Introduction
Allergic rhinitis (AR) and asthma are common
diseases with an increasing prevalence, which, seriously impact the
quality of life of patients (1). A
proportion of AR patients develop asymptomatic bronchial
hyperresponsiveness (BHR), and finally exhibit lower airway
symptoms including coughing, wheezing and breath tightness, which
may develop into asthma. Since is has been reported that AR and
asthma are associated ‘one airway, one disease’ (2), an increasing number of studies have
focused on the association between these conditions. The
epidemiology, pathophysiology, anatomy, immunology evidences, the
consistent response of upper and lower airways to the treatment of
steroids (3–5). Furthermore, international guidelines
(ARIA) (6) have confirmed the
association and impact of them on each other.
Patients suffering from AR without asthma may
present with elevated lower airway inflammation (7). A number of mechanisms have been
proposed to underlie the association between AR and asthma. One of
the these suggests that the inflammation extends from upper airway
to lower airways (8,9). Various methods exist for the assessment
of airway inflammations, including biopsy, lavage and cytology
tests (10). These approaches offer
sensitivity, but may be invasive or time-consuming and require
professional or technical operation (11).
The measurement of fractional exhaled nitric oxide
(FeNO) is a simple, safe and noninvasive method to detect airway
inflammation, which is also correlated well with eosinophil count
and eosinophil cationic protein in induced sputum or lavage fluid
(12,13). FeNO is a widely used metric for the
evaluation and management of airway inflammatory diseases, such as
asthma; however, the measurement of FeNO as a predictor for the
diagnosis of BHR or asthma in AR patients has not been well
investigated. The objective of the present study was to evaluate
the measurement of FeNO for predicting BHR in AR patients
asymptomatic for asthma and patients with AR combined with
asthma.
Materials and methods
Subjects
A total of 102 patients with AR (including AR with
asymptomatic BHR), normal controls (atopic or non-atopic) and AR
combined with asthma (AR with asthma) that consulted the
out-patient department in the First Affiliated Hospital of
Guangzhou Medical University (Guangzhou, China) were recruited
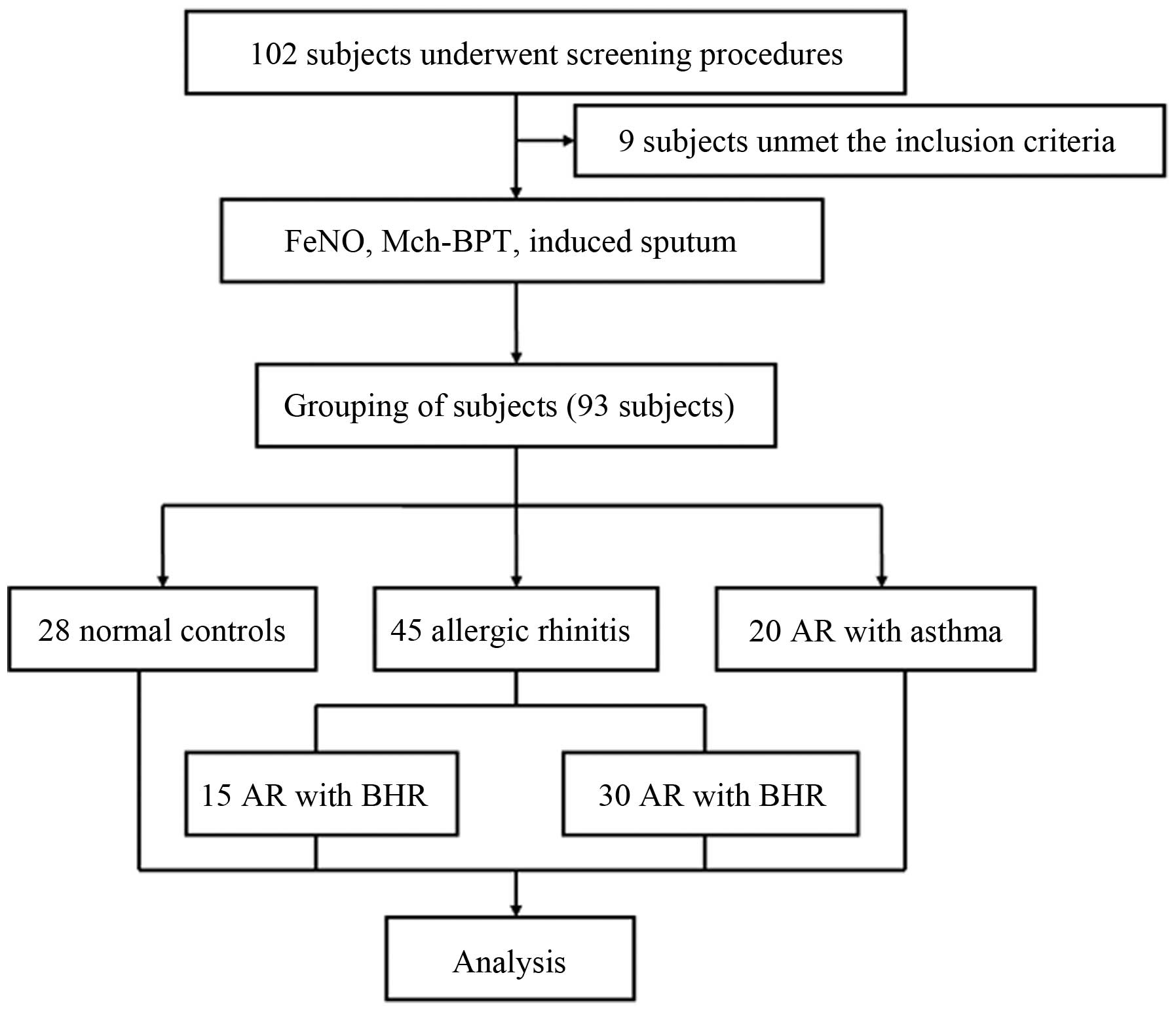
between September 2012 and April 2014 (Fig. 1). After screening, the subjects were
divided into the AR group, AR with asthma group and normal control
group. The AR group was then divided into two sub-groups: AR with
asymptomatic BHR group (AR with BHR) and AR without BHR group. The
diagnosis of AR and asthma were based on the guidelines of ARIA
2010 and GINA 2011 respectively (6,14).
Inclusion criteria: AR patients (male or female) and AR combined
with asthma aged 18–50 years old, with a positive skin prick test
for at least one type of aerogel allergen. The diagnoses of asthma
were required to be >3 months and the patients were not
regularly receiving medication. Exclusion criteria: Patients with
exacerbation of AR or asthma within four weeks; with other
respiratory diseases (e.g. chronic obstructive pulmonary disease);
under the treatment of immunotherapy, leukotriene receptor
antagonist, antihistamines, oral/inhaled corticosteroids or other
medicines that may affect the results of the study within four
weeks prior to the study; pregnant or breast-feeding women.
Study design
The study protocol was registered on https://clinicaltrials.gov/ (NCT01963741) and was
approved by the Ethics Committee of The First Affiliated Hospital
of Guangzhou Medical University (approval no. 2012–18). Written
informed consent was obtained from each participant prior to the
study.
As shown in Fig. 1,
following screening, all enrolled patients underwent measurement of
FeNO, methacholine (Provocholine, Methapharm Inc., Brantford, ON,
Canada) bronchial provocation test (Mch-BPT) and induced sputum.
Eosinophil count in induced sputum was conducted by routing method
(15).
Measurements
FeNO
The measurement of FeNO was performed by using a
NIOX MINO® medical device (Aerocrine AB, Solna, Sweden).
All procedures met the recommendations of the American Thoracic
Society and the European Respiratory Society (ATS/ERS) on FeNO
measurement (16). The normal value
of FeNO in Guangzhou was 17.7 (6.1) ppb, 95%CI (6.1–41.0 ppb)
(17).
Mch-BPT
Spirometry and Mch-BPT were performed using a
spirometer (Jaeger Masterscreen) and automated APS pro system
(CareFusion; BD Biosciences, San Diego, CA, USA), which meet the
joint recommendations of the ATS/ERS (18). At least three maneuvers (≤8) were
required with the variation of forced vital capacity (FVC), and
forced expiratory volume in 1 sec (FEV1) between the
best value best two values <5% or 150 ml. FVC and
FEV1 reported the best records. The predicted values
were calculated based on the equation of Zheng and Zhong (19). The provocation was performed in a
stepwise manner with initial and ultimate cumulative doses 0.390
and 12.520 mmol, respectively.
Sputum induction
Sputum was induced by using 3–4% hypertonic saline
nebulized for 10–20 min. Patients with BHR inhaled 200 µg
salbutamol through a spacer prior to the hypertonic saline
nebulization. The induced sputum was collected, and the
inflammation cells viability was identified by trypan blue; the
sample was considered acceptable when the viability of the
inflammation cells in the sputum was >50% and the ratio of
epithelial cells <20% (15). A
total of 400 non-epithelial cells were counted under 200 times
scope of the microscope with HE staining, the percentages of
eosinophils were reported.
Statistical analysis
Statistical analysis was performed with the SPSS
16.0 version package (SPSS, Inc., Chicago, IL, USA). Data were
expressed as mean ± standard deviation for normally distributed
otherwise median [interquartile range, M (QR)]. The non-normal
distributed FeNO values were log transformed. The comparisons
between groups were used one-way analysis of variance. The
diagnostic value of the FeNO for predicting BHR was revealed by ROC
curve in AR patients. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic and baseline
characteristics
A total of 102 subjects underwent screening, 93
subjects (45 AR patients, 20 AR with asthma patients, and 28 normal
controls) enrolled in this study and included in the analysis. The
AR group was divided into two sub-groups: AR with asymptomatic BHR
group and the AR without BHR group. There were no significant
differences in baseline demographic characteristics (Table I). The spirometric parameters
(FEV1, PEF, MMEF, MEF50% and
MEF25% percent predicted) in the AR with asthma group
were lower compared with that in AR and normal control groups with
significant differences (P<0.05), except FVC percent predicted
and FEV1/FVC% (P>0.05) (Table I). The duration periods of AR and
asthma were 7.5 (9.0) and 5.0 (8.0) years, respectively, in the AR
with asthma group; and the duration period of AR was 9.0 (4.5)
years in AR group, where the values in brackets show the
interquartile range of the duration of the disease. The severities
of AR in the AR with asthma groups were predominantly moderate to
severe.
 | Table I.Demographic and baseline
characteristics. |
Table I.
Demographic and baseline
characteristics.
| Characteristic | AR (n=45) | AR and asthma
(n=20) | Control (n=28) | P-value |
|---|
| Age (year) |
27.9±9.8 |
33.6±10.1 |
30.8±12.1 | >0.05 |
| Gender (male) | 18 | 10 | 16 | >0.05 |
| Height (cm) | 163.4±8.3 | 162.7±9.1 | 164.1±8.2 | >0.05 |
| Weight (kg) |
56.6±9.7 |
53.9±7.0 |
58.4±8.6 | >0.05 |
| FVC pred (%) |
97.15±11.78 |
89.04±9.48 |
99.68±13.17 | >0.05 |
| FEV1 pred
(%) |
94.56±10.83 |
79.07±8.53 |
94.97±9.17 | <0.05 |
| FEV1/FVC
(%) |
83.15±4.87 |
77.35±11.49 |
80.70±6.25 | >0.05 |
| PEF pred (%) |
99.48±12.39 |
83.70±14.01 |
108.61±10.96 | <0.05 |
| MMEF pred (%) |
76.85±14.47 |
49.88±15.62 |
73.77±18.86 | <0.05 |
| MEF50%
pred (%) |
80.19±16.67 |
51.67±14.35 |
78.95±18.87 | <0.05 |
| MEF25%
pred (%) |
73.72±17.23 |
48.90±18.71 |
66.78±20.88 | <0.05 |
| FeNO (ppb) | 29.5
(22.0) | 61.5
(33.0) | 16.0
(10.0) | <0.05 |
| EOs in sputum
(%) |
4.36±4.76 |
18.58±11.26 |
0.45±0.73 | <0.05 |
FeNO levels and diagnostic
sensitivity/specificity
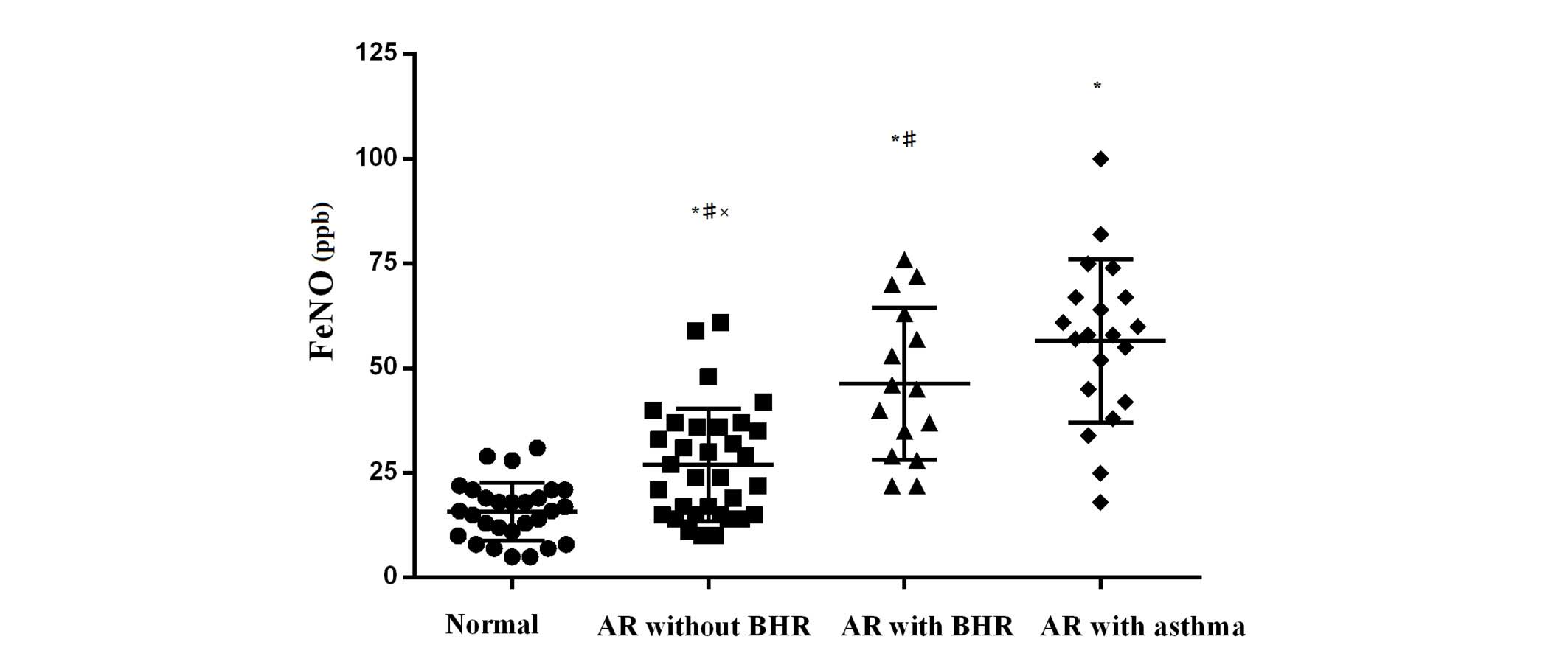
The value of FeNO was significantly higher in the AR
with asthma group compared with the AR group, and higher in the AR
group compared with the control group [61.5 (33.0) vs. 29.5 (22.0)
vs. 16.0 (10.0) ppb; P<0.05]. The values in bracket indicate the
interquartile range. The distribution (including the two sub-groups
of AR) of FeNO is shown in Fig. 2.
An increasing trend was observed in the value of FeNO from normal
controls to AR without BHR, AR + BHR and AR + asthma groups.
Patients with abnormal FeNO values (>25.0 ppb) were
significantly more in the AR with asthma group compared with the
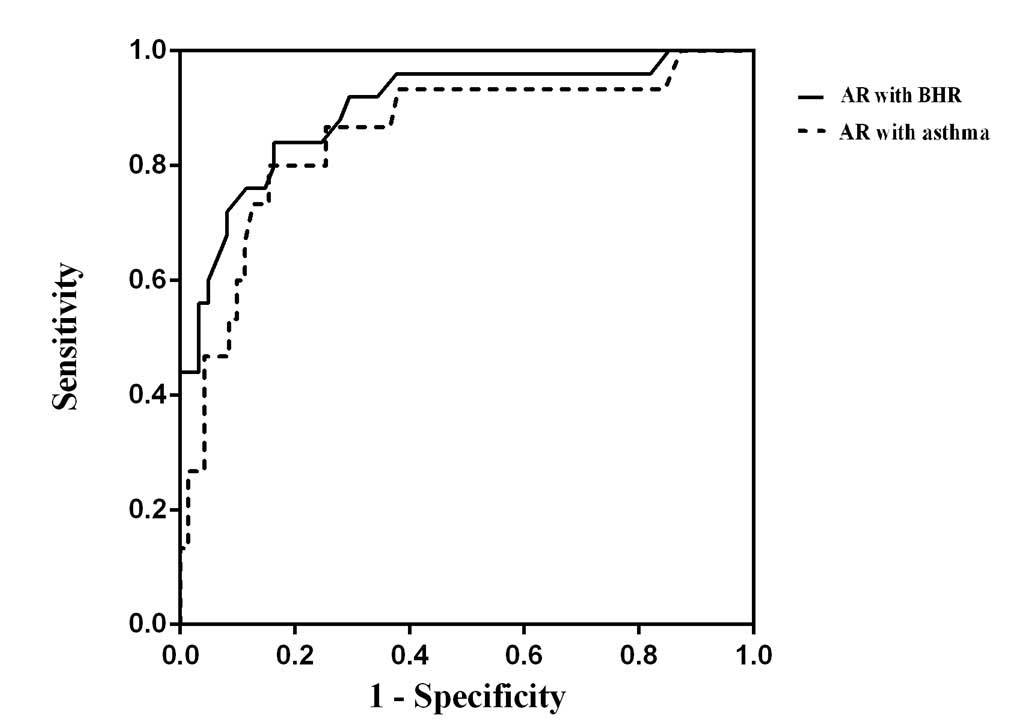
other three groups and subgroups (P<0.05). As shown in Fig. 3, receiver operating characteristic
curves (ROC) revealed a moderately increased screening capability
of FeNO for the diagnosis of BHR/asthma (AUC, 0.910; 95%CI,
0.836–0.984) compared with for asthma only (AUC, 0.873; 95%CI,
0.753–0.992). Furthermore, when taking the cut-off values at 31.5
and 38.0 ppb, the sensitivity and specificity values were 0.750,
0.737 and 0.750, 0.831, respectively.
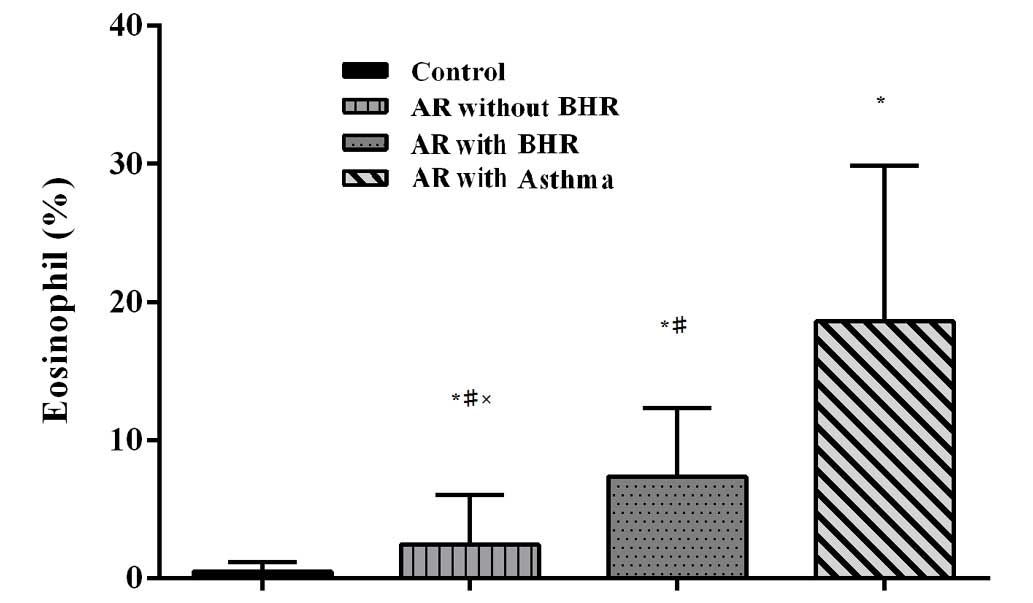
Eosinophil count
The percentages of eosinophils in induced sputum
were 0.45±0.73, 4.36±4.76 and 18.58±11.26% in the control, AR and
AR with asthma groups, respectively (P<0.05). The value of FeNO
and the percentages of eosinophils in induced sputum correlate well
(r=0.70, P<0.01). There is a similar trend of the percentage of
eosinophils in sputum from normal controls to AR without BHR, AR +
BHR and AR with asthma groups (Fig.
4). The AR without BHR exhibited a significantly higher mean
percentage of eosinophils compared with the AR with BHR group
(2.43±3.57 vs. 7.35±4.98%; P=0.047).
Discussion
In the present study, the values of FeNO in the
normal control group were substantially reduced compared with the
AR with asthma groups, which was consistent with the count of
eosinophils in the sputum, suggesting that the value of FeNO may be
used as an indicator of eosinophil inflammation in lower airways.
The high diagnostic value of FeNO in predicting BHR or asthma in AR
patients was revealed by the ROC curve. When taking the cut-off
value to be 31.5 ppb, the sensitivity and specificity were 0.846
and 0.817 for diagnosing BHR, while the sensitivity and specificity
were 0.857 and 0.847 for the diagnosis of asthma (cut-off value,
38.0 ppb). A prior study demonstrated that the eosinophil
inflammation in the lower airways may be correlated with BHR
(13), therefore it is possible that
the measurement of FeNO may predict BHR in AR patients with or
without asthma, and the present results were consistent with
previous findings (11,20). Furthermore, the measurement of FeNO
is non-invasive, simple, reproducible and safe. Thus we propose
that the measurement of FeNO may be used for the early detection of
inflammation and BHR in AR patients.
In the present study, the median value of FeNO [29.5
(22.0) ppb] in the AR group was higher compared with the mean value
[17.7 (6.1) ppb] of Guangzhou normal adults, where the values in
bracket are the interquartile range. In addition, the FeNO values
of 10% (3/30) of AR without BHR subjects were higher than the upper
limit of normal values (ULN; 41.0 ppb) of Guangzhou adults, which
suggests that even in AR patients without BHR or lower airway
symptoms there may already detected lower airways inflammation. The
percentages of subjects with higher FeNO values than the ULN values
of Guangzhou adults in AR with asymptomatic BHR and AR + asthma
groups were 60.0 and 65%, increasingly.
As is an independent risk factor of asthma, AR
promotes the development and affects the control of asthma. It has
been demonstrated that moderate/severe persistent AR was a higher
risk factor than seasonal and mild persistent AR for AR patients
developing asthma (21). A
proportion of AR patients experienced AR, AR with asymptomatic BHR,
then with lower airways symptoms and finally developed to asthma.
There is a consecutive process in the progression of AR into
asthma, as previously demonstrated by a long-term follow-up study
(22). This has also been confirmed
through a retrospectively study (23,24). The
present results showed that the mean duration of AR was longer than
asthma in the AR with asthma group, although certain patients
reported that asthma was diagnosed earlier than AR. Although there
are different phenotypes of asthma, and not all asthma phenotypes
are characterized by elevated FeNO levels, the measurement of FeNO
remained useful for detecting BHR in AR, as the majority of atopic
asthmatics exhibited elevated eosinophilic inflammation and FeNO
levels. The present findings demonstrated the use of FeNO in
detecting the consecutive progression of AR to AR with BHR, and
ultimately to AR with asthma, which supports the hypothesis that AR
and asthma represent ‘one airway, one disease’. The early diagnosis
of BHR in AR and resulting early intervention may help to prevent
the development of asthma and other complications from AR.
Several limitations of the study were considered.
Firstly, the present study was a cross-section survey, which can
not directly demonstrate the process of AR developed to asthma.
Secondly, the sample size was limited in each group, although they
were unselected subjects. Thirdly, not all AR patients ultimately
developed asthma, so prospective studies with duration and severity
classification of AR patients are required.
In conclusion, the present results suggest that FeNO
measurement, as a simple and effective method of evaluation lower
airway inflammation, may be employed for predicting BHR/asthma in
patients with AR, and supervising the consecutive process.
Acknowledgements
This study was supported by Changjiang Scholars and
Innovative Research Team in University (grant no. ITR0961); The
National Key Technology R&D Program of the 12th National
Five-year Development Plan (grant no. 2012BAI05B00); The National
Natural Science Foundation of China (grant no. 81300017). The
authors thank Qingxia Liu (Qingyuan People's Hospital), Guo E
(Xiangyang Central Hospital), Wenhua Jian (First Affiliated
Hospital of Guangzhou Medical University), Zhiyu Liang (Yuexiu
People's Hospital), Linting Luo, Diteng Luo, Xiangong Xu, Huayi
Huang, Yongqing Ye and Xianmiao Ye (all from the Guangzhou Medical
University) for their assistance in participant recruitment.
References
|
1
|
Zhang Y and Zhang L: Prevalence of
allergic rhinitis in China. Allergy Asthma Immunol Res. 6:105–113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grossman J: One airway, one disease.
CHEST. 111:(2 Suppl). 11S–16S. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin J, Su N, Liu G, Yin K, Zhou X, Shen H,
Chen P, Chen R, Liu C, Wu C, et al: The impact of concomitant
allergic rhinitis on asthma control: A cross-sectional nationwide
survey in China. J Asthma. 51:34–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonay M, Neukirch C, Grandsaigne M,
Leçon-Malas V, Ravaud P, Dehoux M and Aubier M: Changes in airway
inflammation following nasal allergic challenge in patients with
seasonal rhinitis. Allergy. 61:111–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linneberg A, Nielsen N Henrik, Frølund L,
Madsen F, Dirksen A and Jørgensen T: Copenhagen Allergy Study: The
link between allergic rhinitis and allergic asthma: A prospective
population-based study. The copenhagen allergy study. Allergy.
57:1048–1052. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brozek JL, Bousquet J, Baena-Cagnani CE,
et al: Allergic Rhinitis and its Impact on Asthma (ARIA)
guidelines: 2010 revision. J Allergy Clin Immunol. 126:466–476.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez MJ, Olaguibel JM, Garcia BE, Tabar
AI and Urbiola E: Comparison of allergen-induced changes in
bronchial hyperresponsiveness and airway inflammation between
mildly allergic asthma patients and allergic rhinitis patients.
Allergy. 55:531–539. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irvin CG: The nose: a window into the
asthmatic lung? Clin Exp Allergy. 40:839–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhimrao SK, Wilson SJ and Howarth PH:
Airway inflammation in atopic patients: a comparison of the upper
and lower airways. Otolaryngol Head Neck Surg. 145:396–400. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braunstahl GJ, Fokkens WJ, Overbeek SE,
KleinJan A, Hoogsteden HC and Prins JB: Mucosal and systemic
inflammatory changes in allergic rhinitis and asthma: a comparison
between upper and lower airways. Clin Exp Allergy. 33:579–587.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith AD, Cowan JO, Filsell S, McLachlan
C, Monti-Sheehan G, Jackson P and Taylor DR: Diagnosing asthma:
comparisons between exhaled nitric oxide measurements and
conventional tests. Am J Respir Crit Care Med. 169:473–478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang DY, Goh DY, Ho AK, Chew FT, Yeoh KH
and Lee BW: The upper and lower airway responses to nasal challenge
with house-dust mite Blomia tropicalis. Allergy. 58:78–82. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Graaf-int Veld C, Garrelds IM, Koenders
S and van Wijk R Gerth: Relationship between nasal hyperreactivity,
mediators and eosinophils in patients with perennial allergic
rhinitis and controls. Clin Exp Allergy. 26:903–908. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Global Strategy for Asthma Management and
Prevention, . Global Initiative for Asthma (GINA). 2011, http://www.ginasthma.orgAccessed December
20, 2011.
|
|
15
|
Luo W, Lai KF, Chen RC, Chen QL and Zhong
NS: Establishment of reference values for cellularity in induced
sputum of healthy adults in Guangzhou. Guo Ji Hu Xi Za Zhi She.
16:1213–1215. 2007.(In Chinese).
|
|
16
|
American Thoracic Society; European
Respiratory Society, . ATS/ERS recommendations for standardized
procedures for the online and offline measurement of exhaled lower
respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir
Crit Care Med. 171:912–930. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang CY, Zheng JP, Guan WJ, Xie YQ, Gao
Y, Liu QX, An JY, Yu XX and Liu WT: Reference ranges and predicted
equations for fractional exhaled nitric oxide in adult hans. Zhong
Guo Shi Yong Nei Ke Za Zhi. 8:608–613. 2012.(In Chinese).
|
|
18
|
Miller MR, Hankinson J, Brusasco V, Burgos
F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP,
Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas
D, Pedersen OF, Pellegrino R, Viegi G and Wanger J: ATS/ERS Task
Force: Standardization of spirometry. Eur Respir J. 26:319–338.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng JP and Zhong NS: Normative values of
pulmonary function testing in Chinese adults. Chin Med J (Engl).
115:50–54. 2002.PubMed/NCBI
|
|
20
|
Schneider A, Schwarzbach J, Faderl B,
Welker L, Karsch-Völk M and Jörres RA: FENO measurement and sputum
analysis for diagnosing asthma in clinical practice. Respir Med.
107:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marcucci F, Passalacqua G, Canonica GW,
Frati F, Salvatori S, Dicara G, Petrini I, Bernini M, Novembre E,
Bernardini R, et al: Lower airway inflammation before and after
house dust mite nasal challenge: An age and allergen
exposure-related phenomenon. Respir Med. 101:1600–1608. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Settipane RJ, Hagy GW and Settipane GA:
Long-term risk factors for developing asthma and allergic rhinitis:
a 23-year follow-up study of college students. Allergy Proc.
15:21–25. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stachler RJ: Comorbidities of asthma and
the unified airway. Int Forum Allergy Rhinol. 5:S17–S22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moshe S, Slodownik D, Yagev Y, Segal N,
Tavor M, Afek A and Zack O: Atopy as a risk factor for the
development of asthma in young recruits. J Asthma. 52:453–457.
2015. View Article : Google Scholar : PubMed/NCBI
|