Introduction
Coxsackievirus is a type of non-enveloped, linear,
positive-sense single-stranded RNA virus that can be divided into
group A and B viruses. Group B coxsackieviruses (CVB) include six
serotypes (CVB1 -CVB6). Infants, young children and
immunocompromised individuals are particularly susceptible to
infection, leading to severe morbidity and mortality. CVB primarily
infect organs such as the heart, pleura, pancreas and liver causing
myocarditis (1), pleurodynia,
pericarditis and hepatitis (2–4). CVB3
infection leads to cardiomyocyte death and induces diseases such as
myocarditis and cardiomyopathy (5).
Increasing research has focused on understanding the molecular
mechanisms involved in CVB3 infection.
MicroRNAs (miRNAs) are small non-coding RNAs that
act posttranscriptionally to regulate gene expression (6). miRNAs have critical roles in numerous
biological (6,7) and pathological processes (8–11). The
presence of circulating miRNAs often correlates with the presence
of disease, such as cancer, myocardial infarction and diabetes, and
these have been indicated to be practicable, promising and
noninvasive biomarkers (12).
Previous studies demonstrated that miRNAs regulate
the pathogenesis of viral myocarditis; in the heart tissue of
patients with viral myocarditis, several miRNAs have been observed
to be differentially expressed (13). miR-155 was indicated as a potential
therapeutic target for viral myocarditis as it downregulates
cardiac myoblast cytokine expression during CVB3 infection
(14). Our previous study also
demonstrated that host cellular miRNAs are involved in the
regulation of CVB3 biosynthesis by targeting CVB3-coding genes
(15). However, little is known
about circulating miRNA changes following CVB infection. The
present study endeavored to detect miRNA expression changes in the
peripheral blood of mice infected with CVB3, with the aim to
provide novel insight into the diagnosis and treatment of viral
infectious diseases.
Materials and methods
Animals
A total of 182 BALB/c mice (3–4 days old; weight,
2±0.2 g) were obtained from the Harbin Medical University
Experimental Animal Center, Harbin, Heilongjiang, China. All
experimental protocols were approved by the Experimental Animal
Ethics Committee of Harbin Medical University Harbin, Heilongjiang,
China. The use of animals conformed to the Guide for the Care and
Use of Laboratory Animals, published by the US National Institutes
of Health (16).
Establishment of a CVB3 mouse
infection model
CVB3 was expressed within the pMKS-1 plasmid, which
contained the full-length cDNA of the CVB3 genomic cDNA (obtained
from Dr J. Linsay, Whitton of the Scripps Research Institute, La
Jolla, CA, USA). The CVB3 H3 strain was prepared by passage through
HeLa cells (American Type Culture Collection, Manassas, VA, USA).
HeLa cells were cultured in Dulbecco's Modified Eagle's Medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Biological
Industries, Kibbutz Beit Haemek, Israel) and antibiotics (50 U/ml
penicillin and 0.1 mg/ml streptomycin) at 37°C with 5%
CO2. Two CVB3 variants, EGFP-CVB3 and RLuc-CVB3, were
recovered by transfecting HeLa cells with pEGFP-CVB3 and
pRLuc-CVB3, respectively. Briefly, HeLa cells were seeded in
12-well culture plates at the density of 1×105
cells/well and cultured for 18–24 h. When 60–70% confluence was
reached, the cells were transfected with 0.8 µg pEGFP-CVB3 and
pRLuc-CVB3, and maintained in DMEM supplemented with 5% FBS.
Cytopathic effects in the transfected cells were observed at 24 h
post-transfection. The recovered viruses were purified and titered
by plaque assay. Viral titers were routinely determined by a 50%
tissue culture infectious dose (TCID50) assay of HeLa cell
monolayers. The virus samples were diluted in DMEM. Serially
diluted virus samples (from 1×10−1 to 1×10−9)
were added to the HeLa cells in 96-well plates and the
quadruplicate samples were used at each dilution. The 96-well
plates were incubated for 7 days at 37°C, and the TCID50 values
were measured by counting the cytopathic effects of infected HeLa
cells. The TCID50 values were calculated using ID-505.0 software,
developed at the National Center for Biotechnology Information
(Bethesda, MD, USA). Upon reaching adequate viral copy number,
BALB/c mice were intraperitoneally administered a dose of
2×106 TCID50 of the virus or the same volume of DMEM, in
the case of the normal control (NC) group, which was determined to
be day 0 of the experiment. Mice were sacrificed by decapitation
(17), and the peripheral blood
samples in the CVB3 and negative control (NC) groups were collected
on days 0, 3 and 6 after infection.
Immunohistochemistry
The myocardial tissues were fixed in 10%
neutral-buffered formalin for 48 h and embedded in paraffin by
routine histochemical procedures (18). Hematoxylin and eosin (HE) staining
was used to detect histological changes.
miRNA microarray analysis
The miRNA microarray was conducted by KangChen
Bio-Tech (Shanghai, China). Total RNA was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and miRNA was extracted using an miRNeasy mini kit (Qiagen
GmbH, Hilden, Germany), according to the manufacturer's
instructions. The quantity of isolated RNA was assessed with a
NanoDrop 1000 UV-Vis Spectrophotometer (Nanodrop Technologies;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Quantified
RNA samples (0.3 µl total RNA and 25 µl microRNA) were labeled with
a miRCURY Hy3/Hy5 Power labeling kit (Exiqon A/S, Vedbaek, Denmark)
and hybridized on an miRCURY LNA Array (Exiqon A/S). Subsequent to
washing with water, the microarrays were scanned using a GenePix
4000 microarray scanner (Molecular Devices, LLC, Sunnyvale, CA,
USA) and analyzed using Pro 6.0 software (Molecular Devices, LLC).
miRNAs with reported intensities ≥30 were retained for further
analysis. The raw expression data of miRNAs were normalized by
transforming the expression of each gene into having a mean of 0
and a standard deviation (SD) of 1 (19). Following normalization, the mean of
replicate values of each miRNA were used for statistical analysis.
The presence of differentially expressed miRNAs was determined
using volcano plot filtering, with a threshold of ≥2.0-fold change
and a P-value ≤0.05. Finally, hierarchical clustering was performed
to demonstrate distinguishable miRNA expression profiling among the
samples.
Quantiative polymerase chain reaction
(qPCR)
The total RNA was extracted from the samples using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
generated using a PrimeScript RT reagent kit (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's instructions. Total
RNA (1 µg) was used as template for RT along with antisense primers
and PrimeScript RT Enzyme Mix I (Takara Bio, Inc.). qPCR was
performed in triplicate using SYBR Premix Script Ex Taq II (Takara
Bio, Inc.) according to the manufacturer's instructions. Briefly,
qPCR was performed with 1 µl synthesized cDNA, SYBR PrimeScript Ex
Taq II (Takara Bio, Inc.), and sense/antisense primers for a final
reaction volume of 20 µl. The sequences of the primers were as
follows: miR-216a reverse transcription (RT):
CTCAACTGGTGTCGTGGAGTCGG-CAATTCAGTTGAGCACAGT, forward:
TAATCTCAGCTGCAACTGTGA, reverse: CAGTGCGTGTCGTGGAGT; miR-710: RT:
CTCAACTGGTGTCGTGGAGTC-GGCAATTCAGTTGAGTCAACT, forward:
CCAAGTCTTGGGGAGAGTTGAG, reverse: CAGTGCGTGTCGTGGAGT; miR-377: RT:
GTCGTATCCAGTGCGTGTCGT-GGAGTCGGCAATTGCACTGGATACGACCAAAAG, forward:
GGGGATCACACAAAGGCAA, reverse: CAGTGCGTGTCGTGGAGT; miR-191: RT:
CTCAACTGGTGT-CGTGGAGTCGGCAATTCAGTTGAGAGCTGC, forward:
CAACGGAATCCCAAAAGCAGCTG, reverse: CAGTGCGTGTCGTGGAGT; miR-713: RT:
GTCGTATCCAGT-CGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGCTGTG, forward:
GGGGTGCACTGAAGGCA, reverse: CAGTGCGTGTCGTGGAGT; U6 snRNA served as
an internal control, U6 RT: CGCTTCACGAATTTGCGTGTCAT, forward:
GCTTCGGCAGCACATATACTAAAAT, reverse: CGCTTCACGAATTTGCGTGTCAT.
Thermocycling was carried out in a LightCycler 2.0 (Roche, Basel,
Switzerland) with the following conditions: 40 cycles of 5 sec at
95°C, 20 sec at 55°C and 15 sec at 72°C. The analytical procedures
of dissociation curve was followed by one cycle of 1 sec at 95°C,
20 sec at 65°C and 1 sec at 95°C. The relative RNA expression data
were analyzed using the 2−ΔΔCt method (20).
Target prediction of miRNAs
The predicted targets of the differentially
expressed miRNAs were obtained from the TargetScan (www.targetscan.org/mamm_31/), miRBase (www.mirbase.org) (21),
and miRanda (www.microrna.org/microrna/home.do) (22) databases. The common results obtained
from these databases were regarded as reliable target genes.
Functional assignment of
differentially expressed miRNAs
To determine the biological functions of the
differently expressed miRNAs in this model, gene ontology (GO) and
Kyoto Encyclopedia Genes and Genomes (KEGG) enrichment analyses
were performed. These were used for predicting the target genes of
statistically significantly altered miRNA, and were conducted using
the DAVID Bioinformatics Tool v. 6.7 (http://david.abcc.ncifcrf.gov/). This is a commonly
used functional annotation tool that can assess overrepresentation
of functional categories among a gene set of interest (23).
Statistical analysis
Statistical analyses were performed using SigmaStat
3.1 software (Systat Software, San Jose, CA, USA). The measurement
data are presented as the mean ± SD and determined by Student's
t-test. For functional analyses, Fisher's exact test,
corrected by the false discovery rate method with an adjusted
P-value <0.01 following Bonferroni correction, was used to
determine the probability that each biological function assigned to
that data set was due to chance alone. P-values <0.05 were
considered to represent a statistically significant difference
Results
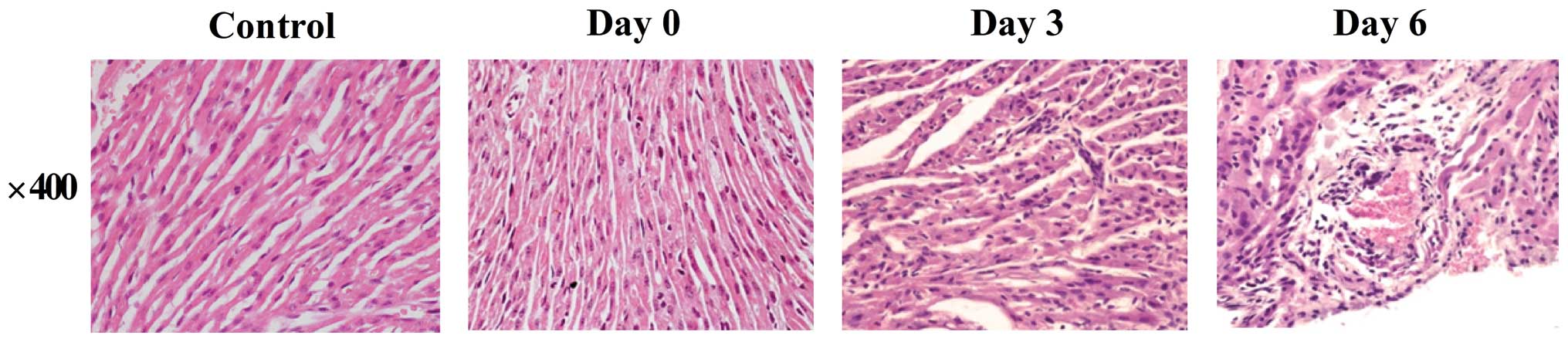
Histopathological changes in the
CVB3-infected mouse heart
HE staining was used to confirm the
histopathological changes in heart tissues. On day 3 after
infection, heart tissues from CVB3-infected mice demonstrated mild
inflammation. On day 6, the heart tissues demonstrated severe
myocardial inflammation, with myocardial cell swelling, and the
myocardial fibers were in a disorganized array (Fig. 1).
Identification of differentially
expressed miRNAs
The miRNA expression profile in CVB3-infected mouse
blood was compared with that of NC mice using a miRCURY LNA Array.
The fold-change was calculated in this comparison in order to
determine the extent and direction of differential expression prior
and subsequent to infection. A total of 96 differentially expressed
miRNAs were identified (33 upregulated and 63 downregulated) on day
3 after infection. A total of 89 differentially expressed miRNAs
were identified (37 upregulated and 52 downregulated) on day 6
after infection. The list of top 10 significantly altered miRNAs
are reported in Tables I and
II. The miRNAs identified as
differentially expressed on day 0 were rejected during the target
prediction and functional analysis.
 | Table I.Significantly upregulated miRNAs. |
Table I.
Significantly upregulated miRNAs.
|
| 3 day |
| 6 day |
|---|
|
|
|
|
|
|---|
| miRNAs | Fold change | P-value | miRNAs | Fold change | P-value |
|---|
| miR-101a-5p | 16.72324 | 0.024329 | miR-883b-5p | 8.899323 | 0.002493 |
| let-7a-5p | 14.94596 | 0.030628 | miR-1192 | 3.343596 | 0.043987 |
| miR-219-5p | 13.38862 | 0.047338 | miR-2137 | 2.100476 | 0.017017 |
| miR-1892 | 12.22507 | 0.014818 | miR-1899 | 2.466724 | 0.010656 |
| miR-9-5p | 11.05589 | 0.000185 | miR-9-3p | 2.376287 | 0.009148 |
| miR-190a-5p | 8.958582 | 0.002267 | miR-713 | 3.690579 | 0.042359 |
| miR-542-3p | 8.310468 | 0.040532 | miR-21a-3p | 3.852925 | 0.002557 |
| miR-24-2-5p | 7.609792 | 0.017971 | miR-465c-5p | 2.588157 | 0.035946 |
| miR-92a-2-5p | 7.551922 | 0.011545 | miR-25-5p | 4.527052 | 0.039037 |
| miR-342-3p | 7.111589 | 0.000863 | miR-147-3p | 2.640046 | 0.043361 |
 | Table II.Significantly downregulated
miRNAs. |
Table II.
Significantly downregulated
miRNAs.
|
| 3 day |
| 6 day |
|---|
|
|
|
|
|
|---|
| miRNAs | Fold change | P-value | miRNAs | Fold change | P-value |
|---|
| miR-1188-3p | 0.048223 | 0.033206 | miR-125b-5 | 0.114178 | 0.047021 |
| miR-135a-5p | 0.078379 | 0.040663 | miR-541-5p | 0.135516 | 0.037748 |
| miR-1190 | 0.091781 | 0.000221 | miR-881-3p | 0.142696 | 0.012731 |
| miR-3067-5p | 0.094127 | 0.012448 | miR-363-3p | 0.164122 | 0.046749 |
|
miR-1982.1–3p/miR-1982.2–3p | 0.110368 | 0.006419 | miR-193a-3p | 0.175409 | 0.023604 |
| miR-1968-3p | 0.110595 | 0.007126 | let-7g-3p | 0.177574 | 0.038513 |
| miR-18b-3p | 0.112993 | 0.009556 | miR-31-5p | 0.186043 | 0.009361 |
| miR-3109-5p | 0.117507 | 0.025232 | miR-3082-5p | 0.199540 | 0.015887 |
| miR-5627-3p | 0.124773 | 0.011820 | miR-669d-5p | 0.219337 | 0.005506 |
| miR-5046 | 0.143941 | 0.004508 | miR-709 | 0.245063 | 0.001812 |
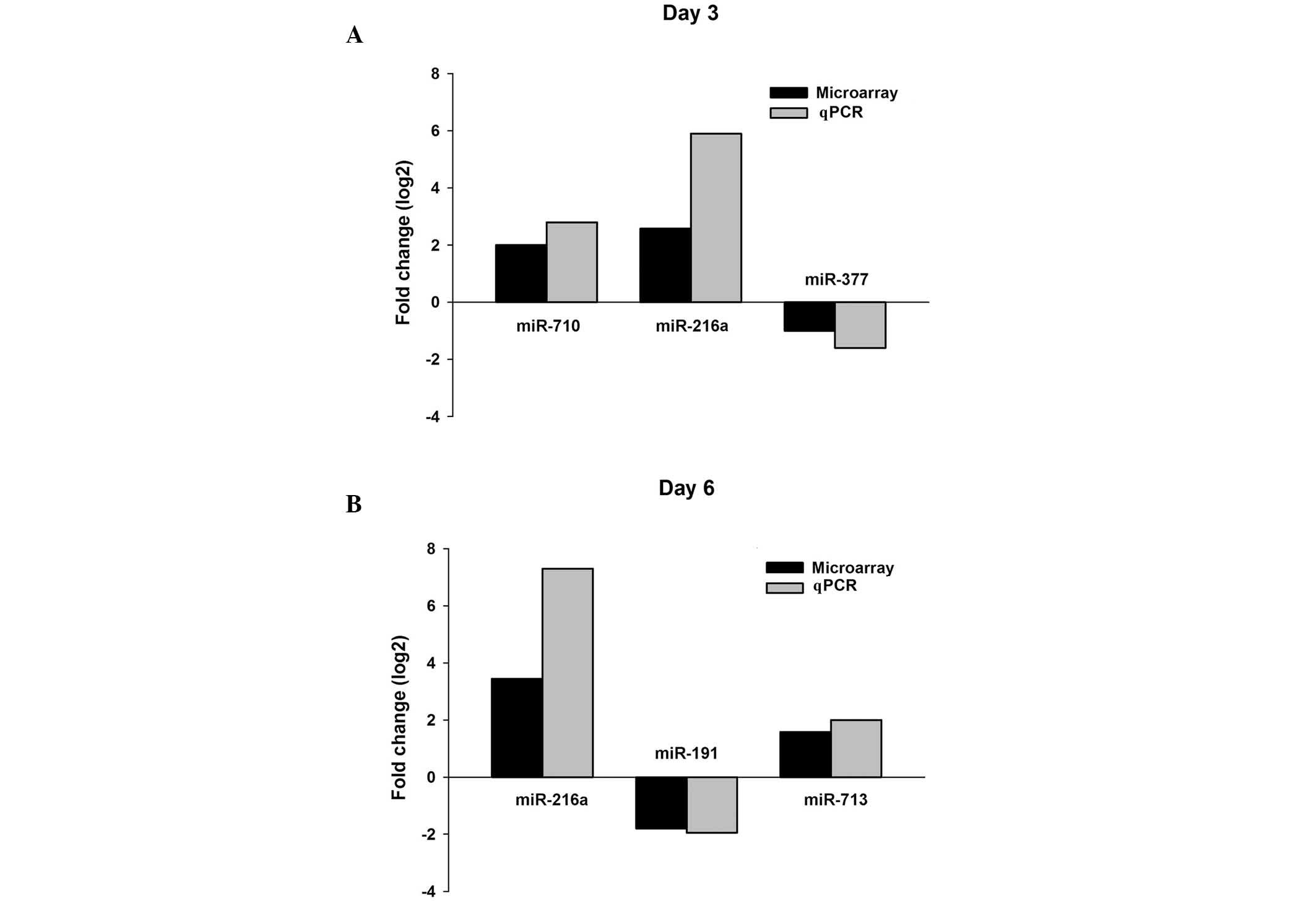
Validation of differentially expressed
miRNAs in CVB3-infected mouse blood by qPCR
Several miRNAs were detected by qPCR in order to
validate the microarray analysis results. These results indicated
that miR-216a and miR-710 were upregulated and miR-337 was
downregulated on day 3 after infection; on day 6 after infection,
miR-216a and miR-713 were reported to be upregulated and miR-191
was downregulated (Fig. 2). The
results of the qPCR for the selected miRNAs were consistent with
the miRNA microarray analysis results (Fig. 2), indicating that the results of the
miRNA microarray are reliable. Amongst these differentially
expressed miRNAs, only miR-216a was upregulated on both day 3 and
day 6 after infection.
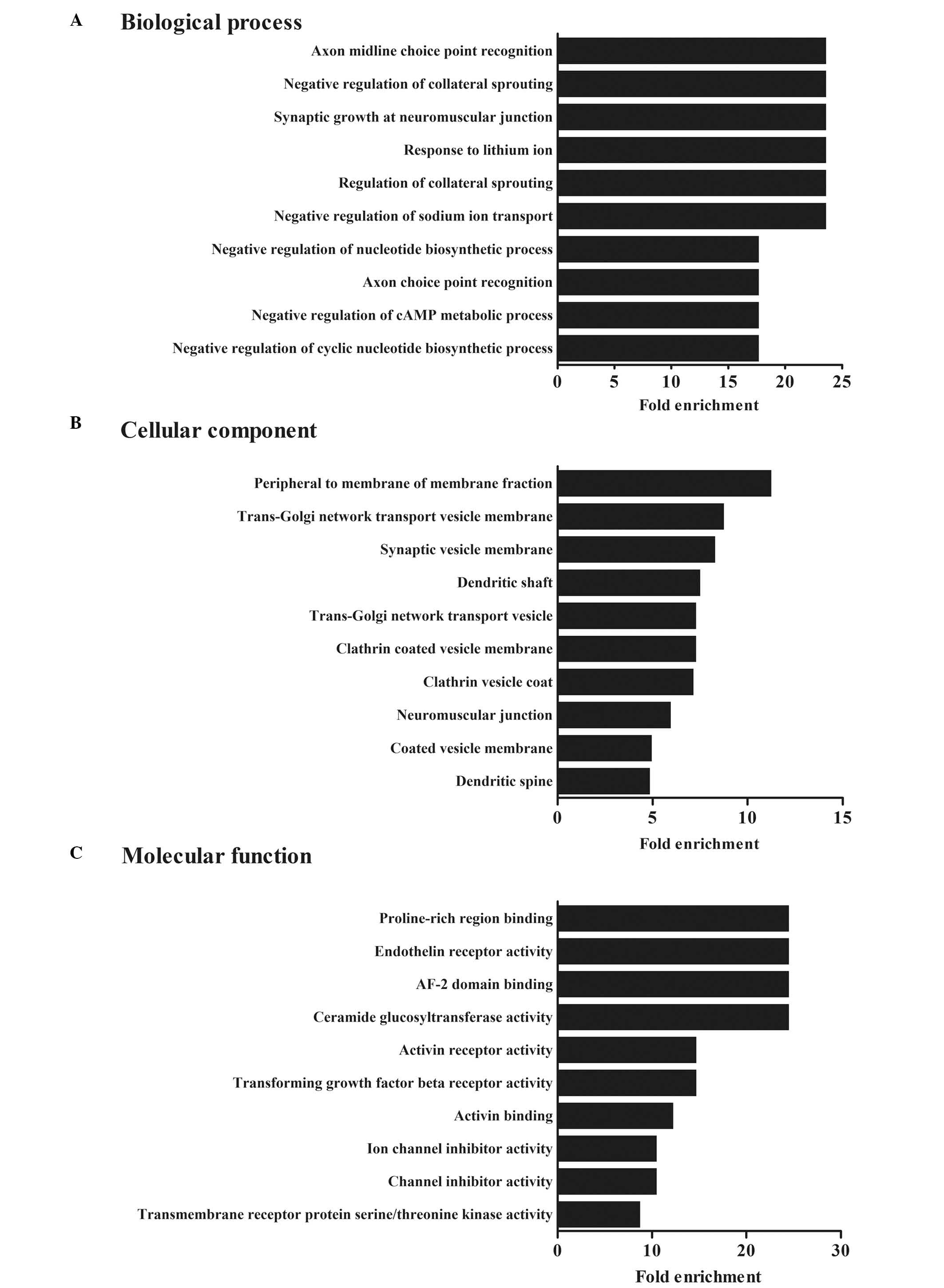
Functional analysis of differentially
expressed miRNAs
To analyze the possible mechanisms involved in CVB3
infection, the predicted miRNA target genes were subjected to GO
enrichment analysis. The results revealed that these predicted
targets were involved in 287 biological processes, including 81
molecular functions and 81 cellular functions (P<0.05). The top
ten biological processes affected by the differentially expressed
miRNAs are reported in Fig. 3. The
biological interpretation of the target genes of differential
miRNAs was further extended using KEGG pathway enrichment analysis.
A total of 66 different metabolic pathways were reported, causative
of hypertrophic, dilated or arrhythmogenic right ventricular
cardiomyopathy (Table III). These
results implied possible roles of alterations to circulating miRNAs
in the regulation of the biological processes involved in CVB3
infection.
 | Table III.Predicted miRNAs involved in CVB3
infection-induced cardiomyopathy. |
Table III.
Predicted miRNAs involved in CVB3
infection-induced cardiomyopathy.
| miRNAs | KEGG terms | Corrected
P-value | Hit gene
symbols |
|---|
| miR-132-3p | Dilated
cardiomyopathy | 0.044589 | Ryr2, Cacna2d1,
Itga9, Gnas, Slc8a1, Actb, Prkacb |
| miR-212-3p | Dilated
cardiomyopathy | 0.022819 | Ryr2, Itgav,
Cacna2d1, Itga9, Slc8a1, Actb, Prkacb |
|
| Hypertrophic
cardiomyopathy | 0.014619 | Itgav, Actb,
Prkaa2, Cacna2d1, Ryr2, Slc8a1, Itga9 |
|
| Arrhythmogenic
right ventricular cardiomyopathy | 0.055986 | Itgav, Actb,
Cacna2d1, Ryr2, Slc8a1, Itga9 |
| miR-335-5p | Hypertrophic
cardiomyopathy | 0.066057 | Itga6, Dag1,
Cacng2, Sgcb, Cacna2d1, Ryr2, Slc8a1 |
|
| Arrhythmogenic
right ventricular cardiomyopathy | 0.004416 | Itga6, Dag1,
Cacng2, Sgcb, Cacna2d1, Ryr2, Slc8a1, Gja1 |
| miR-92a-2-5p | Dilated
cardiomyopathy | 0.049858 | Cacna1c, Cacna2d1,
Sgcb, Itga5, Atp2a2, Slc8a1, Dmd |
|
| Hypertrophic
cardiomyopathy | 0.032248 | Sgcb, Atp2a2,
Cacna2d1, Cacna1c, Itga5, Slc8a1, Dmd |
|
| Arrhythmogenic
right ventricular cardiomyopathy | 0.015516 | Sgcb, Atp2a2,
Cacna2d1, Cacna1c, Itga5, Slc8a1, Dmd |
| miR-9-5p | Dilated
cardiomyopathy | 0.083769 | Dag1, Itga1,
Cacna2d1, Adcy5, Slc8a1, Itgb1, Lmna, Dmd, Itga6, Cacnb4 |
|
| Arrhythmogenic
right ventricular cardiomyopathy | 0.086685 | Itga6, Dag1,
Cacnb4, Itga1, Cacna2d1, Itgb1, Slc8a1, Dmd, Lmna |
Discussion
An infection with CVB3 may induce viral myocarditis,
dilated cardiomyopathy and heart failure (1,2,4). However, little is known about the
mechanisms involved in developing these symptoms. A large number of
miRNAs has been discovered in plants, animal, and certain viruses,
in which they regulate gene expression at a posttranscriptional
level (24–26). In the cardiovascular system, miRNAs
are involved in numerous cardiac pathophysiological processes,
including heart development, cardiac hypertrophy, myocarditis,
cardiomyopathy and heart failure (27–30).
An increasing number of studies have indicated that
miRNAs are involved in the duplication and the pathogenesis of
CVB3. miR-203 is one of the most upregulated miRNAs in
CVB3-infected murine hearts, which enhances CVB3 replication by
targeting the zinc finger protein-148 (31). Myocardial miR-21 expression is
significantly decreased in cases of CVB3-induced myocarditis, and
negatively correlates with disease severity. miR-21 administration
efficiently alleviates CVB3-induced myocarditis by repressing
PDCD4-mediated apoptosis (32). We
previously reported that miR-10a positively modulates gene
expression and affects CVB3 replication during cardiac infection
(33). However, little is known
regarding circulating miRNA changes after CVB3 infection.
The present study demonstrates novel alterations to
miRNA expression in the peripheral blood of mice infected with
CVB3. Subsequent to infection, mouse heart tissues revealed
inflammation, which became more severe over time. An miRNA
microarray analysis demonstrated that miRNAs in the peripheral
blood were differentially expressed on days 3 and 6 after
infection. This differential miR-216a, miR-710, miR-377, miR-191
and miR-713 expression was validated by qPCR.
In the present study, 66 differentially expressed
miRNAs were determined. Among these, miR-132-3p, miR-212-3p,
miR-335-5p, miR-92a-2-5p and miR-9-5p were predicted to be
associated with cardiac pathologies, including dilated
cardiomyopathy, hypertrophic cardiomyopathy and arrhythmogenic
right ventricular cardiomyopathy. These miRNAs have been confirmed
to be differentially expressed and even have an important role in
myocardial diseases. It has previously been reported that miR-132,
miR-212 and miR-9 were involved in the progression of cardiac
hypertrophy (34–37), and circulating levels of miR-92a are
significantly reduced in patients with coronary artery disease
(38). These previous findings, in
conjunction with the current results, indicate that circulating
miRNAs may also have a role in the pathogenesis of cardiovascular
diseases.
Circulating miRNAs may therefore serve as stable
blood-based markers for cancer and cardiovascular diseases
(39). However, the biological
function of circulating miRNAs is largely unknown. The present
findings provide novel evidence for the participation of miRNAs in
CVB3 infection. However, the associated mechanisms, including the
roles of other organs in the pathogenesis of viral myocarditis,
require additional research.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81271825 to Z. Zhong, grant
no. 81101234 to T. Lei), and the Heilongjiang Postdoctoral Grant
(grant no. LBH-Z11076 to T.L). The Heilongjiang Provincial Key
Laboratory of Pathogens and Immunity, and the Heilongjiang
Provincial Science and Technology Innovation Team in Higher
Education Institutes for Infection and Immunity, Harbin Medical
University, Harbin, China are thanked for their technical help.
References
|
1
|
Knowlton KU: CVB infection and mechanisms
of viral cardiomyopathy. Curr Top Microbiol Immunol. 323:315–335.
2008.PubMed/NCBI
|
|
2
|
Kemball CC, Alirezaei M and Whitton JL:
Type B coxsackieviruses and their interactions with the innate and
adaptive immune systems. Future Microbiol. 5:1329–1347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitton JL: Immunopathology during
coxsackievirus infection. Springer Semin Immunopathol. 24:201–213.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitton JL, Cornell CT and Feuer R: Host
and virus determinants of picornavirus pathogenesis and tropism.
Nat Rev Microbiol. 3:765–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yajima T and Knowlton KU: Viral
myocarditis: From the perspective of the virus. Circulation.
119:2615–2624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karp X and Ambros V: Developmental
biology. Encountering microRNAs in cell fate signaling. Science.
310:1288–1289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA.
103:18255–18260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim J, Inoue K, Ishii J, Vanti WB, Voronov
SV, Murchison E, Hannon G and Abeliovich A: A MicroRNA feedback
circuit in midbrain dopamine neurons. Science. 317:1220–1224. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Guire V, Robitaille R, Tétreault N,
Guérin R, Ménard C, Bambace N and Sapieha P: Circulating miRNAs as
sensitive and specific biomarkers for the diagnosis and monitoring
of human diseases: Promises and challenges. Clin Biochem.
46:846–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Xiao Z, He F, Zou J, Wu S and Liu
Z: MicroRNAs regulate the pathogenesis of CVB3-induced viral
myocarditis. Intervirology. 56:104–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao JL and Lin L: MiR-155 and miR-148a
reduce cardiac injury by inhibiting NF-κB pathway during acute
viral myocarditis. Eur Rev Med Pharmacol. 18:2349–2356. 2014.
|
|
15
|
Wang L, Qin Y, Tong L, Wu S, Wang Q, Jiao
Q, Guo Z, Lin L, Wang R, Zhao W and Zhong Z: MiR-342-5p suppresses
coxsackievirus B3 biosynthesis by targeting the 2C-coding region.
Antiviral Res. 93:270–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guidance for the description of
animal research in scientific publications. National Academies
Press; Washington (DC): 2011
|
|
17
|
Lian HY, Gao Y, Jiao GZ, Sun MJ, Wu XF,
Wang TY, Li H and Tan JH: Antioxidant supplementation overcomes the
deleterious effects of maternal restraint stress-induced oxidative
stress on mouse oocytes. Reproduction. 146:559–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhlmann WD: Purification of mouse
alpha1-fetoprotein and preparation of specific peroxidase
conjugates for its cellular localization. Histochemistry.
44:155–167. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou M, Guo M, He DF, Wang XJ, Cui YQ,
Yang HX, Hao DP and Sun J: A potential signature of eight long
non-coding RNAs predicts survival in patients with non-small cell
lung cancer. J Transl Med. 13:2312015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozomara A and Griffiths-Jones S: MiRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42:(Database Issue). D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnes D, Kunitomi M, Vignuzzi M, Saksela
K and Andino R: Harnessing endogenous miRNAs to control virus
tissue tropism as a strategy for developing attenuated virus
vaccines. Cell Host Microbe. 4:239–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J and Wang DZ: MicroRNAs in
cardiovascular development. J Mol Cell Cardiol. 52:949–957. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang ZP, Chen J, Seok HY, Zhang Z,
Kataoka M, Hu X and Wang DZ: MicroRNA-22 regulates cardiac
hypertrophy and remodeling in response to stress. Circ Res.
112:1234–1243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bang C, Batkai S, Dangwal S, Gupta SK,
Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al:
Cardiac fibroblast-derived microRNA passenger strand-enriched
exosomes mediate cardiomyocyte hypertrophy. J Clin Invest.
124:2136–2146. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thum T, Galuppo P, Wolf C, Fiedler J,
Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J,
Haverich A, et al: MicroRNAs in the human heart: A clue to fetal
gene reprogramming in heart failure. Circulation. 116:258–267.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hemida MG, Ye X, Zhang HM, Hanson PJ, Liu
Z, McManus BM and Yang D: MicroRNA-203 enhances coxsackievirus B3
replication through targeting zinc finger protein-148. Cell Mol
Life Sci. 70:277–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He J, Yue Y, Dong C and Xiong S: MiR-21
confers resistance against CVB3-induced myocarditis by inhibiting
PDCD4-mediated apoptosis. Clin Invest Med. 36:E103–E111.
2013.PubMed/NCBI
|
|
33
|
Tong L, Lin L, Wu S, Guo Z, Wang T, Qin Y,
Wang R, Zhong X, Wu X, Wang Y, et al: MiR-10a* up-regulates
coxsackievirus B3 biosynthesis by targeting the 3D-coding sequence.
Nucleic Acids Res. 41:3760–3771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang K, Long B, Zhou J and Li PF: MiR-9
and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem.
285:11903–11912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katare R, Riu F, Mitchell K, Gubernator M,
Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami
AP, et al: Transplantation of human pericyte progenitor cells
improves the repair of infarcted heart through activation of an
angiogenic program involving micro-RNA-132. Circ Res. 109:894–906.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao J, Liang D, Zhang Y, Liu Y, Zhang H,
Liu Y, Li L, Liang X, Sun Y and Chen YH: MicroRNA expression
signature in atrial fibrillation with mitral stenosis. Physiol
Genomics. 43:655–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ucar A, Gupta SK, Fiedler J, Erikci E,
Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann
A, et al: The miRNA-212/132 family regulates both cardiac
hypertrophy and cardiomyocyte autophagy. Nat Commun. 3:10782012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fichtlscherer S, De Rosa S, Fox H,
Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T,
Müller-Ardogan M, et al: Circulating microRNAs in patients with
coronary artery disease. Circ Res. 107:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He
J, Qin YW and Jing Q: Circulating microRNA: A novel potential
biomarker for early diagnosis of acute myocardial infarction in
humans. Eur Heart J. 31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|