscular stem cell transplantation
models, suggesting that spinal cord motoneuron survival is assisted
by growth factor-secreting stem cells acting locally (21), or as far as target muscular regions
(22). It is plausible then, that
BMSC and BMSC + Ch-ABC, promoting growth factor secretion at local
and/or distal ends of the allograft, are similarly supporting
motoneuron survival.
Furthermore, the use of combinatorial ANA therapies
administered in the current study indicated that synaptic profiles
of motoneuron dendrites were markedly improved (Fig. 8). Similarly, the synaptic protein
synaptophysin is enhanced by BMSC + Ch-ABC treatment. Retrograde
transport of neurotrophic factors such as BDNF has previously
demonstrated the capacity to reach motoneruonal synaptic sites
through a process called transsynaptic transcytosis (23), activating a cascade of synaptic
plasticity signals. Another previous study reported that
mesenchymal stem cell secretion of BDNF and GDNF at spinal
motoneuron sites aided synaptophysin-positive nerve terminal
preservation (24) and motoneuron
synaptogenesis (21). Ultimately,
survival and synaptogenesis of the motoneuron are critical to
functional reinnervation of target tissue, and molecular mechanisms
such as those used in the model in the current study likely enhance
functional recovery.
Functional parameters of axonal regeneration have
been previously demonstrated in our BMSC + Ch-ABC model of ANA
(8); however, the molecular
underpinnings had yet to be elucidated. In the current study, it
was revealed that electrophysiological measures of recovered nerve
function are improved, likely as an effect of the local and
retro/anterograde transport of BMSC-assisted growth factors
(Fig. 9). In addition, removal of
proteoglycan obstructions by Ch-ABC contributes to an enhanced
functional recovery. The mechanism by which growth signals trigger
axonal regeneration remains subject to debate. Live, long-term
imaging of axonal lesions has demonstrated that growth factors such
as BDNF are able to promote cytoskeletal elements responsible for
cell growth (25). Specifically, the
number and rate of actin ‘waves’ that form the microfilaments
responsible for axonal morphogenesis and pathfinding, are enhanced
by the introduction of BDNF (26).
Finally, the present study reported that the
restoration of target muscle mass is innervated by axotomized
nerves. Muscular growth and reinnervation are critical components
of functional recovery from PNI. Graft site stimulation of growth
cues were anterogradely propagated to muscle targets. Gene
manipulation-derived delivery of the growth factor VEGF has
previously been demonstrated to aid in peripheral nerve
regeneration through the enhancement of nerve reinnervation and
axonal diameter growth (27).
Similarly, VEGF intervention in a PNI model demonstrated that
denervated muscle atrophy was significantly slowed, although VEGF
overexpression led to worse nerve regeneration compared with
negative controls (28).
Notably, although Ch-ABC and BMSC contributed to the
functional recovery of the nerve, the combination of the two
outweighed the therapeutic benefit of each treatment individually
in almost all parameters of morphological and functional recovery
assessed by the current study. This effect is enduring and abounds
not only in the graft site but extends to both distal regions where
the target muscle and motoneurons also demonstrate therapeutic
phenomena. In the current study, the first reported comprehensive
molecular examination of the BMSC + Ch-ABC ANA in an in vivo
model of sciatic nerve gap was attempted. It was determined that
the combination therapy enhances the growth response of the nerve,
however, also appears to stimulate the growth response of the
target organ and motoneurons. The aforementioned wide-reaching
stimulation of growth factors ultimately contributes to functional
repair of not only the nerve, but also the protection of the
motoneuron and the growth of the target muscle.
Although the current study improved our
understanding of the molecular cues guiding nerve regeneration in
Ch-ABC + BMSC ANA repair, the method by which growth signals are
propagated to the distal ends of the graft remains unclear. We
propose retro- and anterograde transport pathways as a plausible
means of growth signal propagation, though a thorough examination
of these mechanisms remains to be performed. Furthermore, the
convergence of distal and local growth signals render it difficult
to reveal the exact method by which each signaling source is
mechanistically associated with individual parameters of post-graft
recovery. It will be important in the future to analyze these
independently to better gauge how each signaling source
specifically contributes to cellular and functional aspects of
recovery. Investigation of growth factor-activated downstream
signaling is also important to further understand the exact
signaling pathways that mediate anatomical and functional
restoration such as neurite growth. Pharmacological manipulation
and time-course designs are potential approaches to further
investigate the results reported in the current study. Despite
limitations, it is clear that an understanding of how BMSCs and
Ch-ABC mediate growth signaling affects and enhance donor nerve
growth cues is a step toward optimizing the combinatorial therapy
that has to date demonstrated marked efficacy. This endeavor is
worthwhile as a full functional recovery of peripheral nerve injury
remains to be achieved.
Acknowledgements
The present study was supported by grants from the
Regional Science Fund Project of the Natural Science Foundation of
China (grant nos. 81371362 and 81260193), the Natural Science
Foundation of Heilongjiang, China (grant no. H201491) and the
Natural Science Foundation of Ningxia, China (grant no.
NZ12187).
Glossary
Abbreviations
Abbreviations:
|
ANA
|
acellular nerve allograft
|
|
PNI
|
peripheral nerve injury
|
|
Ch-ABC
|
chondroitinase ABC
|
|
BMSC
|
bone marrow stromal cells
|
References
|
1
|
Johnson EO, Zoubos AB and Soucacos PN:
Regeneration and repair of peripheral nerves. Injury. 36(Suppl 4):
S24–S29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Millesi H: Bridging defects: Autologous
nerve graftsHow to improve the results of peripheral nerve surgery.
100. Millesi H and Schmidhammer R: Springer; Vienna: pp. 37–38.
2007, View Article : Google Scholar
|
|
3
|
Tang P and Chauhan A: Decellular Nerve
Allografts. J Am Acad Orthop Surg. 23:641–647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang C, Yao C, He XJ and Li HP: Repair of
subacute spinal cord crush injury by bone marrow stromal cell
transplantation and chondroitinase ABC microinjection in adult
rats. Nan Fang Yi Ke Da Xue Xue Bao. 30:2030–2035. 2010.(In
Chinese). PubMed/NCBI
|
|
5
|
Zhang LX, Tong XJ, Sun XH, Tong L, Gao J,
Jia H and Li ZH: Experimental study of low dose ultrashortwave
promoting nerve regeneration after acellular nerve allografts
repairing the sciatic nerve gap of rats. Cell Mol Neurobiol.
28:501–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Côté MP, Amin AA, Tom VJ and Houle JD:
Peripheral nerve grafts support regeneration after spinal cord
injury. Neurotherapeutics. 8:294–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Zhang H, Zhang G, Ka K and Huang
W: Combining acellular nerve allografts with brain-derived
neurotrophic factor transfected bone marrow mesenchymal stem cells
restores sciatic nerve injury better than either intervention
alone. Neural Regen Res. 9:1814–1819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia H, Wang Y, Tong XJ, Liu GB, Li Q,
Zhang LX and Sun XH: Sciatic nerve repair by acellular nerve
xenografts implanted with BMSCs in rats xenograft combined with
BMSCs. Synapse. 66:256–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Zhang X, Cao R, Yu B, Liang H, Zhou
M, Li D, Wang Y and Liu E: Allografts of the acellular sciatic
nerve and brain-derived neurotrophic factor repair spinal cord
injury in adult rats. PLoS One. 7:e428132012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L,
Chen J, Xu W, Lu S, Zhao Q and Peng J: Recellularized nerve
allografts with differentiated mesenchymal stem cells promote
peripheral nerve regeneration. Neurosci Lett. 514:96–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY,
Wu CW, Wang CC, Wang WY, Huang YS and Hsu SH: Transplantation of
bone marrow stromal cells for peripheral nerve repair. Exp Neuro.
204:443–453. 2007. View Article : Google Scholar
|
|
12
|
Wang Y, Jia H, Li WY, Tong XJ, Liu GB and
Kang SW: Synergistic effects of bone mesenchymal stem cells and
chondroitinase ABC on nerve regeneration after acellular nerve
allograft in rats. Cell Mol Neurobiol. 32:361–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W,
Patel N, et al: Adult bone marrow stromal cells differentiate into
neural cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstein LA, Kurz EM and Sengelaub DR:
Androgen regulation of dendritic growth and retraction in the
development of a sexually dimorphic spinal nucleus. J Neurosci.
10:935–946. 1990.PubMed/NCBI
|
|
15
|
Kurz EM, Bowers CA and Sengelaub DR:
Morphology of rat spinal motoneurons with normal and hormonally
altered specificity. J Comp Neurol. 292:638–650. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byers JS, Huguenard AL, Kuruppu D, Liu NK,
Xu XM and Sengelaub DR: Neuroprotective effects of testosterone on
motoneuron and muscle morphology following spinal cord injury. J
Comp Neurol. 520:2683–2696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YR, Ka K, Zhang GC, Zhang H, Shang
Y, Zhao GQ and Huang WH: Repair of peripheral nerve defects with
chemically extracted acellular nerve allografts loaded with
neurotrophic factors-transfected bone marrow mesenchymal stem
cells. Neural Regen Res. 10:1498–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DiStefano PS, Friedman B, Radziejewski C,
Alexander C, Boland P, Schick CM, Lindsay RM and Wiegand SJ: The
neurotrophins BDNF, NT-3, and NGF display distinct patterns of
retrograde axonal transport in peripheral and central neurons.
Neuron. 8:983–993. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bennett TM, Dowsing BJ, Austin L, Messina
A, Nicola NA and Morrison WA: Anterograde transport of leukemia
inhibitory factor within transected sciatic nerves. Muscle Nerve.
22:78–87. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
vonBartheld CS, Wang X and Butowt R:
Anterograde axonal transport, transcytosis, and recycling of
neurotrophic factors: The concept of trophic currencies in neural
networks. Mol Neurobiol. 24:1–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spejo AB, Carvalho JL, Goes AM and
Oliveira AL: Neuroprotective effects of mesenchymal stem cells on
spinal motoneurons following ventral root axotomy: Synapse
stability and axonal regeneration. Neuroscience. 250:715–732. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki M, McHugh J, Tork C, Shelley B,
Hayes A, Bellantuono I, Aebischer P and Svendsen CN: Direct muscle
delivery of GDNF with human mesenchymal stem cells improves motor
neuron survival and function in a rat model of familial ALS. Mol
Ther. 16:2002–2010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rind HB, Butowt R and von Bartheld CS:
Synaptic targeting of retrogradely transported trophic factors in
motoneurons: Comparison of glial cell line-derived neurotrophic
factor, brain-derived neurotrophic factor, and cardiotrophin-1 with
tetanus toxin. J Neurosci. 25:539–549. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hell RC Rodrigues, Costa MM Silva, Goes AM
and Oliveira AL: Local injection of BDNF producing mesenchymal stem
cells increases neuronal survival and synaptic stability following
ventral root avulsion. Neurobiol Dis. 33:290–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong WK, Cheung AW, Yu SW, Sha O and Cho
EY: Hepatocyte growth factor promotes long-term survival and axonal
regeneration of retinal ganglion cells after optic nerve injury:
Comparison with CNTF and BDNF. CNS Neurosci Ther. 20:916–929. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Difato F, Tsushima H, Pesce M, Benfenati
F, Blau A and Chieregatti E: The formation of actin waves during
regeneration after axonal lesion is enhanced by BDNF. Sci Rep.
1:1832011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haninec P, Kaiser R, Bobek V and Dubový P:
Enhancement of musculocutaneous nerve reinnervation after vascular
endothelial growth factor (VEGF) gene therapy. BMC Neurosci.
13:572012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moimas S, Novati F, Ronchi G, Zacchigna S,
Fregnan F, Zentilin L, Papa G, Giacca M, Geuna S, Perroteau I, et
al: Effect of vascular endothelial growth factor gene therapy on
post-traumatic peripheral nerve regeneration and
denervation-related muscle atrophy. Gene Ther. 20:1014–1021. 2013.
View Article : Google Scholar : PubMed/NCBI
|